Isoselenazole Synthesis by Rh-Catalyzed Direct Annulation of Benzimidates with Sodium Selenite
Abstract
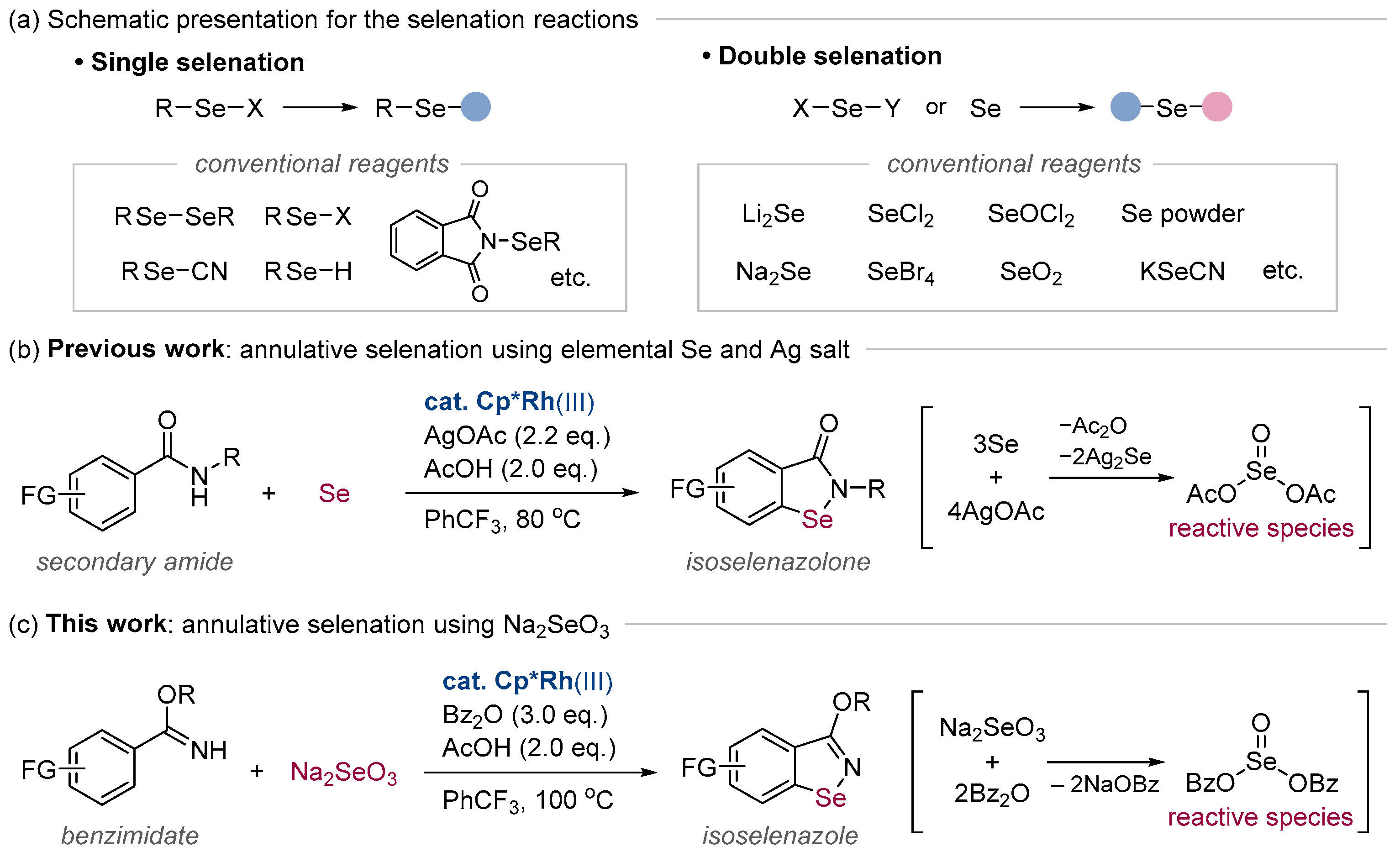
:1. Introduction
2. Materials and Methods
2.1. General Information
2.2. General Procedure for Rh-Catalyzed Annulation of Benzimidates with Sodium Selenite
2.3. Rh-Catalyzed Annulation of 1a with Sodium Selenite (5.0 mmol Scale)
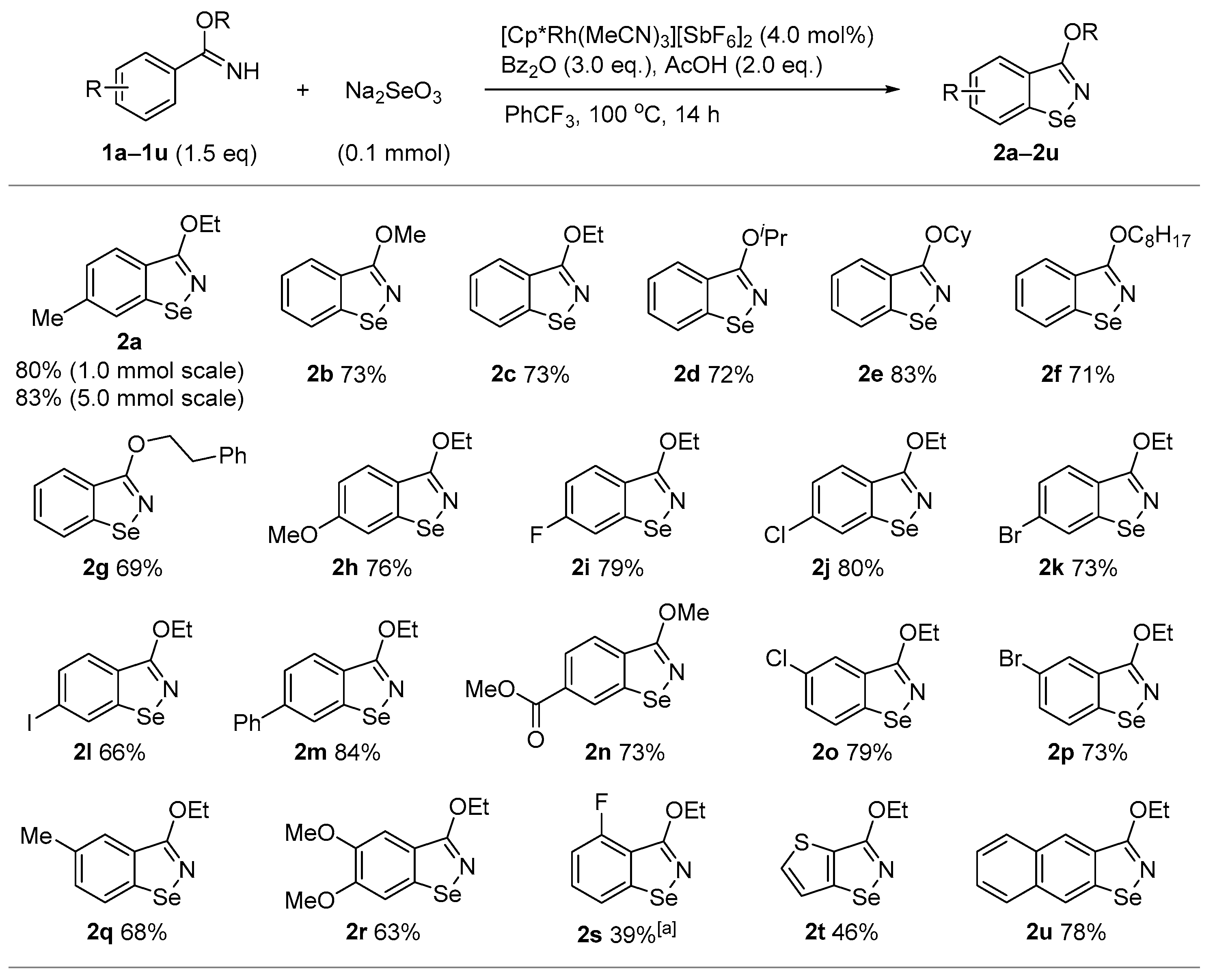
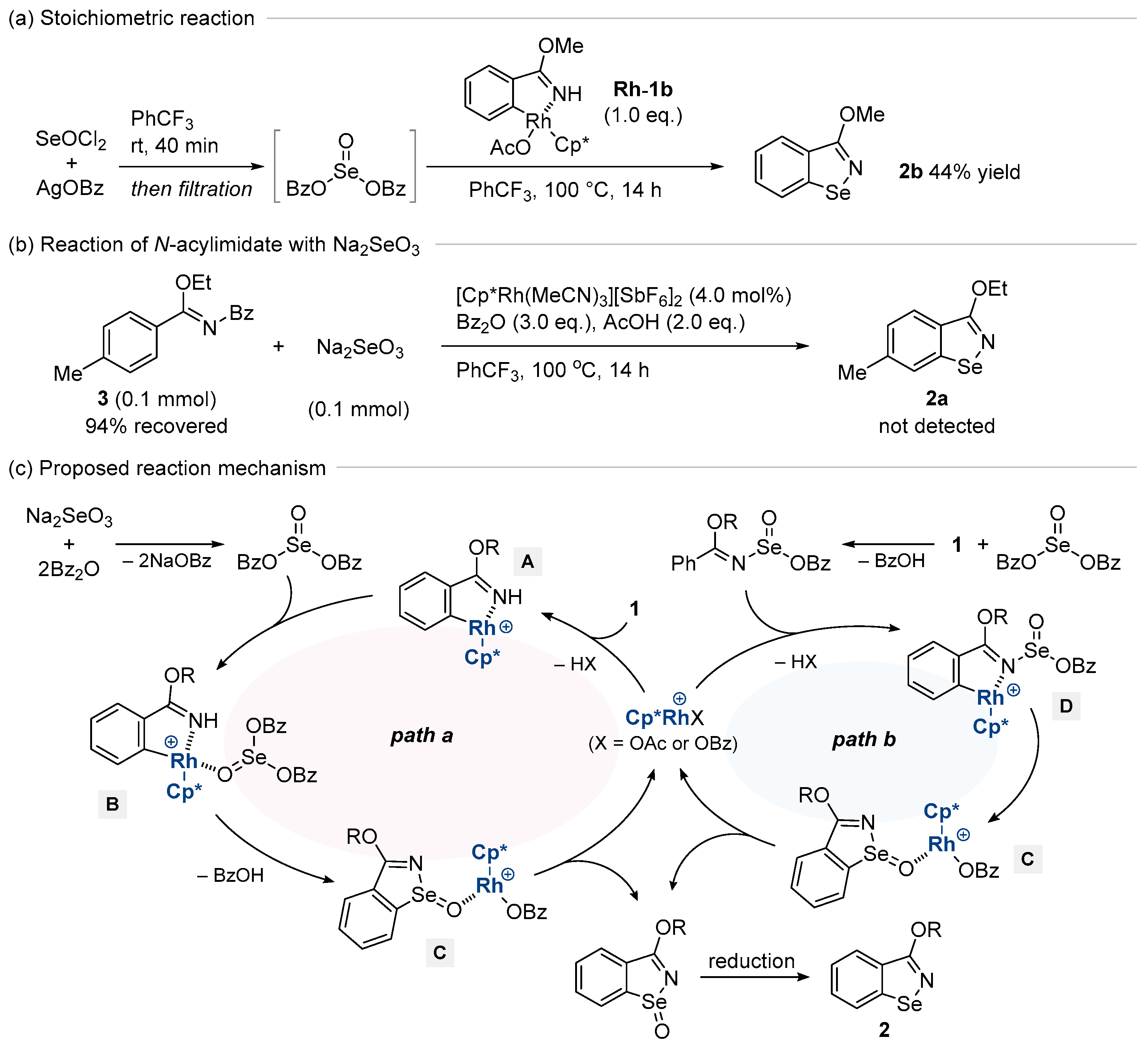
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Beletskaya, I.P.; Ananikov, V.P. Transition-Metal-Catalyzed C–S, C–Se, and C–Te Bond Formations via Cross-Coupling and Atom-Economic Addition Reactions. Achievements and Challenges. Chem. Rev. 2022, 122, 16110–16293. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, C.W.; Zeni, G.; Rocha, J.B.T. Organoselenium and Organotellurium Compounds: Toxicology and Pharmacology. Chem. Rev. 2004, 104, 6255–6285. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Li, Y.; Lu, J.; Jiang, X. Recent progress in selenium-catalyzed organic reactions. Org. Chem. Front. 2019, 6, 2999–3041. [Google Scholar] [CrossRef]
- Singh, F.H.; Wirth, T. Selenium reagents as catalysts. Catal. Sci. Technol. 2019, 9, 1073–1091. [Google Scholar] [CrossRef]
- Manjare, S.T.; Kim, Y.; Churchill, D.G. Selenium- and Tellurium-Containing Fluorescent Molecular Probes for the Detection of Biologically Important Analytes. Acc. Chem. Res. 2014, 47, 2985–2998. [Google Scholar] [CrossRef]
- Brutchey, R.L. Diorganyl Dichalcogenides as Useful Synthons for Colloidal Semiconductor Nanocrystals. Acc. Chem. Res. 2015, 48, 2918–2926. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Y.; Chen, Z.; Pan, X.; Zhang, Z.; Zhu, J.; Zhu, X. Organoselenium chemistry-based polymer synthesis. Org. Chem. Front. 2020, 7, 2815–2841. [Google Scholar] [CrossRef]
- Mugesh, G.; du Mont, W.-W.; Sies, H. Chemistry of Biologically Important Synthetic Organoselenium Compounds. Chem. Rev. 2001, 101, 2125–2180. [Google Scholar] [CrossRef]
- Hou, W.; Dong, H.; Zhang, X.; Wang, Y.; Su, L.; Xu, H. Selenium as an emerging versatile player in heterocycles and natural products modification. Drug Discov. Today 2022, 27, 2268–2277. [Google Scholar] [CrossRef]
- Hou, W.; Xu, H. Incorporating Selenium into Heterocycles and Natural Products From Chemical Properties to Pharmacological Activities. J. Med. Chem. 2022, 65, 4436–4456. [Google Scholar] [CrossRef]
- Sands, K.N.; Rengifo, E.M.; George, G.N.; Pickering, I.J.; Gelfand, B.S.; Back, T.G. The Unexpected Role of SeVI Species in Epoxidations with Benzeneseleninic Acid and Hydrogen Peroxide. Angew. Chem. Int. Ed. 2020, 59, 4283–4287. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Sargsyan, K.; Lin, C.-C.; Chen, T.; Grauffel, C.; Chen, Y.-P.; Yang, W.-Z.; Yuan, H.S.; Lim, C. Multi-targeting of functional cysteines in multiple conserved SARS-CoV-2 domains by clinically safe Zn-ejectors. Chem. Sci. 2020, 11, 9904–9909. [Google Scholar] [CrossRef]
- Ma, W.; Kaplaneris, N.; Fang, X.; Gu, L.; Mei, R.; Ackermann, L. Chelation-assisted transition metal-catalysed C–H chalcogenylations. Org. Chem. Front. 2020, 7, 1022–1060. [Google Scholar] [CrossRef]
- Sonawane, A.D.; Sonawane, R.A.; Ninomiya, M.; Koketsu, M. Diorganyl diselenides: A powerful tool for the construction of selenium containing scaffolds. Dalton Trans. 2021, 50, 12764–12790. [Google Scholar] [CrossRef]
- Gandeepan, P.; Koeller, J.; Ackermann, L. Expedient C–H Chalcogenation of Indolines and Indoles by Positional-Selective Copper Catalysis. ACS Catal. 2017, 7, 1030–1034. [Google Scholar] [CrossRef]
- Vásquez-Céspedes, S.; Ferry, A.; Candish, L.; Glorius, F. Heterogeneously Catalyzed Direct C–H Thiolation of Heteroarenes. Angew. Chem. Int. Ed. 2015, 54, 5772–5776. [Google Scholar] [CrossRef]
- Sinha, S.; Panja, S.; Grover, J.; Hazra, P.S.; Pandit, S.; Bairagi, Y.; Zhang, X.; Maiti, D. Dual Ligand Enabled Nondirected C−H Chalcogenation of Arenes and Heteroarenes. J. Am. Chem. Soc. 2022, 144, 12032–12042. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, N.; Kundu, D.; Ranu, B.C. Copper-Silver Dual Catalyzed Decyanative C–Se Cross-Coupling. Adv. Synth. Catal. 2017, 359, 329–338. [Google Scholar] [CrossRef]
- Tiecco, M.; Carlone, A.; Sternativo, S.; Marini, F.; Bartoli, G.; Melchiorre, P. Organocatalytic Asymmetric α-Selenenylation of Aldehydes. Angew. Chem. Int. Ed. 2007, 46, 6882–6885. [Google Scholar] [CrossRef]
- Xu, H.; Wang, Y.; Dong, H.; Zhang, Y.; Gu, Y.; Zhang, S.; Meng, Y.; Li, J.; Shi, X.J.; Ji, Q.; et al. Selenylation Chemistry Suitable for On-Plate Parallel and On-DNA Library Synthesis Enabling High-Throughput Medicinal Chemistry. Angew. Chem. Int. Ed. 2022, 61, e202206516. [Google Scholar] [CrossRef]
- Xu, H.; Gu, Y.; Zhang, S.; Xiong, H.; Ma, F.; Lu, F.; Ji, Q.; Liu, L.; Ma, P.; Hou, W.; et al. A Chemistry for Incorporation of Selenium into DNA-Encoded Libraries. Angew. Chem. Int. Ed. 2020, 59, 13273–13280. [Google Scholar] [CrossRef] [PubMed]
- Abdo, M.; Knapp, S. Biomimetic Seleninates and Selenonates. J. Am. Chem. Soc. 2008, 130, 9234–9235. [Google Scholar] [CrossRef]
- Krief, A.; Dumont, W.; Delmotte, C. Reaction of Organic Selenocyanates with Hydroxides: The One-Pot Synthesis of Dialkyl Diselenides from Alkyl Bromides. Angew. Chem. Int. Ed. 2000, 39, 1669–1672. [Google Scholar] [CrossRef]
- Wu, G.; Yao, Y.; Zhang, W. An MeSeSO3Na reagent for oxidative aminoselenomethylation of maleimides. Org. Chem. Front. 2021, 8, 6259–6264. [Google Scholar] [CrossRef]
- Wu, G.; Min, L.; Li, H.; Gao, W.; Ding, J.; Huang, X.; Liu, M.; Wu, H. Metal-free synthesis of alkynyl alkyl selenides via three-component coupling of terminal alkynes, Se, and epoxides. Green Chem. 2018, 20, 1560–1563. [Google Scholar] [CrossRef]
- Shin, N.H.; Lim, Y.J.; Kim, C.; Kim, Y.E.; Jeong, Y.R.; Cho, H.; Park, M.-S.; Lee, S.H. An Efficient Method for Selective Syntheses of Sodium Selenide and Dialkyl Selenides. Molecules 2022, 27, 5224. [Google Scholar] [CrossRef]
- Ma, Y.-T.; Liu, M.-C.; Zhou, Y.-B.; Wu, H.-Y. Synthesis of Organoselenium Compounds with Elemental Selenium. Adv. Synth. Catal. 2021, 363, 5386–5406. [Google Scholar] [CrossRef]
- Guan, Y.; Townsend, S.D. Metal-Free Synthesis of Unsymmetrical Organoselenides and Selenoglycosides. Org. Lett. 2017, 19, 5252–5255. [Google Scholar] [CrossRef]
- Wu, B.; Yoshikai, N. Conversion of 2-Iodobiaryls into 2,2″-Diiodobiarylsvia Oxidation-Iodination Sequences: A Versatile Route to Ladder-Type Heterofluorenes. Angew. Chem. Int. Ed. 2015, 54, 8736–8739. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.-X.; Wang, Z.; Xi, Z. Procedure-Controlled Selective Synthesis of 5-Acyl-2-iminothiazolinesand their Selenium and Tellurium Derivatives by Convergent Tandem Annulation. Angew. Chem. Int. Ed. 2011, 50, 8122–8126. [Google Scholar] [CrossRef]
- Wu, G.; Zhou, X.; Wang, C. Copper-catalyzed decarboxylative Se insertion coupling of indoles and propiolic acids. Chin. Chem. Lett. 2022, 33, 4531–4535. [Google Scholar] [CrossRef]
- He, G.; Kang, L.; Delgado, W.T.; Shynkaruk, O.; Ferguson, M.J.; McDonald, R.; Rivard, E. The Marriage of Metallacycle Transfer Chemistry with Suzuki−Miyaura Cross-Coupling to Give Main Group Element-Containing Conjugated Polymers. J. Am. Chem. Soc. 2013, 135, 5360–5363. [Google Scholar] [CrossRef]
- Talukdar, R. A Study on the Reactions of SeO2 with Pyrroles and N-Substituted Indoles in Non-Anhydrous Ethanol under Non-Inert Atmosphere. Asian J. Org. Chem. 2019, 8, 88–92. [Google Scholar] [CrossRef]
- Boyd, G.V.; Doughty, M.; Kenyon, J. The action of selenious acid on alkyl ethers of phenols. J. Chem. Soc. 1949, 2196–2197. [Google Scholar] [CrossRef]
- Xu-Xu, Q.-F.; Nishii, Y.; Uetake, Y.; Sakurai, H.; Miura, M. Synthesis of Benzoisoselenazolones via Rh(III)-Catalyzed Direct Annulative Selenation Using Elemental Selenium. Chem. Eur. J. 2021, 27, 17952–17959. [Google Scholar] [CrossRef]
- Moon, S.; Nishii, Y.; Miura, M. Synthesis of Isothiazoles and Isoselenazoles through Rhodium-Catalyzed Oxidative Annulation with Elemental Sulfur and Selenium. Org. Lett. 2021, 23, 49–53. [Google Scholar] [CrossRef]
- Satoh, T.; Miura, M. Oxidative Coupling of Aromatic Substrates with Alkynes and Alkenes under Rhodium Catalysis. Chem. Eur. J. 2010, 16, 11212–11222. [Google Scholar] [CrossRef] [PubMed]
- Sambiagio, C.; Schönbauer, D.; Blieck, R.; Dao-Huy, T.; Pototschnig, G.; Schaaf, P.; Wiesinger, T.; Zia, M.F.; Wencel-Delord, J.; Besset, T.; et al. A comprehensive overview of directing groups applied in metal-catalysed C–H functionalisation chemistry. Chem. Soc. Rev. 2018, 47, 6603–6743. [Google Scholar] [CrossRef]
- Wang, C.; Chen, F.; Qian, P.; Cheng, J. Recent advances in the Rh-catalyzed cascade arene C–H bond activation/annulation toward diverse heterocyclic compounds. Org. Biomol. Chem. 2021, 19, 1705–1721. [Google Scholar] [CrossRef] [PubMed]
- Barday, M.; Janot, C.; Halcovitch, N.R.; Muir, J.; Aïssa, C. Cross-Coupling of α-Carbonyl Sulfoxonium Ylides with C−H Bonds. Angew. Chem. Int. Ed. 2017, 56, 13117–13121. [Google Scholar] [CrossRef]
- Wang, H.; Li, L.; Yu, S.; Li, Y.; Li, X. Rh(III)-Catalyzed C–C/C–N Coupling of Imidates with α-Diazo Imidamide: Synthesis of Isoquinoline-Fused Indoles. Org. Lett. 2016, 18, 2914–2917. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Gao, F.; Li, C.; Fang, D.; Xie, X.; Zhou, Y.; Liu, H. Synthesis of Highly Fused Pyrano[2,3-b]pyridines via Rh(III)-Catalyzed C–H Activation and Intramolecular Cascade Annulation under Room Temperature. J. Org. Chem. 2020, 85, 6281–6294. [Google Scholar] [CrossRef]
- Baugh, S.D.P.; Jackson, M.R.; Rashad, A.A.; Reitz, A.B.; Lam, P.Y.S.; Jorns, M.S. Synthesis and evaluation of potent novel inhibitors of human sulfide:quinone oxidoreductase. Bioorg. Med. Chem. Lett. 2021, 54, 128443. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, X. Synthesis of 1H-Indazoles from Imidates and Nitrosobenzenes via Synergistic Rhodium/Copper Catalysis. Org. Lett. 2016, 18, 2102–2105. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.-J.; Finger, L.H.; Messinis, A.M.; Kuniyil, R.; Oliveira, J.C.A.; Ackermann, L. Flow Rhodaelectro-Catalyzed Alkyne Annulations by Versatile C–H Activation: Mechanistic Support for Rhodium(III/IV). J. Am. Chem. Soc. 2019, 141, 17198–17206. [Google Scholar] [CrossRef]
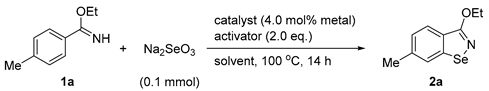
| Entry | Catalyst | Activator | Solvent | Yield [b] |
|---|---|---|---|---|
| 1 | [Cp*Rh(MeCN)3][SbF6]2 | AcCl | PhCF3 | n.d. |
| 2 | [Cp*Rh(MeCN)3][SbF6]2 | PhCOF | PhCF3 | 38% |
| 3 | [Cp*Rh(MeCN)3][SbF6]2 | Ac2O | PhCF3 | 36% |
| 4 | [Cp*Rh(MeCN)3][SbF6]2 | Piv2O | PhCF3 | 28% |
| 5 | [Cp*Rh(MeCN)3][SbF6]2 | Bz2O | PhCF3 | 58% (54%) |
| 6 [c] | [Cp*Rh(MeCN)3][SbF6]2 | Bz2O | PhCF3 | 62% |
| 7 [c,d] | [Cp*Rh(MeCN)3][SbF6]2 | Bz2O | PhCF3 | 84% (82%) |
| 8 [c,d] | [Cp*Rh(MeCN)3][SbF6]2 | Bz2O | dioxane | 84% (80%) |
| 9 [c,d] | [Cp*Rh(MeCN)3][SbF6]2 | Bz2O | MeCN | 80% |
| 10 [c,d,e] | [Cp*IrCl2]2 | Bz2O | PhCF3 | 27% |
| 11 [c,d,e] | Cp*Co(CO)I2 | Bz2O | PhCF3 | n.d. |
| 12 [c,d,e] | [Ru(p-cymene)Cl2]2 | Bz2O | PhCF3 | 6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu-Xu, Q.-F.; Nishii, Y.; Miura, M. Isoselenazole Synthesis by Rh-Catalyzed Direct Annulation of Benzimidates with Sodium Selenite. Chemistry 2023, 5, 2068-2074. https://doi.org/10.3390/chemistry5040140
Xu-Xu Q-F, Nishii Y, Miura M. Isoselenazole Synthesis by Rh-Catalyzed Direct Annulation of Benzimidates with Sodium Selenite. Chemistry. 2023; 5(4):2068-2074. https://doi.org/10.3390/chemistry5040140
Chicago/Turabian StyleXu-Xu, Qing-Feng, Yuji Nishii, and Masahiro Miura. 2023. "Isoselenazole Synthesis by Rh-Catalyzed Direct Annulation of Benzimidates with Sodium Selenite" Chemistry 5, no. 4: 2068-2074. https://doi.org/10.3390/chemistry5040140






