Effects of Natural and Artificial Surfactants on Diffusive Boundary Dynamics and Oxygen Exchanges across the Air–Water Interface
Abstract
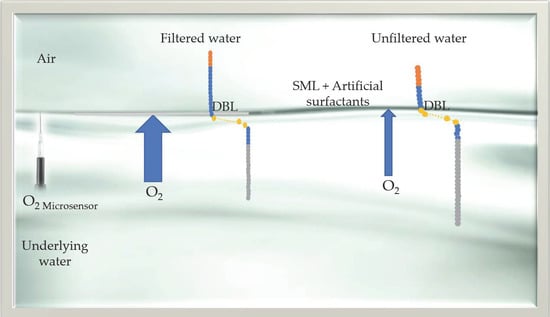
1. Introduction
2. Materials and Methods
2.1. Sample Collection
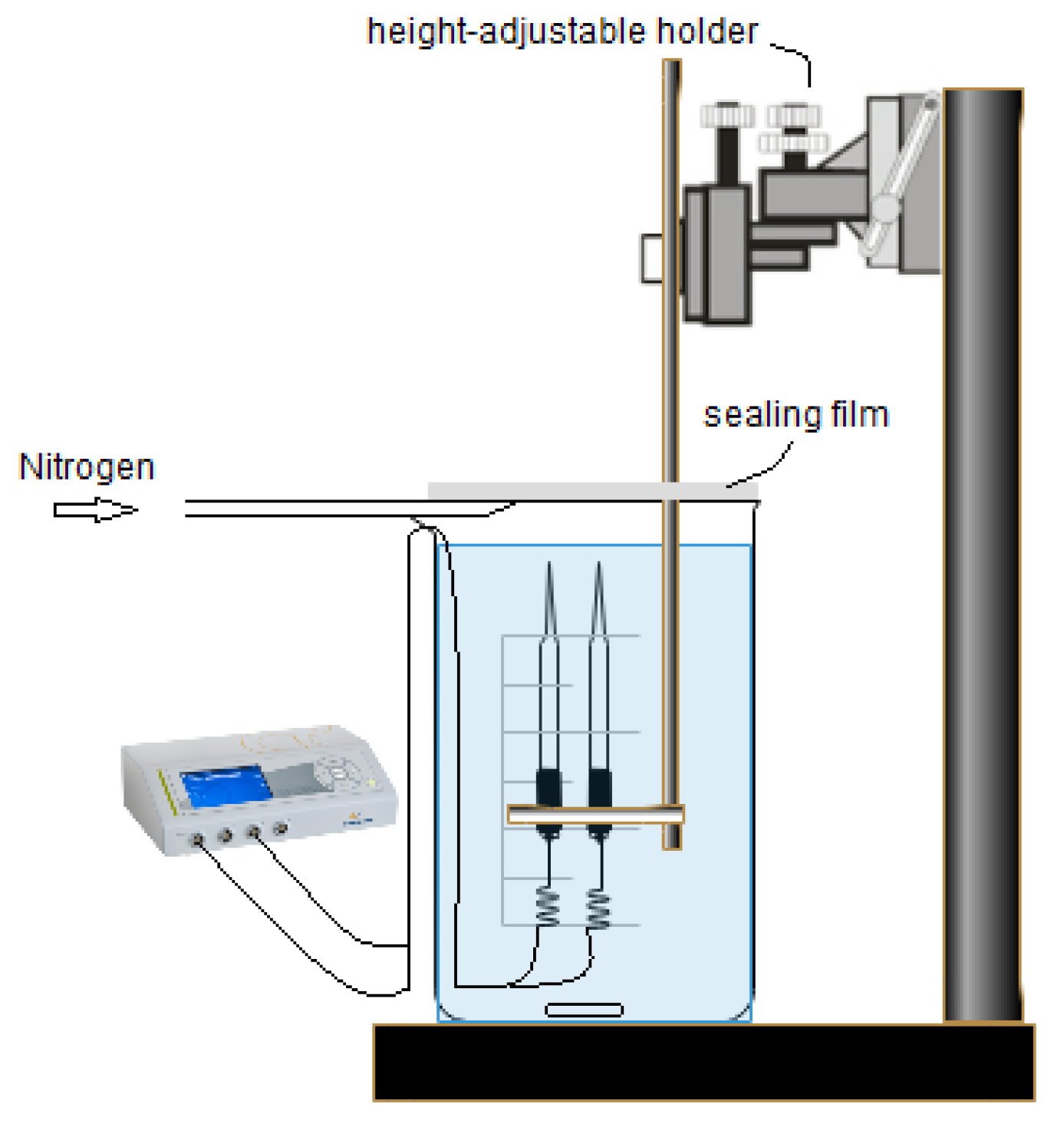
2.2. Measurement of O2 Concentration and Diffusive Boundary Layer (DBL) in Natural Seawater and Deionized Water
2.3. Surface Tension Measurements
2.3.1. Surface Tension as a Function of Temperature and Salinity
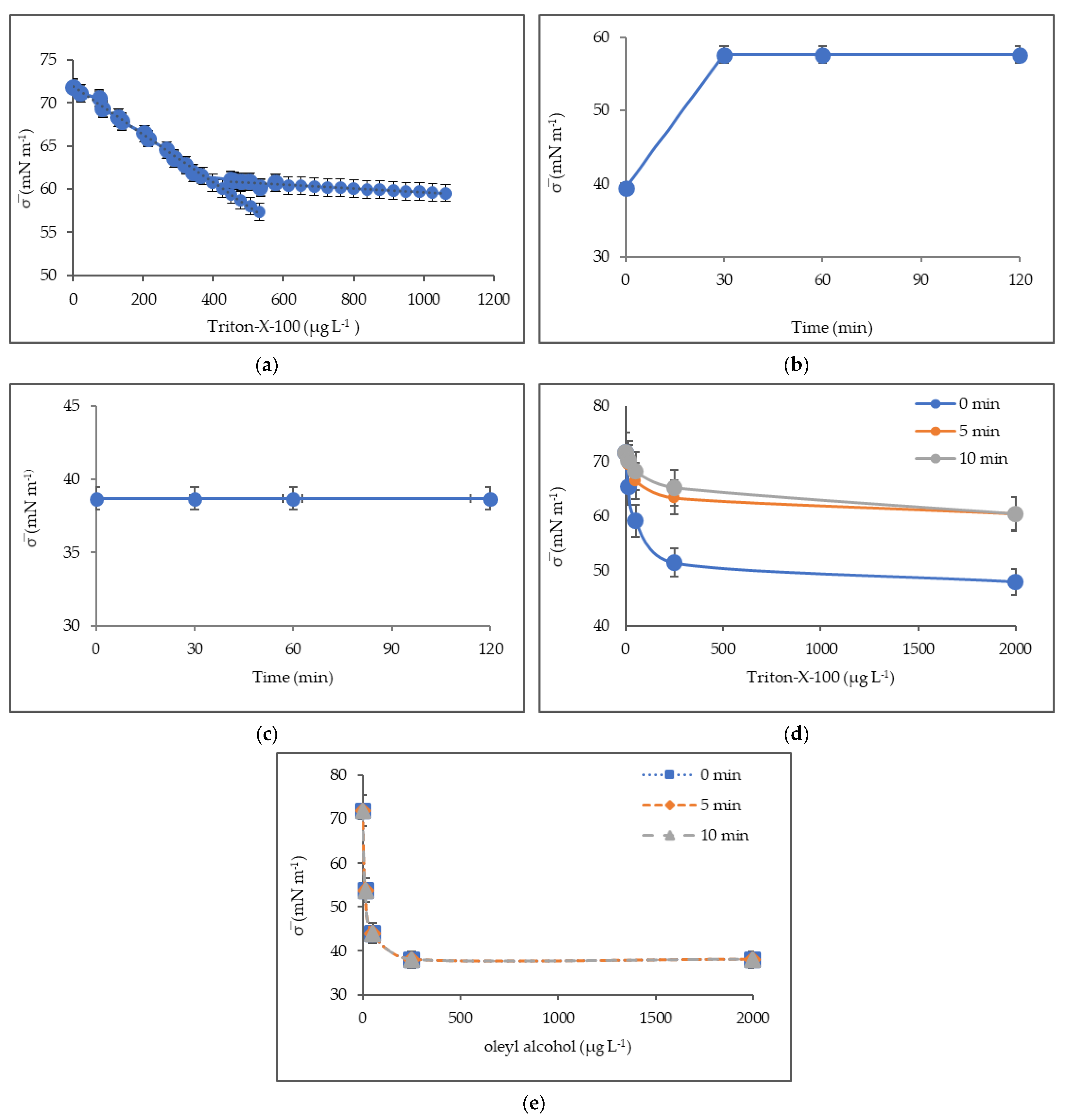
2.3.2. Surface Tension of Deionized Water with Triton-X-100 and Oleyl Alcohol
3. Results
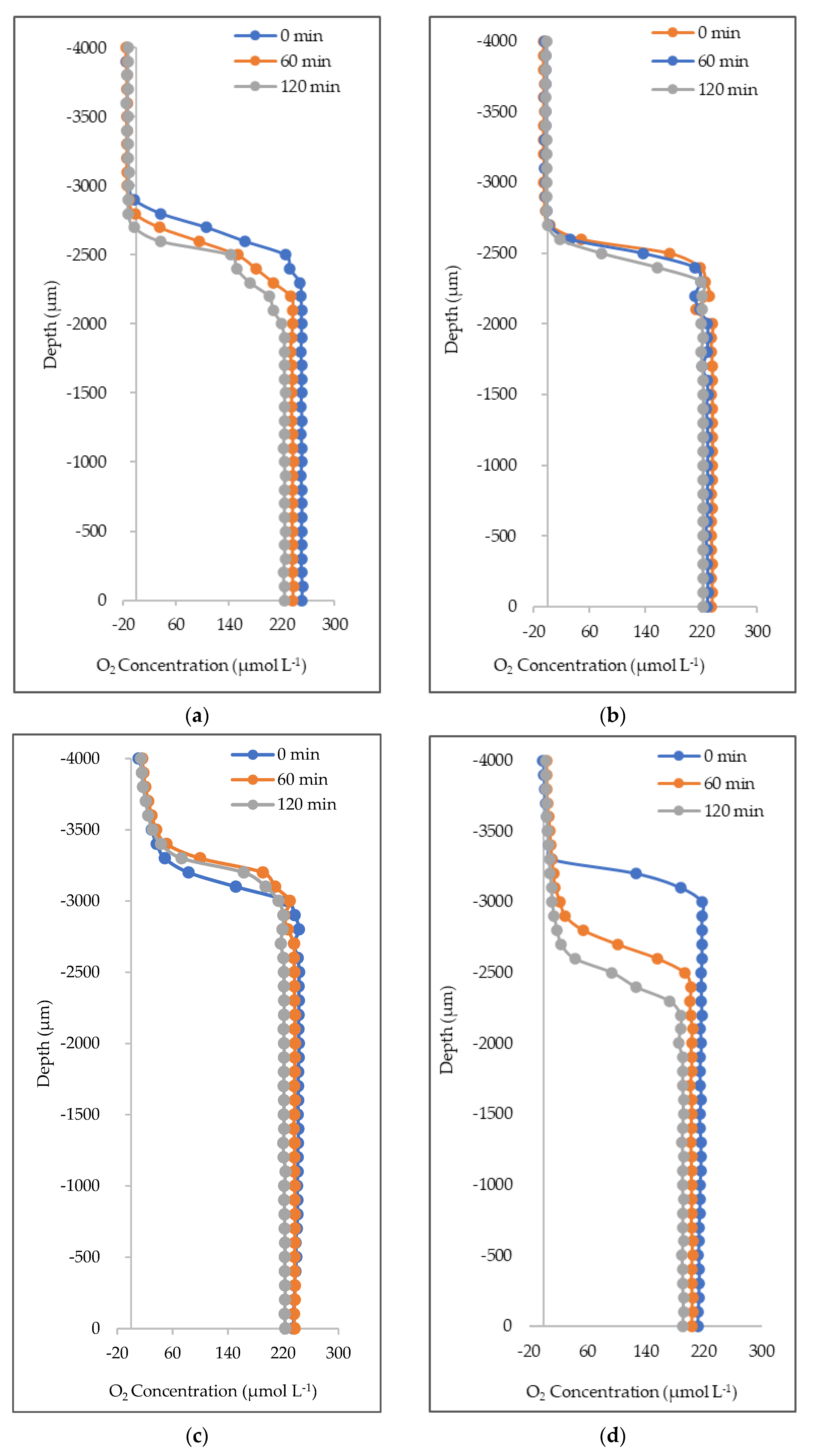
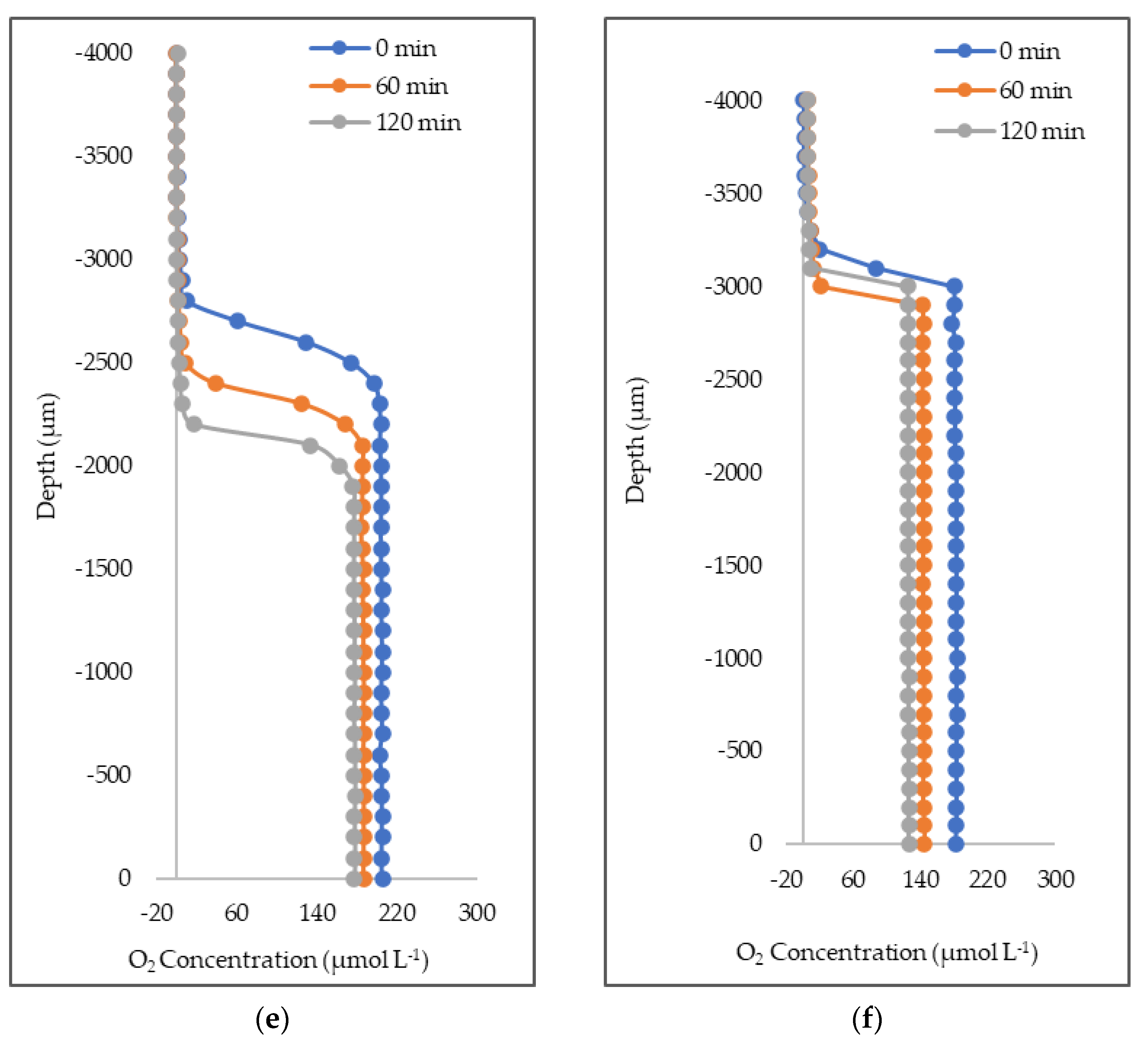
3.1. O2 Concentration Loss Diffusive Outflux in Natural Seawater and Deionized Water
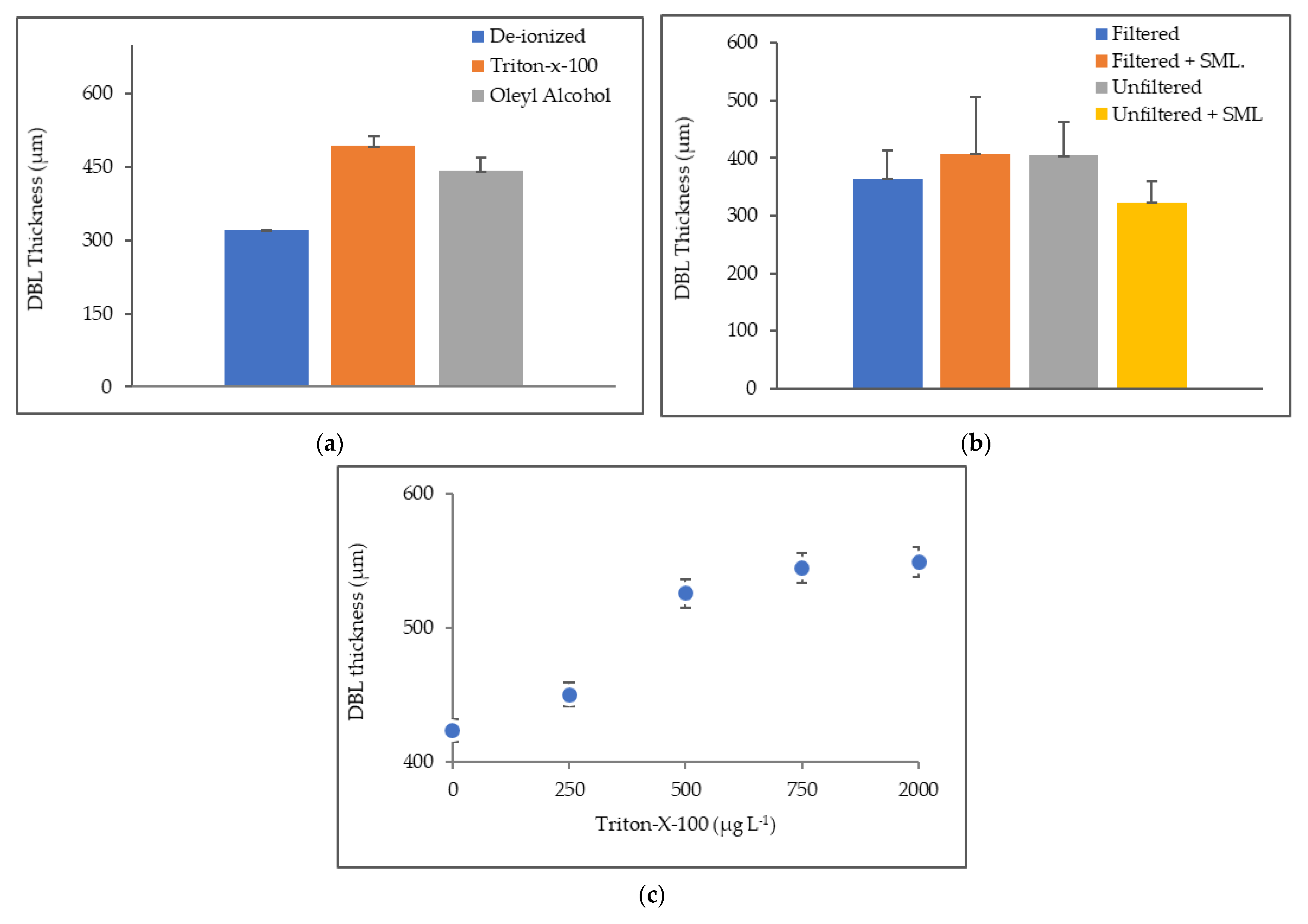
3.2. Thickness of Diffusive Boundary Layer (DBL)
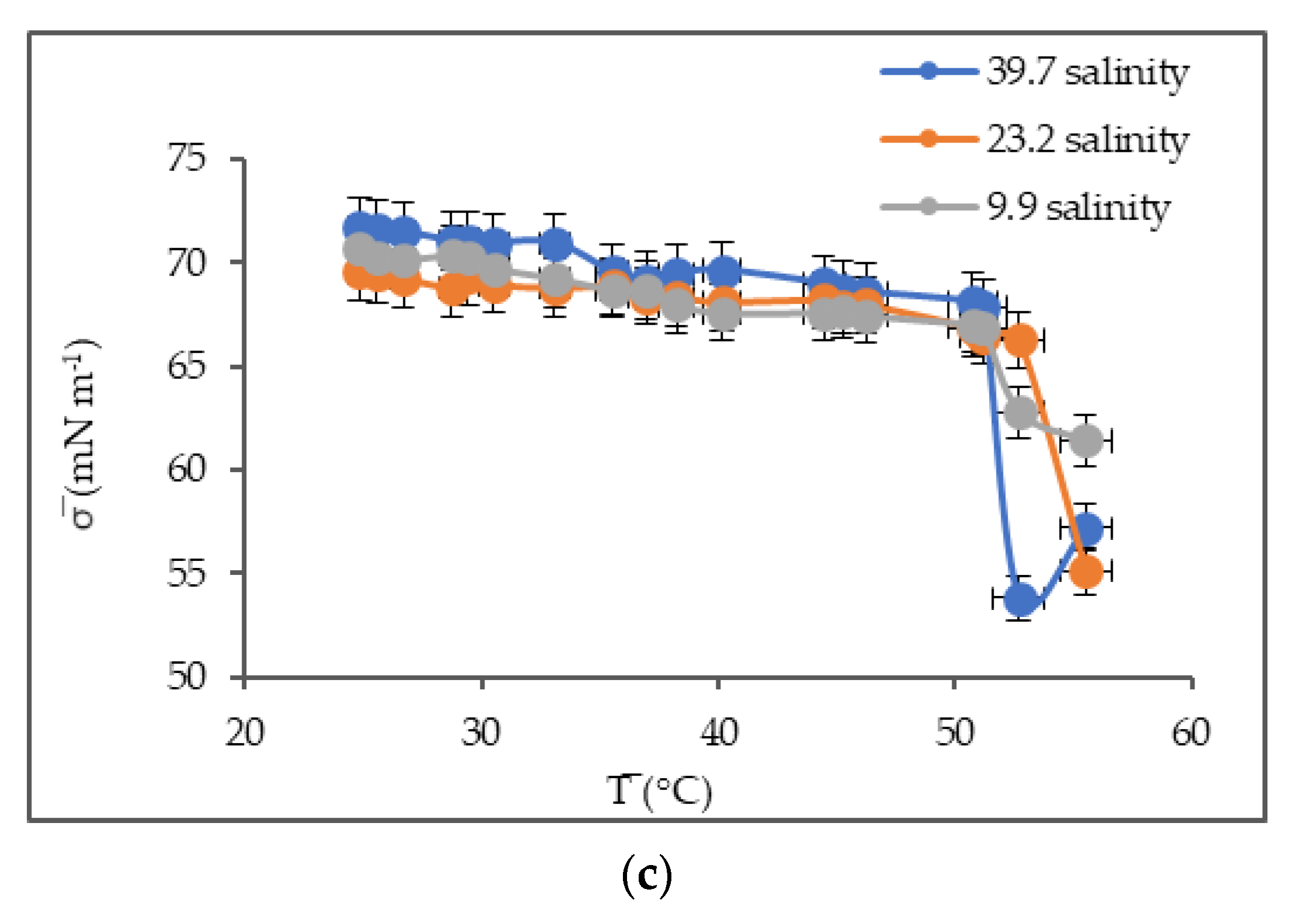
3.3. Surface Tension as a Function of Temperature and Salinity
3.4. Surface Tension of Deionized Water with Triton-X-100 and Oleyl Alcohol
4. Discussion
4.1. Oxygen Concentration, Exchange, and Suppression
4.2. Thickness of Diffusive Boundary Layer (DBL)
4.3. Surface Tension
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wurl, O.; Ekau, W.; Landing, W.M.; Zappa, C.J.; Bowman, J. Sea surface microlayer in a changing ocean—A perspective. Elem. Sci. Anthr. 2017, 5, 31. [Google Scholar] [CrossRef]
- Schimpf, U.; Garbe, C.; Jähne, B. Investigation of transport processes across the sea surface microlayer by infrared imagery. J. Geophys. Res. Ocean. 2004, 109, C08S13. [Google Scholar] [CrossRef]
- Mustaffa, N.I.H.; Ribas-Ribas, M.; Banko-Kubis, H.M.; Wurl, O. Global reduction of in situ CO2 transfer velocity by natural surfactants in the sea-surface microlayer. Proc. R. Soc. A 2020, 476, 20190763. [Google Scholar] [CrossRef]
- Garbe, C.S.; Rutgersson, A.; Boutin, J.; De Leeuw, G.; Delille, B.; Fairall, C.W.; Gruber, N.; Hare, J.; Ho, D.T.; Johnson, M.T.; et al. Transfer across the air-water interface. In Ocean-Atmosphere Interactions of Gases and Particles; Springer: Berlin/Heidelberg, Germany, 2014; pp. 55–112. [Google Scholar]
- Bock, E.J.; Hara, T.; Frew, N.M.; McGillis, W.R. Relationship between air-sea gas transfer and short wind waves. J. Geophys. Res. Ocean. 1999, 104, 25821–25831. [Google Scholar] [CrossRef]
- Zappa, C.J.; Raymond, P.A.; Terray, E.A.; McGillis, W.R. Variation in surface turbulence and the gas transfer velocity over a tidal cycle in a macro-tidal estuary. Estuaries 2003, 26, 1401–1415. [Google Scholar] [CrossRef]
- Jahne, B.; Münnich, K.O.; Siegenthaler, U. Measurements of gas exchange and momentum transfer in a circular wind-water tunnel. Tellus 1979, 31, 321–329. [Google Scholar] [CrossRef]
- Schrage, R.W. A Theoretical Study of Interphase Mass Transfer; Columbia University Press: New York, NY, USA, 1953. [Google Scholar]
- Mallinger, W.D.; Mickelson, T.P. Experiments with monomolecular films on the surface of the open sea. J. Phys. Oceanogr. 1973, 3, 328–336. [Google Scholar] [CrossRef][Green Version]
- Gundersen, J.K.; Jorgensen, B.B. Microstructure of diffusive boundary layers and the oxygen uptake of the sea floor. Nature 1990, 345, 604–607. [Google Scholar] [CrossRef]
- Rahlff, J.; Stolle, C.; Giebel, H.A.; Ribas-Ribas, M.; Damgaard, L.R.; Wurl, O. Oxygen profiles across the sea-surface microlayer—Effects of diffusion and biological activity. Front. Mar. Sci. 2019, 6, 11. [Google Scholar] [CrossRef]
- Revsbech, N.P.; Madsen, B.; Jørgensen, B.B. Oxygen production and consumption in sediments determined at high spatial resolution by computer simulation of oxygen microelectrode data. Limnol. Oceanogr. 1986, 31, 293–304. [Google Scholar] [CrossRef]
- Ribas-Ribas, M.; Helleis, F.; Rahlff, J.; Wurl, O. Air-water CO2-exchange in a large annular wind-wave tank and the effects of surfactants. Front. Mar. Sci. 2018, 5, 457. [Google Scholar] [CrossRef]
- Wurl, O.; Miller, L.; Röttgers, R.; Vagle, S. The distribution and fate of surface-active substances in the sea-surface microlayer and water column. Mar. Chem. 2009, 115, 1–9. [Google Scholar] [CrossRef]
- Jarvis, N.L.; Garrett, W.D.; Scheiman, M.A.; Timmons, C.O. Surface chemical characterization of surface-active material in seawater. Limnol. Oceanogr. 1967, 12, 88–96. [Google Scholar] [CrossRef]
- Serafini, P.; Leyes, M.F.; Pereyra, R.B.; Schulz, E.P.; Durand, G.A.; Schulz, P.C.; Ritacco, H.A. The aqueous Triton X-100–dodecyltrimethylammonium bromidemicellar mixed system. Experimental results and thermodynamic analysis. Colloids Surf. A Physicochem. Eng. Asp. 2018, 559, 127–135. [Google Scholar] [CrossRef]
- Knepper, T.P.; de Voogt, P.; Barcelo, D. Analysis and Fate of Surfactants in the Aquatic Environment; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Nayar, K.G.; Panchanathan, D.; McKinley, G.H.; Lienhard, J.H. Surface tension of seawater. J. Phys. Chem. Ref. Data 2014, 43, 043103. [Google Scholar] [CrossRef]
- Schmidt, R.; Schneider, B. The effect of surface films on the air–sea gas exchange in the Baltic Sea. Mar. Chem. 2011, 126, 56–62. [Google Scholar] [CrossRef]
- Schückel, U.; Beck, M.; Kröncke, I. Spatial variability in structural and functional aspects of macrofauna communities and their environmental parameters in the Jade Bay (Wadden Sea Lower Saxony, southern North Sea). Helgol. Mar. Res. 2013, 67, 121–136. [Google Scholar] [CrossRef]
- Harvey, G.W.; Burzell, L.A. A simple microlayer method for small samples 1. Limnol. Oceanogr. 1972, 17, 156–157. [Google Scholar] [CrossRef]
- Wurl, O.; Stolle, C.; Van Thuoc, C.; Thu, P.T.; Mari, X. Biofilm-like properties of the sea surface and predicted effects on air–sea CO2 exchange. Prog. Oceanogr. 2016, 144, 15–24. [Google Scholar] [CrossRef]
- Romano, J.C. Sea-surface slick occurrence in the open sea (Mediterranean, Red Sea, Indian Ocean) in relation to wind speed. Deep. Sea Res. Part I Oceanogr. Res. Pap. 1996, 43, 411–423. [Google Scholar] [CrossRef]
- Petters, S.S.; Petters, M.D. Surfactant effect on cloud condensation nuclei for two-component internally mixed aerosols. J. Geophys. Res. Atmos. 2016, 121, 1878–1895. [Google Scholar] [CrossRef]
- Saunders, P.M. The accuracy of measurement of salinity, oxygen and temperature in the deep ocean. J. Phys. Oceanogr. 1986, 16, 189–195. [Google Scholar] [CrossRef]
- Gallaher, W.U. Dissolved Oxygen Changes During Filtration. J. Am. Water Work. Assoc. 1927, 17, 476–480. [Google Scholar] [CrossRef]
- Parker, C.A.; O’Reilly, J.E. Oxygen depletion in Long Island Sound: A historical perspective. Estuaries 1991, 14, 248–264. [Google Scholar] [CrossRef]
- Romanescu, G.; Stoleriu, C.C. Seasonal Variation of Temperature, p H, and Dissolved Oxygen Concentration in Lake Rosu, Romania. CLEAN–Soil Air Water 2014, 42, 236–242. [Google Scholar] [CrossRef]
- Sieburth, J.M. Microbiological and Organic-Chemical Processes in the Surface and Mixed Layers in Air-Sea Exchange of Gases and Particles 1983; Springer: Dordrecht, The Netherlands; pp. 121–172.
- Obernosterer, I.; Catala, P.; Reinthaler, T.; Herndl, G.J.; Lebaron, P. Enhanced heterotrophic activity in the surface microlayer of the Mediterranean Sea. Aquat. Microb. Ecol. 2005, 39, 293–302. [Google Scholar] [CrossRef]
- Le Moigne, F.A.; Cisternas-Novoa, C.; Piontek, J.; Maßmig, M.; Engel, A. On the effect of low oxygen concentrations on bacterial degradation of sinking particles. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yates, K.K.; Dufore, C.; Smiley, N.; Jackson, C.; Halley, R.B. Diurnal variation of oxygen and carbonate system parameters in Tampa Bay and Florida Bay. Mar. Chem. 2007, 104, 110–124. [Google Scholar] [CrossRef]
- Goldman, J.C.; Dennett, M.R.; Frew, N.M. Surfactant effects on air-water gas exchange under turbulent conditions. Deep. Sea Res. Part A Oceanogr. Res. Pap. 1988, 35, 1953–1970. [Google Scholar] [CrossRef]
- Tsunogai, S.; Tanaka, N. Flux of oxygen across the air-sea interface as determined by the analysis of dissolved components in sea water. Geochem. J. 1980, 14, 227–234. [Google Scholar] [CrossRef]
- Zhengbin, Z.; Liansheng, L.; Zhijian, W.; Jun, L.; Haibing, D. Physicochemical studies of the sea surface microlayer: I. Thickness of the sea surface microlayer and its experimental determination. J. Colloid Interface Sci. 1998, 204, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Frew, N.M.; Goldman, J.C.; Dennett, M.R.; Johnson, A.S. Impact of phytoplankton-generated surfactants on air-sea gas exchange. J. Geophys. Res. Ocean. 1990, 95, 3337–3352. [Google Scholar] [CrossRef]
- Carpenter, L.J.; Archer, S.D.; Beale, R. Ocean-atmosphere trace gas exchange. Chem. Soc. Rev. 2012, 41, 6473–6506. [Google Scholar] [CrossRef] [PubMed]
- Broecker, H.C.; Petermann, J.; Siems, W. The influence of wind on CO2-exchange in a wind-wave tunnel, including the effects of monolayers. J. Mar. Res. 1978, 36, 595–610. [Google Scholar]
- Brockmann, U.H.; Huhnerfuss, H.; Kattner, G.; Broecker, H.C.; Hentzschel, G. Artificial surface films in the sea area near Sylt 1. Limnol. Oceanogr. 1982, 27, 1050–1058. [Google Scholar] [CrossRef]
- Salter, M.E.; Upstill-Goddard, R.C.; Nightingale, P.D.; Archer, S.D.; Blomquist, B.; Ho, D.T.; Huebert, B.; Schlosser, P.; Yang, M. Impact of an artificial surfactant release on air-sea gas fluxes during Deep Ocean Gas Exchange Experiment II. J. Geophys. Res. Ocean. 2011, 116, C11016. [Google Scholar] [CrossRef]
- Barger, W.R.; Garrett, W.D.; Mollo-Christensen, E.L.; Ruggles, K.W. Effects of an artificial sea slick upon the atmosphere and the ocean. J. Appl. Meteorol. 1970, 9, 396–400. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, W.; Liu, L.; Liu, C.; Chen, F. Direct determination of thickness of sea surface microlayer using a pH microelectrode at original location. Sci. China Ser. B Chem. 2003, 46, 339–351. [Google Scholar] [CrossRef]
- Peng, T.H.; Broecker, W.S.; Mathieu, G.G.; Li, Y.H.; Bainbridge, A.E. Radon evasion rates in the Atlantic and Pacific Oceans as determined during the GEOSECS program. J. Geophys. Res. Ocean. 1979, 84, 2471–2486. [Google Scholar] [CrossRef]
- Dragcevic, D.; Vukovic, M.; Cukman, D.; Pravdic, V. Properties of the seawater-air interface. Dynamic surface tension studies 1. Limnol. Oceanogr. 1979, 24, 1022–1030. [Google Scholar] [CrossRef]
- Frew, N.M.; Nelson, R.K. Scaling of marine microlayer film surface pressure-area isotherms using chemical attributes. J. Geophys. Res. Ocean. 1992, 97, 5291–5530. [Google Scholar] [CrossRef]
- Zhang, X.; Han, J.; Zhang, X.; Shen, J.; Chen, Z.; Chu, W.; Kang, J.; Zhao, S.; Zhou, Y. Application of Fourier transform ion cyclotron resonance mass spectrometry to characterize natural organic matter. Chemosphere 2020, 260, 127458. [Google Scholar] [CrossRef] [PubMed]
- Wurl, O.; Sin, T.M. Analysis of dissolved and particulate organic carbon with the HTCO technique. In Practical Guidelines for the Analysis of Seawater; CRC Press: Boca Raton, FL, USA, 2009; pp. 45–60. [Google Scholar]
- Gebhart, B.; Pera, L. The nature of vertical natural convection flows resulting from the combined buoyancy effects of thermal and mass diffusion. Int. J. Heat Mass Transf. 1971, 14, 2025–2050. [Google Scholar] [CrossRef]
- Richards, T.W.; Coombs, L.B. The surface tensions of water, methyl, ethyl and isobutyl alcohols, ethyl butyrate, benzene and toluene. J. Am. Chem. Soc. 1915, 37, 1656–1676. [Google Scholar] [CrossRef][Green Version]
- Harkins, W.D.; Young, T.F.; Cheng, L.H. The ring method for the determination of surface tension. Science 1926, 64, 333–336. [Google Scholar] [CrossRef]
- Vargaftik, N.B.; Volkov, B.N.; Voljak, L.D. International tables of the surface tension of water. J. Phys. Chem. Ref. Data 1983, 12, 817–820. [Google Scholar] [CrossRef]
- Jian, C.; Poopari, M.R.; Liu, Q.; Zerpa, N.; Zeng, H.; Tang, T. Mechanistic understanding of the effect of temperature and salinity on the water/toluene interfacial tension. Energy Fuels 2016, 30, 10228–10235. [Google Scholar] [CrossRef]
- Sghaier, N.; Prat, M.; Nasrallah, S.B. On the influence of sodium chloride concentration on equilibrium contact angle. Chem. Eng. J. 2006, 122, 47–53. [Google Scholar] [CrossRef]
- Wang, X.; Chen, C.; Poeschl, U.; Su, H.; Cheng, Y. Molecular Dynamics Simulation of Surface Tension of NaCl Aqueous Solution at 298.15 K: From Diluted to Highly Supersaturated Concentrations. EGUGA 2017, 19, 1015. [Google Scholar]
- Zhang, C.; Carloni, P. Salt effects on water/hydrophobic liquid interfaces: A molecular dynamics study. J. Phys. Condens. Matter 2012, 24, 124109. [Google Scholar] [CrossRef]
- Pereira, R.; Ashton, I.; Sabbaghzadeh, B.; Shutler, J.D.; Upstill-Goddard, R.C. Reduced air–sea CO2 exchange in the Atlantic Ocean due to biological surfactants. Nat. Geosci. 2018, 11, 492–496. [Google Scholar] [CrossRef]

| Sample Dates | Samples | Periods |
|---|---|---|
| 6 November 2017 | Unfiltered natural | |
| 7 November 2017 | seawater | Autumn |
| 7 November 2017 | ||
| 6 November 2017 | Unfiltered natural | Autumn |
| 22 January 2018 | seawater and | Winter |
| 6 July 2018 | unfiltered SML | Summer |
| 24 January 2018 | Filtered natural | Winter |
| 31 January 2018 | seawater | Winter |
| 5 July 2018 | Summer | |
| 24 January 2018 | Filtered natural seawater | Winter |
| 5 July 2018 | And filtered SML | Summer |
| 5 November 2018 | Autumn | |
| November 2018 | Deionized water | - |
| November 2018 | Triton-X-100 | - |
| November 2018 | oleyl alcohol | - |
| November 2019 | Natural seawater | - |
| November 2019 | Artificial seawater | - |
| Artificial Seawater | Temperature (°C) | Salinity |
|---|---|---|
| No dilution | 20.7 | 39.7 |
| 1:1 | 20.7 | 23.2 |
| 1:4 | 20.7 | 9.9 |
| Autumn | Autumn | Winter | Winter | Winter | Summer | Summer | Summer | |
|---|---|---|---|---|---|---|---|---|
| Time (min) | Unfiltered (µmol L−1) | Unfiltered + SML (µmol L−1) | Unfiltered + SML (µmol L−1) | Filtered (µmol L−1) | Filtered + SML (µmol L−1) | Unfiltered + SML (µmol L−1) | Filtered (µmol L−1) | Filtered+ SML. (µmol L−1) |
| 0 | 250.1 ± 3 | 235.2 ± 5 | 239.0 ± 3 | 234.6 ± 28 | 186.9 ± 2 | 205.6 ± 1 | 182.8 ± 0 | 211.1 ± 70 |
| 60 | 237.8 ± 2 | 228.8 ± 1 | 236.6 ± 1 | 217.9 ± 19 | 182.9 ± 1 | 187.6 ± 0 | 146.9 ± 0 | 201.2 ± 72 |
| 120 | 226.3 ± 3 | 222.5 ± 0 | 222.1 ± 1 | 197.3 ± 8 | 183.8 ± 2 | 177.8 ± 0 | 126.0 ± 0 | 188.8 ± 72 |
| Loss per hour | 11.9 ± 0 | 6.4 ± 5 | 8.5 ± 2 | 18.7 ± 20 | 1.6 ± 0 | 13.9 ± 1 | 28.4 ± 0 | 11.2 ± 1 |
| % loss | 9.6 | 5.4 | 7.1 | 15.9 | 1.7 | 13.6 | 31.1 | 10.6 |
| O2 Outflux (µmol O2 L−1 min−1) | ||||||||
| t0-t120 | −0.19 | −0.10 | −0.14 | −0.31 | −0.02 | −0.23 | −0.47 | −0.19 |
| Temperature (°C) | ||||||||
| 0 | 13.6 ± 2 | 13.1 ± 0 | 9.8 ± 0 | 13.4 ± 3 | 15.9 ± 1 | 17.3 ± 1 | 17.9 ± 0 | 17.2 ± 0 |
| 60 | 15.09 ± 1 | 14.7 ± 0 | 11.7 ± 0 | 14.4± 0 | 15.9 ± 1 | 16.6 ± 0 | 17.6 ± 0 | 17.1 ± 0 |
| 120 | 15.8 ± 1 | 16.1 ± 0 | 13.0 ± 0 | 15.2 ± 2 | 15.9 ± 0 | 16.4± 0 | 17.6 ± 0 | 17.3 ± 0 |
| Time (min) | Deionized Water (µmol L−1) | Deionized Water + Triton-X-100 (µmol L−1) | Deionized Water + Oleyl Alcohol (µmol L−1) |
|---|---|---|---|
| 0 | 237.3 ± 4 | 253.3 ± 40 | 241.8 ± 6 |
| 60 | 215.8 ± 8 | 243.9 ± 32 | 251.0 ± 7 |
| 120 | 198.2 ± 7 | 228.9 ± 17 | 240.8 ± 8 |
| loss per hour | 19.4 ± 3 | 12.2 ± 23 | 0.5 ± 0 |
| % loss | 16.5 | 9.6 | 0.4 |
| O2 outflux (µmol O2 L−1 min−1) | |||
| t0-t120 | −0.32 | −0.20 | −0.009 |
| Temperature (°C) | |||
| 0 | 21.1 ± 1 | 19.1 ± 1 | 19.6 ± 2 |
| 60 | 20.0 ± 1 | 18.4 ± 1 | 20.0 ± 2 |
| 120 | 19.6 ± 2 | 19.1 ± 2 | 20.8 ± 2 |
| Diffusive Boundary Layers | |||
|---|---|---|---|
| Time (min) | Deionized Water (µm) | Deionized Water + Oleyl Alcohol (µm) | Deionized Water + Triton-X-100 (µm) |
| 30 | 319 ± 28 | 423 ± 10 | 473 ± 6 |
| 60 | 294 ± 16 | 481 ± 72 | 457 ± 29 |
| 120 | 352 ± 28 | 424 ± 8 | 550 ± 5 |
| Diffusive Boundary Layers | ||||
|---|---|---|---|---|
| Time (min) | Filtered (µm) | Filtered + SML. (µm) | Unfiltered (µm) | Unfiltered + SML (µm) |
| 30 | 416 ± 53 | 499 ± 49 | 348 ± 41 | 351 ± 41 |
| 60 | 357 ± 54 | 303 ± 31 | 459 ± 24 | 333 ± 45 |
| 120 | 318 ± 6 | 412 ± 13 | 383 ± 32 | 283 ± 58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adenaya, A.; Haack, M.; Stolle, C.; Wurl, O.; Ribas-Ribas, M. Effects of Natural and Artificial Surfactants on Diffusive Boundary Dynamics and Oxygen Exchanges across the Air–Water Interface. Oceans 2021, 2, 752-771. https://doi.org/10.3390/oceans2040043
Adenaya A, Haack M, Stolle C, Wurl O, Ribas-Ribas M. Effects of Natural and Artificial Surfactants on Diffusive Boundary Dynamics and Oxygen Exchanges across the Air–Water Interface. Oceans. 2021; 2(4):752-771. https://doi.org/10.3390/oceans2040043
Chicago/Turabian StyleAdenaya, Adenike, Michaela Haack, Christian Stolle, Oliver Wurl, and Mariana Ribas-Ribas. 2021. "Effects of Natural and Artificial Surfactants on Diffusive Boundary Dynamics and Oxygen Exchanges across the Air–Water Interface" Oceans 2, no. 4: 752-771. https://doi.org/10.3390/oceans2040043
APA StyleAdenaya, A., Haack, M., Stolle, C., Wurl, O., & Ribas-Ribas, M. (2021). Effects of Natural and Artificial Surfactants on Diffusive Boundary Dynamics and Oxygen Exchanges across the Air–Water Interface. Oceans, 2(4), 752-771. https://doi.org/10.3390/oceans2040043