DNA Nanotechnology-Empowered Fluorescence Imaging of APE1 Activity
Abstract
:1. Introduction
2. Origin of AP Sites and Mechanism of Cleavage of AP Site by APE1
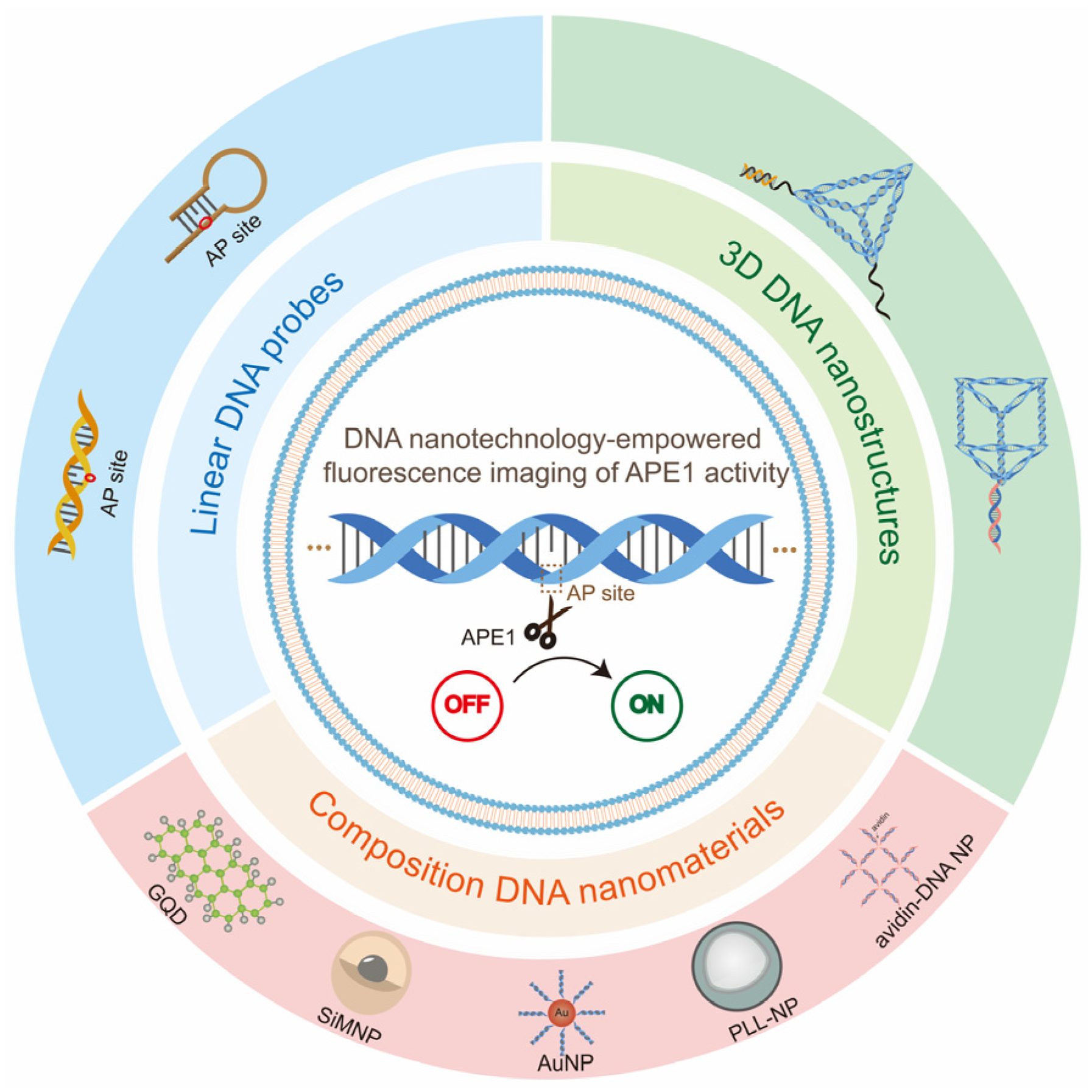
3. DNA-Nanotechnology-Empowered Fluorescence Imaging of APE1 Activity
3.1. Linear DNA-Empowered Fluorescence Imaging of APE1 Activity
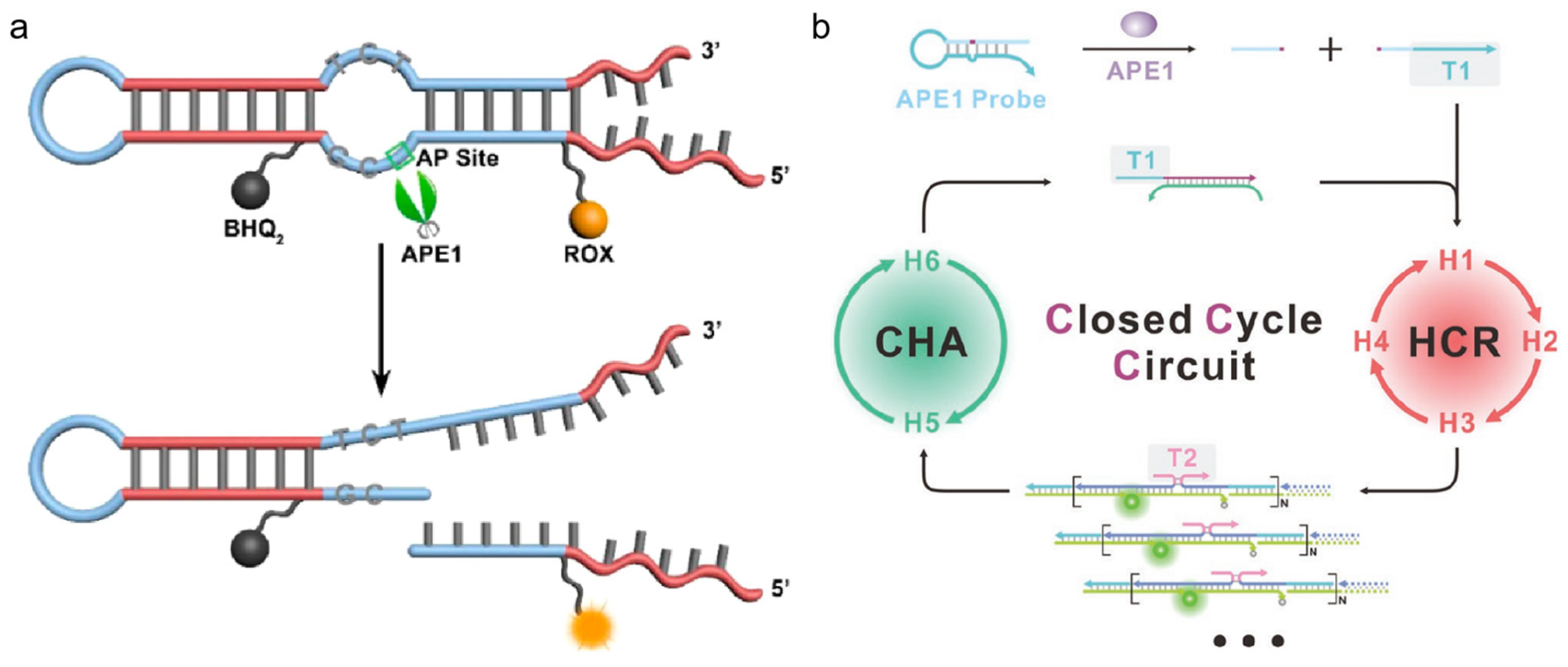
3.1.1. Hairpin-DNA-Based Nucleic Acid Probes
3.1.2. Double-Stranded Linear-DNA-Based Nucleic Acid Probes
3.2. Composite DNA-Nanomaterials-Empowered Fluorescence Imaging of APE1 Activity
3.2.1. DNA/Inorganic Nanomaterial Composites
3.2.2. DNA/Organic Nanomaterial Composites
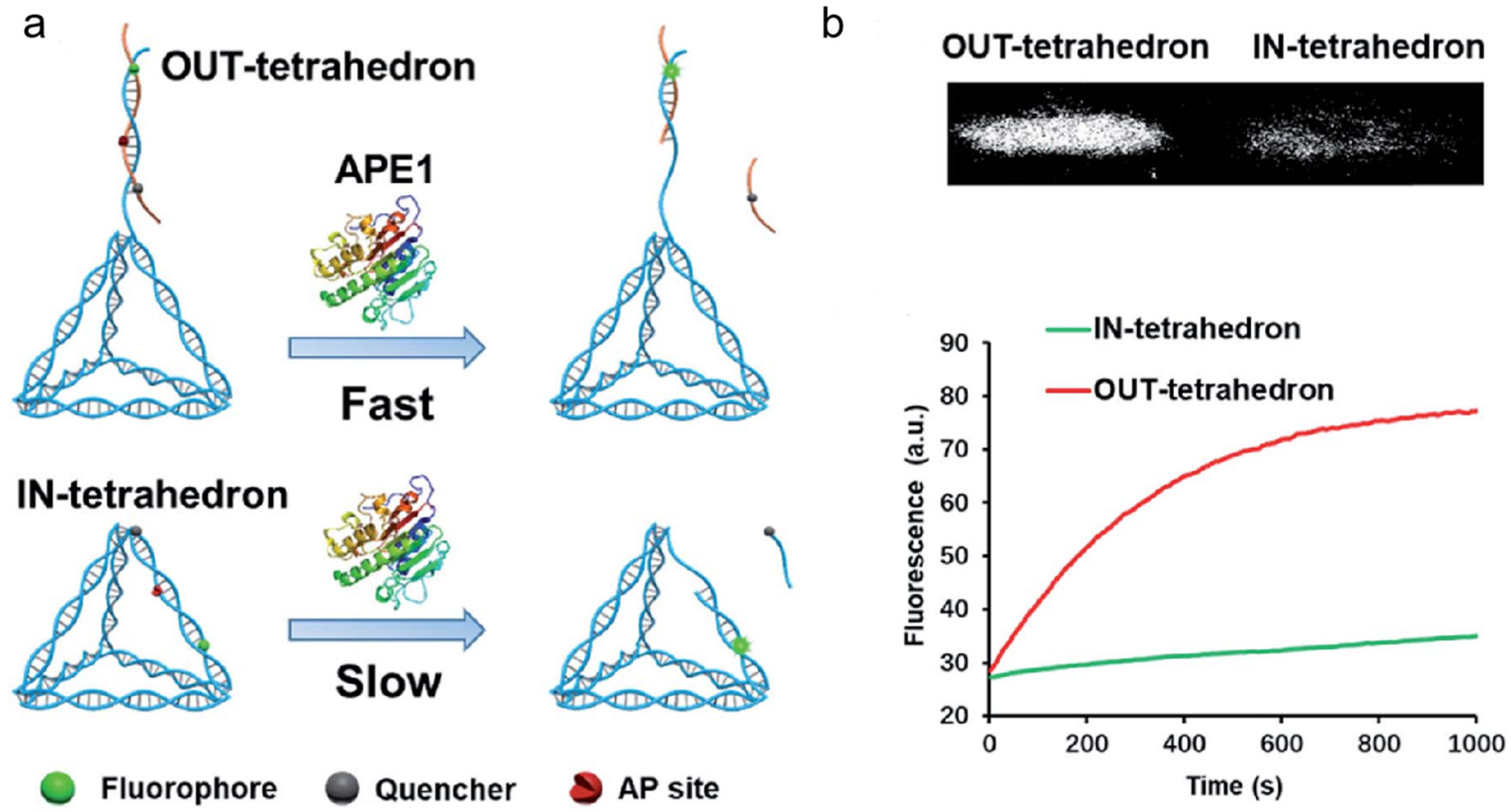
3.3. 3D DNA-Nanostructure-Empowered Fluorescence Imaging of APE1 Activity
4. Discussion and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Izumi, T.; Mitra, S. Deletion analysis of human AP-endonuclease: Minimum sequence required for the endonuclease activity. Carcinogenesis 1998, 19, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Lopez, D.J.; Rodriguez, J.A.; Banuelos, S. Molecular mechanisms regulating the DNA repair protein APE1: A focus on its flexible N-terminal tail domain. Int. J. Mol. Sci. 2021, 22, 6308. [Google Scholar] [CrossRef] [PubMed]
- Demple, B.; Herman, T.; Chen, D.S. Cloning and expression of APE, the cDNA encoding the major human apurinic endonuclease: Definition of a family of DNA repair enzymes. Proc. Natl. Acad. Sci. USA 1991, 88, 11450–11454. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.T.; Coutinho, L.G.; de Oliveira, L.O.A.; de Souza Timoteo, A.R.; Farias, G.C.; Agnez-Lima, L.F. APE1/Ref-1 role in inflammation and immune response. Front. Immunol. 2022, 13, 793096. [Google Scholar] [CrossRef] [PubMed]
- Barnes, T.; Kim, W.C.; Mantha, A.K.; Kim, S.E.; Izumi, T.; Mitra, S.; Lee, C.H. Identification of Apurinic/apyrimidinic endonuclease 1 (APE1) as the endoribonuclease that cleaves c-myc mRNA. Nucleic Acids Res. 2009, 37, 3946–3958. [Google Scholar] [CrossRef]
- Tell, G.; Pellizzari, L.; Cimarosti, D.; Pucillo, C.; Damante, G. Ref-1 controls pax-8 DNA-binding activity. Biochem. Biophys. Res. Commun. 1998, 252, 178–183. [Google Scholar] [CrossRef]
- Berquist, B.R.; McNeill, D.R.; Wilson III, D.M. Characterization of abasic endonuclease activity of human Ape1 on alternative substrates, as well as effects of ATP and sequence context on AP site incision. J. Mol. Biol. 2008, 379, 17–27. [Google Scholar] [CrossRef]
- Cohen, I. DNA damage talks to inflammation. Cytokine Growth Factor Rev. 2017, 33, 35–39. [Google Scholar] [CrossRef]
- Zou, G.M.; Luo, M.H.; Reed, A.; Kelley, M.R.; Yoder, M.C. Ape1 regulates hematopoietic differentiation of embryonic stem cells through its redox functional domain. Blood 2007, 109, 1917–1922. [Google Scholar] [CrossRef] [PubMed]
- Fishel, M.L.; He, Y.; Reed, A.M.; Chin-Sinex, H.; Hutchins, G.D.; Mendonca, M.S.; Kelley, M.R. Knockdown of the DNA repair and redox signaling protein Ape1/Ref-1 blocks ovarian cancer cell and tumor growth. DNA Repair 2008, 7, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Fishel, M.L.; Jiang, Y.; Rajeshkumar, N.V.; Scandura, G.; Sinn, A.L.; He, Y.; Shen, C.; Jones, D.R.; Pollok, K.E.; Ivan, M.; et al. Impact of APE1/Ref-1 redox inhibition on pancreatic tumor growth. Mol. Cancer Ther. 2011, 10, 1698–1708. [Google Scholar] [CrossRef] [PubMed]
- Dianov, G.; Lindahl, T. Reconstitution of the DNA base excision-repair pathway. Curr. Biol. 1994, 4, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Schermerhorn, K.M.; Delaney, S. A chemical and kinetic perspective on base excision repair of DNA. Acc. Chem. Res. 2014, 47, 1238–1246. [Google Scholar] [CrossRef]
- Zou, G.M.; Karikari, C.; Kabe, Y.; Handa, H.; Anders, R.A.; Maitra, A. The Ape-1/Ref-1 redox antagonist E3330 inhibits the growth of tumor endothelium and endothelial progenitor cells: Therapeutic implications in tumor angiogenesis. J. Cell. Physiol. 2009, 219, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Xu, M.; Wang, Y.; Xu, J.; Yuan, Y. An APE1 inhibitor reveals critical roles of the redox function of APE1 in KSHV replication and pathogenic phenotypes. PLoS Pathog. 2017, 13, e1006289. [Google Scholar] [CrossRef] [PubMed]
- Pines, A.; Perrone, L.; Bivi, N.; Romanello, M.; Damante, G.; Gulisano, M.; Kelley, M.R.; Quadrifoglio, F.; Tell, G. Activation of APE1/Ref-1 is dependent on reactive oxygen species generated after purinergic receptor stimulation by ATP. Nucleic Acids Res. 2005, 33, 4379–4394. [Google Scholar] [CrossRef] [PubMed]
- Mohni, K.N.; Wessel, S.R.; Zhao, R.; Wojciechowski, A.C.; Luzwick, J.W.; Layden, H.; Eichman, B.F.; Thompson, P.S.; Mehta, K.P.M.; Cortez, D. HMCES maintains genome integrity by shielding abasic sites in single-strand DNA. Cell 2019, 176, 144–153. [Google Scholar] [CrossRef]
- Lindahl, T. Instability and decay of the primary structure of DNA. Nature 1993, 362, 709–715. [Google Scholar] [CrossRef]
- Lindahl, T.; Nyberg, B. Rate of depurination of native deoxyribonucleic acid. Biochemistry 1972, 11, 3610–3618. [Google Scholar] [CrossRef]
- Sczepanski, J.T.; Wong, R.S.; McKnight, J.N.; Bowman, G.D.; Greenberg, M.M. Rapid DNA-protein cross-linking and strand scission by an abasic site in a nucleosome core particle. Proc. Natl. Acad. Sci. USA 2010, 107, 22475–22480. [Google Scholar] [CrossRef]
- Hoitsma, N.M.; Whitaker, A.M.; Beckwitt, E.C.; Jang, S.; Agarwal, P.K.; Houten, B.V.; Freudenthal, B.D. AP-endonuclease 1 sculpts DNA through an anchoring tyrosine residue on the DNA intercalating loop. Nucleic Acids Res. 2020, 48, 7345–7355. [Google Scholar] [CrossRef] [PubMed]
- Drohat, A.C.; Coey, C.T. Role of base excision “repair” enzymes in erasing epigenetic marks from DNA. Chem. Rev. 2016, 116, 12711–12729. [Google Scholar] [CrossRef]
- Lindahl, T. An N-glycosidase from Escherichia coli that releases free uracil from DNA containing deaminated cytosine residues. Proc. Natl. Acad. Sci. USA 1974, 71, 3649–3653. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y.; Nash, R.A.; Klungland, A.; Schar, P.; Barnes, D.E.; Lindahl, T. Reconstitution of DNA base excision-repair with purified human proteins: Interaction between DNA polymerase β and the XRCC1 protein. EMBO J. 1996, 15, 6662–6670. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Demple, B. Knockout and inhibition of Ape1: Roles of Ape1 in base excision DNA repair and modulation of gene expression. Antioxidants 2022, 11, 1817. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, S.; Xing, D. New horizons for the roles and association of APE1/Ref-1 and ABCA1 in atherosclerosis. J. Inflamm. Res. 2021, 14, 5251–5271. [Google Scholar] [CrossRef]
- Weaver, T.M.; Hoitsma, N.M.; Spencer, J.J.; Gakhar, L.; Schnicker, N.J.; Freudenthal, B.D. Structural basis for APE1 processing DNA damage in the nucleosome. Nat. Commun. 2022, 13, 5390. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, A.M.; Freudenthal, B.D. APE1: A skilled nucleic acid surgeon. DNA Repair 2018, 71, 93–100. [Google Scholar] [CrossRef]
- Fan, Z.; Beresford, P.J.; Zhang, D.; Xu, Z.; Novina, C.D.; Yoshida, A.; Pommier, Y.; Lieberman, J. Cleaving the oxidative repair protein Ape1 enhances cell death mediated by granzyme A. Nat. Immunol. 2003, 4, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Hong, J.; Peng, D.; Bhat, A.A.; Chen, Z.; Zaika, A.; El-Rifai, W.M. A new function of APE1 in barrett’s esophagus and esophageal adenocarcinoma: APE1 upregulates MMP2 and MMP14 to promote invasion. Gastroenterology 2017, 152, S237. [Google Scholar] [CrossRef]
- Antoniali, G.; Serra, F.; Lirussi, L.; Tanaka, M.; D’Ambrosio, C.; Zhang, S.; Radovic, S.; Dalla, E.; Ciani, Y.; Scaloni, Y.; et al. Mammalian APE1 controls miRNA processing and its interactome is linked to cancer RNA metabolism. Nat. Commun. 2017, 8, 797. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, X.; Lu, X.; Dai, N.; Zhang, S.; Cheng, Y.; Zhang, L.; Yang, Y.; Liu, Y.; Yang, Z.; et al. APE1 deficiency promotes cellular senescence and premature aging features. Nucleic Acids Res. 2018, 46, 5664–5677. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Shi, W.; Li, X.; Ma, H. Recognition moieties of small molecular fluorescent probes for bioimaging of enzymes. Acc. Chem. Res. 2019, 52, 1892–1904. [Google Scholar] [CrossRef] [PubMed]
- Dischler, A.M.; Maslar, D.; Zhang, C.; Qin, Y. Development and characterization of a red fluorescent protein-based sensor RZnP1 for the detection of cytosolic Zn2+. ACS Sens. 2022, 7, 3838–3845. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.H.; Willner, B.; Willner, I. DNA nanotechnology: From sensing and DNA machines to drug-delivery systems. ACS Nano 2013, 7, 8320–8332. [Google Scholar] [CrossRef]
- Xie, N.; Huang, J.; Yang, X.; Yang, Y.; Quan, K.; Ou, M.; Fang, H.; Wang, K. Competition-mediated FRET-switching DNA tetrahedron molecular beacon for intracellular molecular detection. ACS Sens. 2016, 1, 1445–1452. [Google Scholar] [CrossRef]
- Lei, Y.; Qian, Z.; Tang, J.; He, X.; Shi, H.; Ye, X.; Yan, L.; He, D.; Wang, K. DNA nanotriangle-scaffolded activatable aptamer probe with ultralow background and robust stability for cancer theranostics. Theranostics 2018, 8, 4062–4071. [Google Scholar] [CrossRef]
- Zou, S.; Lei, Y.; Ma, W.; Chen, B.; Cheng, H.; Jia, R.; Li, Z.; He, X.; Wang, K. Extracellular pH-manipulated in situ reconfiguration of aptamer functionalized DNA monomer enables specifically improved affinity, detection and drug delivery. Analyst 2020, 145, 2562–2569. [Google Scholar] [CrossRef]
- Huang, J.; Wu, Y.; He, H.; Ma, W.; Liu, J.; Cheng, H.; Sun, H.; He, X.; Wang, K. Acidic microenvironment triggered in situ assembly of activatable three-arm aptamer nanoclaw for contrast-enhanced imaging and tumor growth inhibition in vivo. Theranostics 2022, 12, 3474–3487. [Google Scholar] [CrossRef]
- Wang, F.; Willner, B.; Willner, I. DNA nanotechnology with one-dimensional self-assembled nanostructures. Curr. Opin. Biotechnol. 2013, 24, 562–574. [Google Scholar] [CrossRef]
- Liu, B.; Yang, Z.; Huang, T.; Li, M.M.; Duan, W.; Xie, B.; Chen, J.X.; Dai, Z.; Chen, J. Label-free and highly sensitive APE1 detection based on rolling circle amplification combined with G-quadruplex. Talanta 2022, 244, 123404. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ma, Y.; Li, Y.; Xiong, M.; Li, X.; Zhang, L.; Zhao, S. Sensitive and label-free fluorescence detection of apurinic/apyrimidinic endonuclease 1 activity based on isothermal amplified-generation of G-quadruplex. New J. Chem. 2017, 41, 1893–1896. [Google Scholar] [CrossRef]
- Jans, H.; Huo, Q. Gold nanoparticle-enabled biological and chemical detection and analysis. Chem. Soc. Rev. 2012, 41, 2849–2866. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.C.; Wright, D.W. Size-dependent cellular uptake of DNA functionalized gold nanoparticles. Small 2016, 12, 5592–5600. [Google Scholar] [CrossRef]
- Liang, M.; Li, N.; Liu, F.; Zeng, N.; Yu, C.; Li, S. Apurinic/apyrimidinic endonuclease triggered doxorubicin-releasing DNA nanoprism for target therapy. Cell Cycle 2022, 21, 2627–2634. [Google Scholar] [CrossRef] [PubMed]
- Atamna, H.; Cheung, I.; Ames, B.N. A method for detecting abasic sites in living cells: Age-dependent changes in base excision repair. Proc. Natl. Acad. Sci. USA 2000, 97, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Khodyreva, S.; Lavrik, O. Non-canonical interaction of DNA repair proteins with intact and cleaved AP sites. DNA Repair 2020, 90, 102847. [Google Scholar] [CrossRef]
- Abe, Y.S.; Sasaki, S. DNA cleavage at the AP site via β-elimination mediated by the AP site-binding ligands. Bioorg. Med. Chem. 2016, 24, 910–914. [Google Scholar] [CrossRef]
- Boiteux, S.; Guillet, M. Abasic sites in DNA: Repair and biological consequences in Saccharomyces cerevisiae. DNA Repair 2004, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.S.; Sasaki, S. The adduct formation between the thioguanine-polyamine ligands and DNA with the AP site under UVA irradiated and non-irradiated conditions. Bioorg. Med. Chem. 2019, 27, 115160. [Google Scholar] [CrossRef]
- Kuznetsova, A.A.; Fedorova, O.S.; Kuznetsov, N.A. Kinetic features of 3′-5′ exonuclease activity of human AP-endonuclease APE1. Molecules 2018, 23, 2101. [Google Scholar] [CrossRef] [PubMed]
- Long, K.; Gu, L.; Zhang, Z.; Li, E.; Zhang, Y.; He, L.; Pan, F.; Guo, Z.; Hu, Z. Small-molecule inhibition of APE1 induces apoptosis, pyroptosis, and necroptosis in non-small cell lung cancer. Cell Death Dis. 2021, 12, 503. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, S.B.; Samanta, D.; Mirkin, C.A. DNA-based nanostructures for live-cell analysis. J. Am. Chem. Soc. 2020, 142, 11343–11356. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, N.; Fu, S.; Deng, Y.; Yu, C.; Su, X. Base excision repair-inspired DNA motor powered by intracellular apurinic/apyrimidinic endonuclease. Nanoscale 2019, 11, 1343–1350. [Google Scholar] [CrossRef]
- Strohsahl, C.M.; Krauss, T.D.; Miller, B.L. Identification of high-stringency DNA hairpin probes by partial gene folding. Biosens. Bioelectron. 2007, 23, 233–240. [Google Scholar] [CrossRef]
- Bidar, N.; Amini, M.; Oroojalian, F.; Baradaran, B.; Hosseini, S.S.; Shahbazi, M.A.; Hashemzaei, M.; Mokhtarzadeh, A.; Hamblin, M.R.; de la Guardia, M. Molecular beacon strategies for sensing purpose. TrAC Trend. Anal. Chem. 2021, 134, 116143. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, W.; Luo, H.Q.; Li, N.B. Label-free cascade amplification strategy for sensitive visual detection of thrombin based on target-triggered hybridization chain reaction-mediated in situ generation of DNAzymes and Pt nanochains. Biosens. Bioelectron. 2016, 80, 463–470. [Google Scholar] [CrossRef]
- Yang, D.; Ning, L.; Gao, T.; Ye, Z.; Li, G. Enzyme-free dual amplification strategy for protein assay by coupling toehold-mediated DNA strand displacement reaction with hybridization chain reaction. Electrochem. Commun. 2015, 58, 33–36. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, X.; Yang, X.; Liu, P.; Wang, K.; Huang, J.; Liu, J.; Song, C.; Wang, J. Colorimetric detection of mercury ion based on unmodified gold nanoparticles and target-triggered hybridization chain reaction amplification. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 136, 283–287. [Google Scholar] [CrossRef]
- Zhou, Q.; Lin, Y.; Lin, Y.; Wei, Q.; Chen, G.; Tang, D. In situ amplified electrochemical aptasensing for sensitive detection of adenosine triphosphate by coupling target-induced hybridization chain reaction with the assembly of silver nanotags. Talanta 2016, 146, 23–28. [Google Scholar] [CrossRef]
- Gao, Z.; Qiu, Z.; Lu, M.; Shu, J.; Tang, D. Hybridization chain reaction-based colorimetric aptasensor of adenosine 5′-triphosphate on unmodified gold nanoparticles and two label-free hairpin probes. Biosens. Bioelectron. 2017, 89, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Miao, X.; Zhu, A.; Ling, L. Hybridization chain reaction and gold nanoparticles dual signal amplification for sensitive glucose detection. Biochem. Eng. J. 2015, 103, 205–210. [Google Scholar] [CrossRef]
- Dai, J.; Xing, C.; Lin, Y.; Huang, Y.; Yang, Y.; Chen, Z.; Lu, C.; Yang, H. Localized DNA catalytic hairpin assembly reaction on DNA origami for tumor-associated microRNA detection and imaging in live cells. Sens. Actuators B Chem. 2021, 344, 130195. [Google Scholar] [CrossRef]
- Miao, P. Magnetic multipedal DNA walking nanomachine driven by catalytic hairpin assembly. Anal. Chem. 2023, 95, 6760–6764. [Google Scholar] [CrossRef] [PubMed]
- Okabe, K.; Harada, Y.; Zhang, J.; Tadakuma, H.; Tani, T.; Funatsu, T. Real time monitoring of endogenous cytoplasmic mRNA using linear antisense 2′-O-methyl RNA probes in living cells. Nucleic Acids Res. 2011, 39, e20. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Guan, R.; Guo, M.; Ning, P.; Shao, R.; Meng, X. A FRET based two-photon fluorescent probe for ratiometric detection of Pd2+ in living cells and in vivo. Sens. Actuators B Chem. 2018, 254, 949–955. [Google Scholar] [CrossRef]
- He, J.H.; Cheng, Y.Y.; Zhang, Q.Q.; Liu, H.; Huang, C.Z. Carbon dots-based fluorescence resonance energy transfer for the prostate specific antigen (PSA) with high sensitivity. Talanta 2020, 219, 121276. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, D.; Luo, Z.; Duan, Y. A dual-functional fluorescent biosensor based on enzyme-involved catalytic hairpin assembly for the detection of APE1 and miRNA-21. Analyst 2022, 147, 2834–2842. [Google Scholar] [CrossRef]
- Fang, S.; Chen, L.; Zhao, M. Unimolecular chemically modified DNA fluorescent probe for one-step quantitative measurement of the activity of human apurinic/apyrimidinic endonuclease 1 in biological samples. Anal. Chem. 2015, 87, 11952–11956. [Google Scholar] [CrossRef]
- Chai, Q.; Chen, J.; Zeng, S.; Zhu, T.; Chen, J.; Qi, C.; Mao, G.; Liu, Y. Closed cyclic DNA machine for sensitive logic operation and APE1 detection. Small 2023, 19, e2207736. [Google Scholar] [CrossRef]
- Simeonov, A.; Kulkarni, A.; Dorjsuren, D.; Jadhav, A.; Shen, M.; McNeill, D.R.; Austin, C.P.; Wilson III, D.M. Identification and characterization of inhibitors of human apurinic/apyrimidinic endonuclease APE1. PLoS ONE 2009, 4, e5740. [Google Scholar] [CrossRef] [PubMed]
- Seiple, L.A.; Cardellina II, J.H.; Akee, R.; Stivers, J.T. Potent inhibition of human apurinic/apyrimidinic endonuclease 1 by arylstibonic acids. Mol. Pharmacol. 2008, 73, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xie, Q.; Qin, Y.; Tong, C.; Liu, B.; Wang, W. Real-time monitoring and effector screening of APE1 based on rGO assisted DNA nanoprobe. Anal. Biochem. 2021, 633, 114394. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Cao, X.; Zheng, J.; Zhu, C.; Zhang, R.; Sun, Y.; Yang, Z.; Tang, Z.; Wang, J.; Zhao, M. A DNA/RNA hybrid fluorescent probe for high-throughput quantification of the activity of human apurinic/apyrimidinic endonuclease 1 in subcellular extracts. Biosens. Bioelectron. 2023, 14, 100329. [Google Scholar] [CrossRef]
- Li, X.; Xiong, M.; Huang, Y.; Zhang, L.; Zhao, S. Simple label-free fluorescence detection of apurinic/apyrimidinic endonuclease 1 activity and its inhibitor using the abasic site-binding fluorophore. Anal. Methods 2019, 11, 739–743. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Tang, H.; Yang, B.; Zhao, Y.; Wu, P. Evaluation of the sequence-dependent relative activity of APE1 for optimal biosensing design. Biosens. Bioelectron. 2022, 214, 114539. [Google Scholar] [CrossRef]
- Moon, W.J.; Liu, J. Interfacing catalytic DNA with nanomaterials. Adv. Mater. Interfaces 2020, 7, 2001017. [Google Scholar] [CrossRef]
- Lv, Z.; Zhu, Y.; Li, F. DNA Functional nanomaterials for controlled delivery of nucleic acid-based drugs. Front. Bioeng. Biotechnol. 2021, 9, 720291. [Google Scholar] [CrossRef]
- Baron, R.; Willner, B.; Willner, I. Biomolecule-nanoparticle hybrids as functional units for nanobiotechnology. Chem. Commun. 2007, 4, 323–332. [Google Scholar] [CrossRef]
- Samanta, A.; Medintz, I.L. Nanoparticles and DNA-a powerful and growing functional combination in bionanotechnology. Nanoscale 2016, 8, 9037–9095. [Google Scholar] [CrossRef]
- Heuer-Jungemann, A.; Linko, V. Engineering inorganic materials with DNA nanostructures. ACS Cent. Sci. 2021, 7, 1969–1979. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Z.; Willner, I. Dynamic reconfigurable DNA nanostructures, networks and materials. Angew. Chem. Int. Ed. 2023, 62, e202215332. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Lin, L.; Chao, Z.; Shao, C.; Chen, Z.; Wei, Z.; Lu, J.; Huang, Y.; Li, L.; Liu, Q.; et al. Organic spherical nucleic acids for the transport of a NIR-II-emitting dye across the blood-brain barrier. Angew. Chem. Int. Ed. 2020, 59, 9702–9710. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, D.; Calandra, P.; Pasqua, L.; Magazu, S. Self-assembly of organic nanomaterials and biomaterials: The bottom-up approach for functional nanostructures formation and advanced applications. Materials 2020, 13, 1048. [Google Scholar] [CrossRef] [PubMed]
- Ahirwar, S.; Mallick, S.; Bahadur, D. Electrochemical method to prepare graphene quantum dots and graphene oxide quantum dots. ACS Omega 2017, 2, 8343–8353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ba, S.; Yang, Z.; Wang, T.; Lee, J.Y.; Li, T.; Shao, F. Graphene quantum dot-based nanocomposites for diagnosing cancer biomarker APE1 in living cells. ACS Appl. Mater. Interfaces 2020, 12, 13634–13643. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, A.; Wang, Y.; Qiu, Z.; Li, Y.; Yang, H.; Fu, M.; Liu, M.; Yu, Y.; Gao, F. Smart programmable scalable dual-mode diagnostic logic nanoflare strategy for dual-tumor marker detection. Anal. Chem. 2022, 94, 9715–9723. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Wang, W.; Min, Q.; Zhang, J.R.; Zhu, J.J. A telomerase-assisted strategy for regeneration of DNA nanomachines in living cells. Angew. Chem. Int. Ed. 2023, 62, e202213884. [Google Scholar] [CrossRef]
- Du, K.; Feng, J.; Gao, X.; Zhang, H. Nanocomposites based on lanthanide-doped upconversion nanoparticles: Diverse designs and applications. Light Sci. Appl. 2022, 11, 222. [Google Scholar] [CrossRef]
- Liang, G.; Wang, H.; Shi, H.; Wang, H.; Zhu, M.; Jing, A.; Li, J.; Li, G. Recent progress in the development of upconversion nanomaterials in bioimaging and disease treatment. J. Nanobiotechnol. 2020, 18, 154. [Google Scholar] [CrossRef]
- Shah, F.; Logsdon, D.; Messmann, R.A.; Fehrenbacher, J.C.; Fishel, M.L.; Kelley, M.R. Exploiting the Ref-1-APE1 node in cancer signaling and other diseases: From bench to clinic. NPJ Precis. Oncol. 2017, 1, 19. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Zhao, J.; Yuan, J.; Zhao, Y.; Li, L. Organelle-specific photoactivation of DNA nanosensors for precise profiling of subcellular enzymatic activity. Angew. Chem. Int. Ed. 2021, 60, 8923–8931. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Shao, Y.; Chai, X.; Zhao, Y.; Li, L. Spatially selective monitoring of subcellular enzyme dynamics in response to mitochondria-targeted photodynamic therapy. Angew. Chem. Int. Ed. 2022, 61, e202203238. [Google Scholar] [CrossRef]
- Qian, R.; Ding, L.; Ju, H. Switchable fluorescent imaging of intracellular telomerase activity using telomerase-responsive mesoporous silica nanoparticle. J. Am. Chem. Soc. 2013, 135, 13282–13285. [Google Scholar] [CrossRef] [PubMed]
- Torney, F.; Trewyn, B.G.; Lin, V.S.; Wang, K. Mesoporous silica nanoparticles deliver DNA and chemicals into plants. Nat. Nanotechnol. 2007, 2, 295–300. [Google Scholar] [CrossRef] [PubMed]
- He, X.X.; Wang, K.; Tan, W.; Liu, B.; Lin, X.; He, C.; Li, D.; Huang, S.; Li, J. Bioconjugated nanoparticles for DNA protection from cleavage. J. Am. Chem. Soc. 2003, 125, 7168–7169. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Liu, Y.; Huang, S.; Fang, S.; Zhao, M. A specific DNA-nanoprobe for tracking the activities of human apurinic/apyrimidinic endonuclease 1 in living cells. Nucleic Acids Res. 2017, 45, e45. [Google Scholar] [CrossRef] [PubMed]
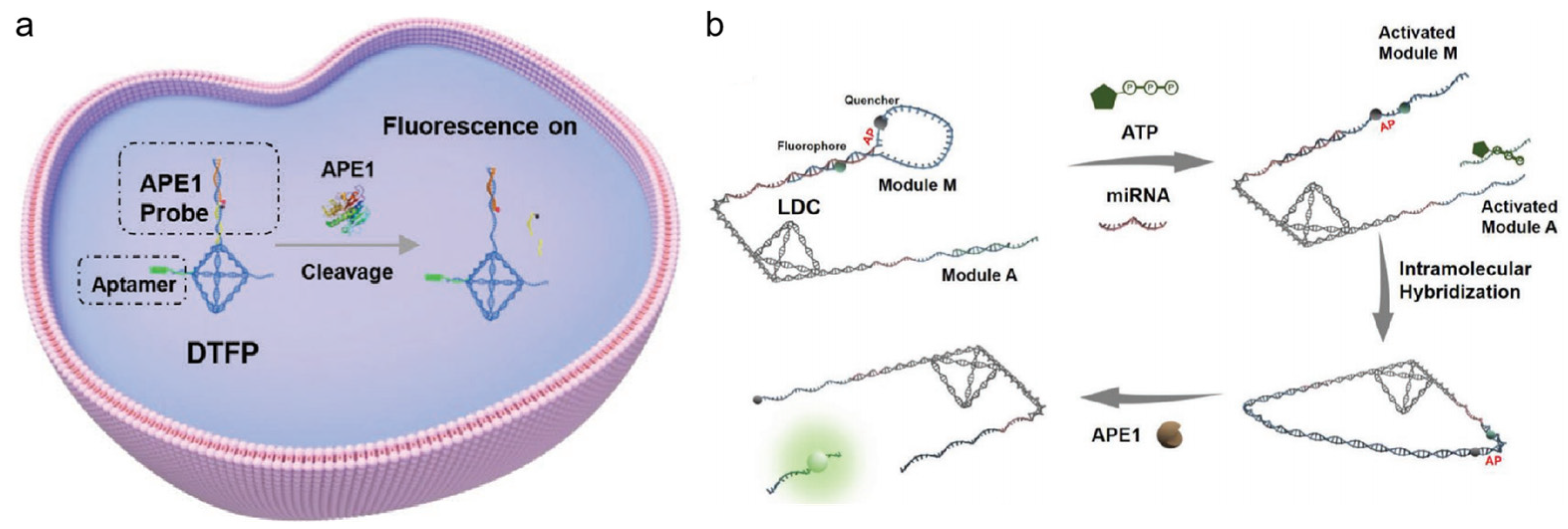
- Li, Z.; Feng, X.; Hu, W.; Li, L. An activatable DNA nanodevice for correlated imaging of apoptosis-related dual proteins. Nanoscale 2022, 14, 6465–6470. [Google Scholar] [CrossRef] [PubMed]
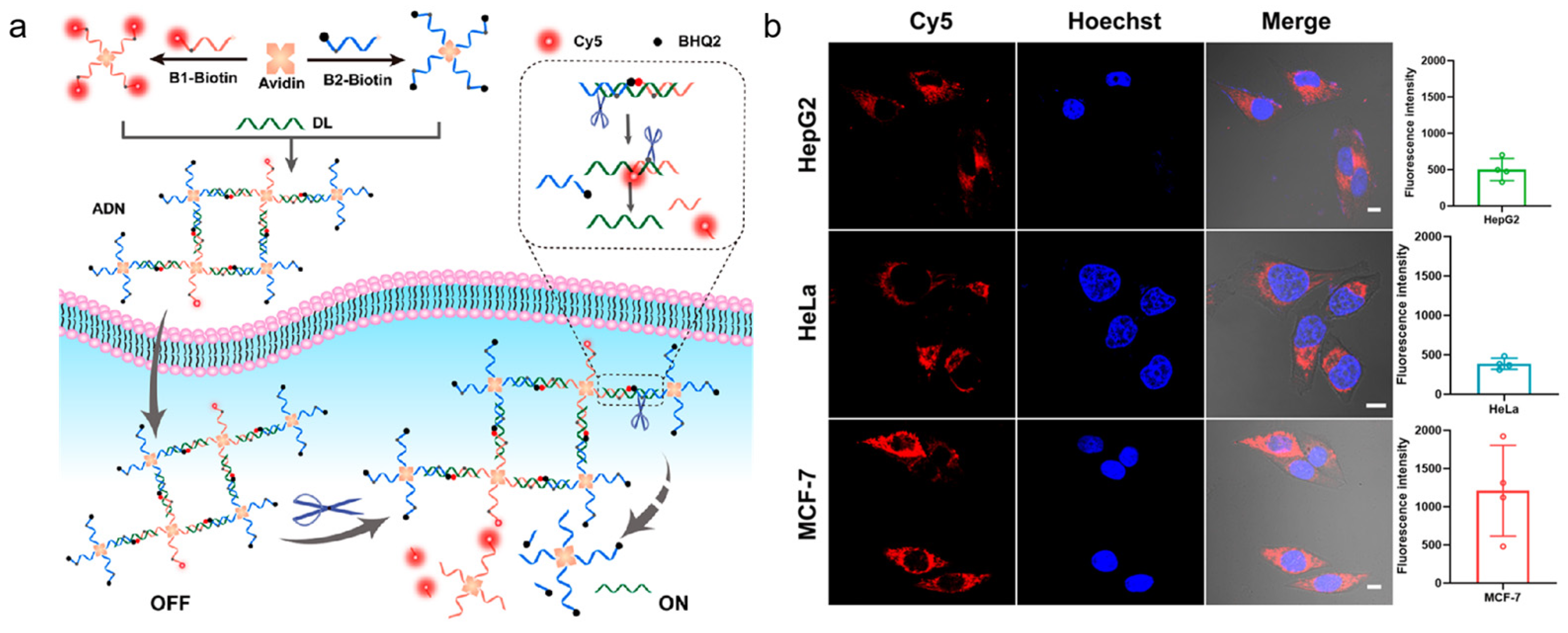
- Li, J.J.; Du, W.F.; Liu, Y.N.; Wang, F.; Tang, L.J.; Jiang, J.H. Protein-scaffolded DNA nanostructures for imaging of apurinic/apyrimidinic endonuclease 1 activity in live cells. Anal. Chem. 2023, 95, 3551–3555. [Google Scholar] [CrossRef]
- Zhang, T.; Tian, T.; Zhou, R.; Li, S.; Ma, W.; Zhang, Y.; Liu, N.; Shi, S.; Li, Q.; Xie, X.; et al. Design, fabrication and applications of tetrahedral DNA nanostructure-based multifunctional complexes in drug delivery and biomedical treatment. Nat. Protoc. 2020, 15, 2728–2757. [Google Scholar] [CrossRef]
- Xu, W.; He, W.; Du, Z.; Zhu, L.; Huang, K.; Lu, Y.; Luo, Y. Functional nucleic acid nanomaterials: Development, properties, and applications. Angew. Chem. Int. Ed. 2021, 60, 6890–6918. [Google Scholar] [CrossRef] [PubMed]
- Mou, Q.; Ma, Y.; Pan, G.; Xue, B.; Yan, D.; Zhang, C.; Zhu, X. DNA trojan horses: Self-assembled floxuridine-containing DNA polyhedra for cancer therapy. Angew. Chem. Int. Ed. 2017, 56, 12528–12532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ptacin, J.L.; Fischer, E.C.; Aerni, H.R.; Caffaro, C.E.; Jose, K.S.; Feldman, A.W.; Turner, C.R.; Romesberg, F.E. A semi-synthetic organism that stores and retrieves increased genetic information. Nature 2017, 551, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Zhang, T.; Shi, S.; Gao, Y.; Cai, X.; Lin, Y. A dynamic DNA tetrahedron framework for active targeting. Nat. Protoc. 2023, 18, 1028–1055. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Liu, S.; Fang, H.; Yang, Y.; Quan, K.; Li, J.; Yang, X.; Wang, K.; Huang, J. Three-dimensional molecular transfer from DNA nanocages to inner gold nanoparticle surfaces. ACS Nano 2019, 13, 4174–4182. [Google Scholar] [CrossRef]
- Du, R.R.; Cedrone, E.; Romanov, A.; Falkovich, R.; Dobrovolskaia, M.A.; Bathe, M. Innate immune stimulation using 3D wireframe DNA origami. ACS Nano 2022, 16, 20340–20352. [Google Scholar] [CrossRef]
- Cai, R.; Zhang, S.; Chen, L.; Li, M.; Zhang, Y.; Zhou, N. Self-assembled DNA nanoflowers triggered by a DNA walker for highly sensitive electrochemical detection of staphylococcus aureus. ACS Appl. Mater. Interfaces 2021, 13, 4905–4914. [Google Scholar] [CrossRef]
- Zhang, L.; Abdullah, R.; Hu, X.; Bai, H.; Fan, H.; He, L.; Liang, H.; Zou, J.; Liu, Y.; Sun, Y.; et al. Engineering of bioinspired, size-controllable, self-degradable cancer-targeting DNA nanoflowers via the incorporation of an artificial sandwich base. J. Am. Chem. Soc. 2019, 141, 4282–4290. [Google Scholar] [CrossRef]
- Wang, D.X.; Wang, J.; Wang, Y.X.; Du, Y.C.; Huang, Y.; Tang, A.N.; Cui, Y.X.; Kong, D.M. DNA nanostructure-based nucleic acid probes: Construction and biological applications. Chem. Sci. 2021, 12, 7602–7622. [Google Scholar] [CrossRef]
- Lu, N.; Pei, H.; Ge, Z.; Simmons, C.R.; Yan, H.; Fan, C. Charge transport within a three-dimensional DNA nanostructure framework. J. Am. Chem. Soc. 2012, 134, 13148–13151. [Google Scholar] [CrossRef]
- Goodman, R.P.; Schaap, I.A.T.; Tardin, C.F.; Erben, C.M.; Berry, R.M.; Schmidt, C.F.; Turberfield, A.J. Rapid chiral assembly of rigid DNA building blocks for molecular nanofabrication. Science 2005, 310, 1661–1665. [Google Scholar] [CrossRef] [PubMed]
- Walsh, A.S.; Yin, H.; Erben, C.M.; Wood, M.J.; Turberfield, A.J. DNA cage delivery to mammalian cells. ACS Nano 2011, 5, 5427–5432. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Li, J.; Li, Q.; Huang, Q.; Shi, J.; Yan, H.; Fan, C. Single-particle tracking and modulation of cell entry pathways of a tetrahedral DNA nanostructure in live cells. Angew. Chem. Int. Ed. 2014, 53, 7745–7750. [Google Scholar] [CrossRef] [PubMed]
- Keum, J.W.; Bermudez, H. Enhanced resistance of DNA nanostructures to enzymatic digestion. Chem. Commun. 2009, 7, 7036–7038. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, G.; Wang, T.; Fu, J.; Li, R.; Song, L.; Wang, Z.G.; Ding, B.; Chen, H. Enhanced stability of DNA nanostructures by incorporation of unnatural base pairs. ChemPhysChem 2017, 18, 2977–2980. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, L.; Qin, Z.; Karges, J.; Xiao, H.; Su, X. Unraveling and overcoming platinum drug-resistant cancer tumors with DNA nanostructures. Adv. Funct. Mater. 2022, 33, 2208797. [Google Scholar] [CrossRef]
- Kou, Q.; Wang, L.; Zhang, L.; Ma, L.; Fu, S.; Su, X. Simulation-assisted localized DNA logical circuits for cancer biomarkers detection and imaging. Small 2022, 18, e2205191. [Google Scholar] [CrossRef]
- Tang, Y.; Lu, B.; Zhu, Z.; Li, B. Establishment of a universal and rational gene detection strategy through three-way junction-based remote transduction. Chem. Sci. 2018, 9, 760–769. [Google Scholar] [CrossRef]
- Dorjsuren, D.; Kim, D.; Vyjayanti, V.N.; Maloney, D.J.; Jadhav, A.; Wilson III, D.M.; Simeonov, A. Diverse small molecule inhibitors of human apurinic/apyrimidinic endonuclease APE1 identified from a screen of a large public collection. PLoS ONE 2012, 7, e47974. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, Y.; Wang, C.; Li, L.; Xu, L.; Yu, Y.; Su, X. Probing and regulating the activity of cellular enzymes by using DNA tetrahedron nanostructures. Chem. Sci. 2019, 10, 5959–5966. [Google Scholar] [CrossRef]
- Lv, M.M.; Liu, J.W.; Yu, R.Q.; Jiang, J.H. A bipedal DNA nanowalker fueled by catalytic assembly for imaging of base-excision repairing in living cells. Chem. Sci. 2020, 11, 10361–10366. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Luo, R.; Li, Y.; Fan, J.; Hu, Y.; Tong, C.; Liu, B.; Li, D. Activity assay and intracellular imaging of APE1 assisted with tetrahedral DNA nanostructure modified-dnazyme and molecular beacon. Sens. Actuat. B-Chem. 2020, 317, 128203. [Google Scholar] [CrossRef]
- Zhou, X.M.; Zhuo, Y.; Tu, T.T.; Yuan, R.; Chai, Y.Q. Construction of fast-walking tetrahedral DNA walker with four arms for sensitive detection and intracellular imaging of apurinic/apyrimidinic endonuclease 1. Anal. Chem. 2022, 94, 8732–8739. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, L.; Sun, H.; Xu, S.; Li, K.; Su, X. Screening and application of inhibitory aptamers for DNA repair protein apurinic/apyrimidinic endonuclease 1. Int. J. Biol. Macromol. 2023, 242, 124918. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Liu, Y.; Zhang, L.; Liu, J.; Su, X. Programming dissipation systems by DNA timer for temporally regulating enzyme catalysis and nanostructure assembly. ACS Nano 2022, 16, 14274–14283. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, H.; Liu, X.; Wu, Y.; Qi, L.; Huang, J.; Zhou, Y.; Zeng, J.; Wang, K.; He, X. DNA Nanotechnology-Empowered Fluorescence Imaging of APE1 Activity. Chemistry 2023, 5, 1815-1831. https://doi.org/10.3390/chemistry5030124
He H, Liu X, Wu Y, Qi L, Huang J, Zhou Y, Zeng J, Wang K, He X. DNA Nanotechnology-Empowered Fluorescence Imaging of APE1 Activity. Chemistry. 2023; 5(3):1815-1831. https://doi.org/10.3390/chemistry5030124
Chicago/Turabian StyleHe, Hui, Xiaojun Liu, Yuchen Wu, Lanlin Qi, Jin Huang, Yan Zhou, Jiahao Zeng, Kemin Wang, and Xiaoxiao He. 2023. "DNA Nanotechnology-Empowered Fluorescence Imaging of APE1 Activity" Chemistry 5, no. 3: 1815-1831. https://doi.org/10.3390/chemistry5030124




