Rare Nuclearities and Unprecedented Structural Motifs in Manganese Cluster Chemistry from the Combined Use of Di-2-Pyridyl Ketone with Selected Diols †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials, Physical and Spectroscopic Measurements
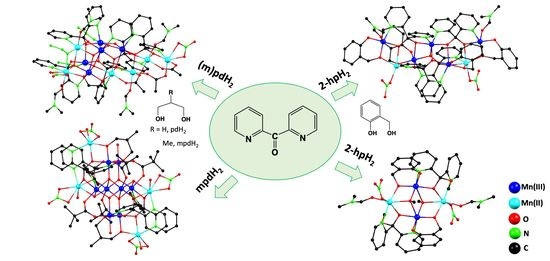
2.2. Syntheses
2.3. Single-Crystal X-ray Crystallography
3. Results and Discussion
3.1. Synthetic Comments
3.2. Description of Structures
3.3. Solid-State Magnetic Susceptibility Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Papatriantafyllopoulou, C.; Moushi, E.E.; Christou, G.; Tasiopoulos, A.J. Filling the Gap between the Quantum and Classical Worlds of Nanoscale Magnetism: Giant Molecular Aggregates Based on Paramagnetic 3d Metal Ions. Chem. Soc. Rev. 2016, 45, 1597–1628. [Google Scholar] [CrossRef]
- Maniaki, D.; Pilichos, E.; Perlepes, S.P. Coordination Clusters of 3d-Metals That Behave as Single-Molecule Magnets: Synthetic Routes and Strategies. Front. Chem. 2018, 6, 461. [Google Scholar] [CrossRef]
- Dearle, A.E.; Cutler, D.J.; Coletta, M.; Lee, E.; Dey, S.; Sanz, S.; Fraser, H.W.L.; Nichol, G.S.; Rajaraman, G.; Schnack, J.; et al. An [FeIII30] Molecular Metal Oxide. Chem. Commun. 2022, 58, 52–55. [Google Scholar] [CrossRef]
- Deng, Y.; Wu, Y.; Li, Z.; Jagličić, Z.; Gupta, R.K.; Tung, C.; Sun, D. Synthesis, Structure, and Optical-Response Magnetic Property of a Heteroarene-azo Functionalized Mn19 Cluster. Chin. J. Chem. 2023, 41, 1667–1672. [Google Scholar] [CrossRef]
- Lee, K.H.K.; Aebersold, L.; Peralta, J.E.; Abboud, K.A.; Christou, G. Synthesis, Structure, and Magnetic Properties of an Fe36 Dimethylarsinate Cluster: The Largest ‘Ferric Wheel’. Inorg. Chem. 2022, 61, 17256–17267. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Stull, J.A.; Yano, J.; Stamatatos, T.C.; Pringouri, K.; Stich, T.A.; Abboud, K.A.; Britt, R.D.; Yachandra, V.K.; Christou, G. Synthetic Model of the Asymmetric [Mn3CaO4] Cubane Core of the Oxygen-Evolving Complex of Photosystem II. Proc. Natl. Acad. Sci. USA 2012, 109, 2257–2262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, C.; Dong, H.; Shen, J.-R.; Dau, H.; Zhao, J. A Synthetic Mn4Ca-Cluster Mimicking the Oxygen-Evolving Center of Photosynthesis. Science 2015, 348, 690–693. [Google Scholar] [CrossRef]
- Signorella, S.; Hureau, C. Bioinspired Functional Mimics of the Manganese Catalases. Coord. Chem. Rev. 2012, 256, 1229–1245. [Google Scholar] [CrossRef]
- Najafpour, M.M.; Zaharieva, I.; Zand, Z.; Maedeh Hosseini, S.; Kouzmanova, M.; Hołyńska, M.; Tranca, I.; Larkum, A.W.; Shen, J.-R.; Allakhverdiev, S.I. Water-Oxidizing Complex in Photosystem II: Its Structure and Relation to Manganese-Oxide Based Catalysts. Coord. Chem. Rev. 2020, 409, 213183. [Google Scholar] [CrossRef]
- Thuijs, A.E.; Li, X.-G.; Wang, Y.-P.; Abboud, K.A.; Zhang, X.G.; Cheng, H.-P.; Christou, G. Molecular Analogue of the Perovskite Repeating Unit and Evidence for Direct MnIII-CeIV-MnIII Exchange Coupling Pathway. Nat. Commun. 2017, 8, 500. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.-K.; Su, H.-F.; Xu, J.-H.; Wang, W.-G.; Kurmoo, M.; Lin, S.-C.; Tan, Y.-Z.; Jia, J.; Sun, D.; Zheng, L.-S. Hierarchical Assembly of a {MnII15MnIII4} Brucite Disc: Step-by-Step Formation and Ferrimagnetism. J. Am. Chem. Soc. 2016, 138, 1328–1334. [Google Scholar] [CrossRef]
- Das Gupta, S.; Stewart, R.L.; Chen, D.-T.; Abboud, K.A.; Cheng, H.-P.; Hill, S.; Christou, G. Long-Range Ferromagnetic Exchange Interactions Mediated by Mn–CeIV–Mn Superexchange Involving Empty 4f Orbitals. Inorg. Chem. 2020, 59, 8716–8726. [Google Scholar] [CrossRef]
- Zabala-Lekuona, A.; Seco, J.M.; Colacio, E. Single-Molecule Magnets: From Mn12-ac to Dysprosium Metallocenes, a Travel in Time. Coord. Chem. Rev. 2021, 441, 213984. [Google Scholar] [CrossRef]
- Coronado, E. Molecular Magnetism: From Chemical Design to Spin Control in Molecules, Materials and Devices. Nat. Rev. Mater. 2020, 5, 87–104. [Google Scholar] [CrossRef]
- Abbasi, P.; Quinn, K.; Alexandropoulos, D.I.; Damjanović, M.; Wernsdorfer, W.; Escuer, A.; Mayans, J.; Pilkington, M.; Stamatatos, T.C. Transition Metal Single-Molecule Magnets: A {Mn31} Nanosized Cluster with a Large Energy Barrier of ∼60 K and Magnetic Hysteresis at ∼5 K. J. Am. Chem. Soc. 2017, 139, 15644–15647. [Google Scholar] [CrossRef]
- Evangelisti, M.; Brechin, E.K. Recipes for Enhanced Molecular Cooling. Dalton Trans. 2010, 39, 4672–4676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evangelisti, M.; Luis, F.; de Jongh, L.J.; Affronte, M. Magnetothermal Properties of Molecule-Based Materials. J. Mater. Chem. 2006, 16, 2534–2549. [Google Scholar] [CrossRef] [Green Version]
- Oyarzabal, I.; Zabala-Lekuona, A.; Mota, A.J.; Palacios, M.A.; Rodríguez-Diéguez, A.; Lorusso, G.; Evangelisti, M.; Rodríguez-Esteban, C.; Brechin, E.K.; Seco, J.M.; et al. Magneto-thermal Properties and Slow Magnetic Relaxation in Mn(ii)Ln(iii) Complexes: Influence of Magnetic Coupling on the Magneto-caloric Effect. Dalton Trans. 2022, 51, 12954–12967. [Google Scholar] [CrossRef] [PubMed]
- Aromí, G.; Brechin, E.K. Synthesis of 3d Metallic Single-Molecule Magnets. In Single-Molecule Magnets and Related Phenomena; Winpenny, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–67. [Google Scholar]
- Milios, C.J.; Winpenny, R.E.P. Cluster-Based Single-Molecule Magnets. In Molecular Nanomagnets and Related Phenomena; Gao, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–109. [Google Scholar]
- Pedersen, K.S.; Bendix, J.; Clérac, R. Single-molecule Magnet Engineering: Building-block Approaches. Chem. Commun. 2014, 50, 4396–4415. [Google Scholar] [CrossRef] [Green Version]
- Bagai, R.; Christou, G. The Drosophila of Single-Molecule Magnetism: [Mn12O12(O2CR)16(H2O)4]. Chem. Soc. Rev. 2009, 38, 1011–1026. [Google Scholar] [CrossRef]
- Gatteschi, D.; Sessoli, R. Quantum Tunneling of Magnetization and Related Phenomena in Molecular Materials. Angew. Chem. Int. Ed. 2003, 42, 268–297. [Google Scholar] [CrossRef]
- Bogani, L.; Wernsdorfer, W. Molecular Spintronics Using Single-Molecule Magnets. Nat. Mater. 2008, 7, 179. [Google Scholar] [CrossRef]
- Urdampilleta, M.; Klyatskaya, S.; Cleuziou, J.P.; Ruben, M.; Wernsdorfer, W. Supramolecular Spin Valves. Nat. Mater. 2011, 10, 502. [Google Scholar] [CrossRef]
- Tiron, R.; Wernsdorfer, W.; Aliaga-Alcalde, N.; Christou, G. Quantum Tunneling in a Three-Dimensional Network of Exchange-Coupled Single-Molecule Magnets. Phys. Rev. B 2003, 68, 140407. [Google Scholar] [CrossRef] [Green Version]
- Hill, S.; Edwards, R.S.; Aliaga-Alcalde, N.; Christou, G. Quantum Coherence in an Exchange-Coupled Dimer of Single-Molecule Magnets. Science 2003, 302, 1015–1018. [Google Scholar] [CrossRef] [Green Version]
- Vincent, R.; Klyatskaya, S.; Ruben, M.; Wernsdorfer, W.; Balestro, F. Electronic Read-out of a Single Nuclear Spin Using a Molecular Spin Transistor. Nature 2012, 488, 357. [Google Scholar] [CrossRef]
- Aguilà, D.; Barrios, L.A.; Velasco, V.; Roubeau, O.; Repollés, A.; Alonso, P.J.; Sesé, J.; Teat, S.J.; Luis, F.; Aromí, G. Heterodimetallic [LnLn’] Lanthanide Complexes: Toward a Chemical Design of Two-Qubit Molecular Spin Quantum Gates. J. Am. Chem. Soc. 2014, 136, 14215–14222. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Pineda, E.; Godfrin, C.; Balestro, F.; Wernsdorfer, W.; Ruben, M. Molecular Spin Qudits for Quantum Algorithms. Chem. Soc. Rev. 2018, 47, 501–513. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Pineda, E.; Wernsdorfer, W. Measuring Molecular Magnets for Quantum Technologies. Nat. Rev. Phys. 2021, 3, 645–659. [Google Scholar] [CrossRef]
- Aravena, D.; Ruiz, E. Spin Dynamics in Single-Molecule Magnets and Molecular Qubits. Dalton Trans. 2020, 49, 9916–9928. [Google Scholar] [CrossRef]
- Sessoli, R.; Gatteschi, D.; Caneschi, A.; Novak, M.A. Magnetic Bistability in a Metal-ion Cluster. Nature 1993, 365, 141. [Google Scholar] [CrossRef]
- Christou, G.; Gatteschi, D.; Hendrickson, D.N.; Sessoli, R. Single-Molecule Magnets. MRS Bull. 2000, 25, 66–71. [Google Scholar] [CrossRef]
- Tasiopoulos, A.J.; Vinslava, A.; Wernsdorfer, W.; Abboud, K.A.; Christou, G. Giant Single-Molecule Magnets: A {Mn84} Torus and Its Supramolecular Nanotubes. Angew. Chem. Int. Ed. 2004, 43, 2117–2121. [Google Scholar] [CrossRef] [Green Version]
- Hale, A.R.; Abboud, K.A.; Christou, G. Synthetic Factors Determining the Curvature and Nuclearity of the Giant Mn70 and Mn84 Clusters with a Torus Structure of ∼4 nm Diameter. Inorg. Chem. 2023, 62, 6020–6031. [Google Scholar] [CrossRef]
- Vinslava, A.; Tasiopoulos, A.J.; Wernsdorfer, W.; Abboud, K.A.; Christou, G. Molecules at the Quantum-Classical Nanoparticle Interface: Giant Mn70 Single-Molecule Magnets of ∼4 nm Diameter. Inorg. Chem. 2016, 55, 3419–3430. [Google Scholar] [CrossRef]
- Manoli, M.; Alexandrou, S.; Pham, L.; Lorusso, G.; Wernsdorfer, W.; Evangelisti, M.; Christou, G.; Tasiopoulos, A.J. Magnetic ‘Molecular Oligomers’ Based on Decametallic Supertetrahedra: A Giant Mn49 Cuboctahedron and Its Mn25Na4 Fragment. Angew. Chem. Int. Ed. 2016, 55, 679–684. [Google Scholar] [CrossRef] [Green Version]
- Moushi, E.E.; Masello, A.; Wernsdorfer, W.; Nastopoulos, V.; Christou, G.; Tasiopoulos, A.J. A Mn15 single-molecule magnet consisting of a supertetrahedron incorporated in a loop. Dalton Trans. 2010, 39, 4978–4985. [Google Scholar] [CrossRef]
- Charalambous, M.; Zartilas, S.M.; Moushi, E.E.; Papatriantafyllopoulou, C.; Manos, M.J.; Stamatatos, T.C.; Mukherjee, S.; Nastopoulos, V.; Christou, G.; Tasiopoulos, A.J. Discrete and encapsulated molecular grids: Homometallic Mn15 and heterometallic Mn24Ni2 aggregates. Chem. Commun. 2014, 50, 9090–9093. [Google Scholar] [CrossRef]
- Charalambous, M.; Moushi, E.E.; Nguyen, T.N.; Mowson, A.M.; Christou, G.; Tasiopoulos, A.J. [Mn14] “Structural Analogues” of Well-Known [Mn12] Single-Molecule Magnets. Eur. J. Inorg. Chem. 2018, 2018, 3905–3912. [Google Scholar] [CrossRef]
- Skordi, K.; Papatriantafyllopoulou, C.; Zartilas, S.; Poole, K.M.; Nastopoulos, V.; Christou, G.; Tasiopoulos, A.J. Homometallic {Mn10} and heterometallic {Mn6Ca4} supertetrahedra exhibiting an unprecedented {MnIII9MnII} oxidation state level and heterometal ions distribution. Polyhedron 2018, 151, 433–440. [Google Scholar] [CrossRef]
- Manoli, M.; Inglis, R.; Manos, M.J.; Nastopoulos, V.; Wernsdorfer, W.; Brechin, E.K.; Tasiopoulos, A.J. A [Mn32] Double-Decker Wheel. Angew. Chem. Int. Ed. 2011, 50, 4441–4444. [Google Scholar] [CrossRef] [Green Version]
- Manoli, M.; Inglis, R.; Manos, M.J.; Papaefstathiou, G.S.; Brechin, E.K.; Tasiopoulos, A.J. A 1-D coordination polymer based on a Mn40 octagonal super-structure. Chem. Commun. 2013, 49, 1061–1063. [Google Scholar] [CrossRef] [Green Version]
- Manoli, M.; Inglis, R.; Piligkos, S.; Yanhua, L.; Wernsdorfer, W.; Brechin, E.K.; Tasiopoulos, A.J. A hexameric [MnIII18Na6] wheel based on [MnIII3O]7+ sub-units. Chem. Commun. 2016, 52, 12829–12832. [Google Scholar] [CrossRef] [Green Version]
- Stamatatos, T.C.; Efthymiou, C.G.; Stoumpos, C.C.; Perlepes, S.P. Adventures in the Coordination Chemistry of Di-2-pyridyl Ketone and Related Ligands: From High-Spin Molecules and Single-Molecule Magnets to Coordination Polymers, and from Structural Aesthetics to an Exciting New Reactivity Chemistry of Coordinated Ligands. Eur. J. Inorg. Chem. 2009, 2009, 3361–3391. [Google Scholar]
- Mayans, J.; Font-Bardia, M.; Escuer, A. Lithium cations in a self-assembled electrostatic nanocapsule. Dalton Trans. 2019, 48, 16158–16161. [Google Scholar] [CrossRef] [PubMed]
- Papaefstathiou, G.S.; Perlepes, S.P. Families of Polynuclear Manganese, Cobalt, Nickel and Copper Complexes Stabilized by Various Forms of Di-2-pyridyl Ketone. Comments Inorg. Chem. 2002, 23, 249–274. [Google Scholar] [CrossRef]
- Tasiopoulos, A.J.; Perlepes, S.P. Diol-type ligands as central ‘players’ in the chemistry of high-spin molecules and single-molecule magnets. Dalton Trans. 2008, 41, 5537–5555. [Google Scholar] [CrossRef]
- Savva, M.; Skordi, K.; Fournet, A.D.; Thuijs, A.E.; Christou, G.; Perlepes, S.P.; Papatriantafyllopoulou, C.; Tasiopoulos, A.J. Heterometallic MnIII4Ln2 (Ln = Dy, Gd, Tb) Cross-Shaped Clusters and Their Homometallic MnIII4MnII2 Analogues. Inorg. Chem. 2017, 56, 5657–5668. [Google Scholar] [CrossRef]
- Skordi, K.; Anastassiades, A.; Fournet, A.D.; Kumar, R.; Schulze, M.; Wernsdorfer, W.; Christou, G.; Nastopoulos, V.; Perlepes, S.P.; Papatriantafyllopoulou, C.; et al. High nuclearity structurally—Related Mn supertetrahedral T4 aggregates. Chem. Commun. 2021, 57, 12484–12487. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532. [Google Scholar] [CrossRef]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A powerful new program for the analysis of anisotropic monomeric and exchange-coupled polynuclear d- and f-block complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Loeffen, P. ; Oxford Diffraction. CrysAlis CCD and CrysAlis RED; Oxford Diffraction Ltd.: Abingdon, UK, 2008. [Google Scholar]
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A.; Burla, M.C.; Polidori, G.; Camalli, M. SIR92—A program for automatic solution of crystal structures by direct methods. J. Appl. Crystallogr. 1994, 27, 435. [Google Scholar] [CrossRef]
- Shelxl, S.G.M. Program for the Refinement of Crystal Structures; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXL-2014, Program for the Refinement of Crystal Structures; University of Göttingen: Göttingen, Germany, 2014. [Google Scholar]
- Oxford Diffraction. CrysAlis CCD and CrysAlis RED, Version 1.71; Oxford Diffraction: Oxford, UK, 2007. [Google Scholar]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; Streek, J.V.D. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef] [Green Version]
- Brandenburg, K.; Putz, H. Diamond; Crystal Impact GbR: Bonn, Germany, 2006. [Google Scholar]
- van der Sluis, P.; Spek, A.L. BYPASS: An effective method for the refinement of crystal structures containing disordered solvent regions. Acta Crystallogr. Sect. A 1990, 46, 194–201. [Google Scholar] [CrossRef]
- Liu, W.; Thorp, H.H. Bond valence sum analysis of metal-ligand bond lengths in metalloenzymes and model complexes. 2. Refined distances and other enzymes. Inorg. Chem. 1993, 32, 4102–4105. [Google Scholar] [CrossRef]
- Brown, I.D.; Altermatt, D. Bond-valence parameters obtained from a systematic analysis of the Inorganic Crystal Structure Database. Acta Crystallogr. Sect. B 1985, 41, 244–247. [Google Scholar] [CrossRef] [Green Version]
- Koumousi, E.S.; Lazari, G.; Grammatikopoulos, S.; Papatriantafyllopoulou, C.; Manos, M.J.; Perlepes, S.P.; Tasiopoulos, A.J.; Christou, G.; Stamatatos, T.C. Rare nuclearities in Mn/oxo cluster chemistry: Synthesis and characterization of a mixed-valence {MnII/III11} complex bearing acetate and salicylhydroximate(-3) bridging/chelating ligands. Polyhedron 2021, 206, 115298. [Google Scholar] [CrossRef]
- Yoo, J.; Yamaguchi, A.; Nakano, M.; Krzystek, J.; Streib, W.E.; Brunel, L.-C.; Ishimoto, H.; Christou, G.; Hendrickson, D.N. Mixed-Valence Tetranuclear Manganese Single-Molecule Magnets. Inorg. Chem. 2001, 40, 4604–4616. [Google Scholar] [CrossRef] [Green Version]
- Yang, E.-C.; Harden, N.; Wernsdorfer, W.; Zakharov, L.; Brechin, E.K.; Rheingold, A.L.; Christou, G.; Hendrickson, D.N. Mn4 single-molecule magnets with a planar diamond core and S=9. Polyhedron 2003, 22, 1857–1863. [Google Scholar] [CrossRef]
- Wittick, L.M.; Jones, L.F.; Jensen, P.; Moubaraki, B.; Spiccia, L.; Berry, K.J.; Murray, K.S. New mixed-valence MnII2MnIII2 clusters exhibiting an unprecedented MnII/III oxidation state distribution in their magnetically coupled cores. Dalton Trans. 2006, 12, 1534–1543. [Google Scholar] [CrossRef] [PubMed]
- Ako, A.M.; Mereacre, V.; Hewitt, I.J.; Clérac, R.; Lecren, L.; Anson, C.E.; Powell, A.K. Enhancing single molecule magnet parameters. Synthesis, crystal structures and magnetic properties of mixed-valent Mn4 SMMs. J. Mater. Chem. 2006, 16, 2579–2586. [Google Scholar] [CrossRef]
- Karotsis, G.; Teat, S.J.; Wernsdorfer, W.; Piligkos, S.; Dalgarno, S.J.; Brechin, E.K. Calix[4]arene-Based Single-Molecule Magnets. Angew. Chem. Int. Ed. 2009, 48, 8285–8288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, A.; Abboud, K.A.; Christou, G. New Mixed-Valent Mn Clusters from the Use of N,N,N′,N′-Tetrakis(2-hydroxyethyl)ethylenediamine (edteH4): Mn3, Mn4, Mn6, and Mn10. Inorg. Chem. 2011, 50, 12774–12784. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.; Abboud, K.A.; Christou, G. A Mn4 single-molecule magnet with the defective-dicubane structure from the use of pyrenecarboxylic acid. Polyhedron 2013, 66, 171–178. [Google Scholar] [CrossRef]
- Papaefstathiou, G.S.; Escuer, A.; Raptopoulou, C.P.; Terzis, A.; Perlepes, S.P.; Vicente, R. Defective Double-Cubane, Tetranuclear Manganese(II) and Cobalt(II) Complexes with Simultaneous μ1,1-Azido and μ-O Bridges. Eur. J. Inorg. Chem. 2001, 2001, 1567–1574. [Google Scholar] [CrossRef]
- Stamatatos, T.C.; Adam, R.; Raptopoulou, C.P.; Psycharis, V.; Ballesteros, R.; Abarca, B.; Perlepes, S.P.; Boudalis, A.K. The first member of a second generation family of ligands derived from metal-ion assisted reactivity of di-2,6-(2-pyridylcarbonyl)pyridine: Synthesis and characterization of a MnII/III4 rhombus. Inorg. Chem. Commun. 2012, 15, 73–77. [Google Scholar] [CrossRef]
- Vignesh, K.R.; Langley, S.K.; Gartshore, C.J.; Moubaraki, B.; Murray, K.S.; Rajaraman, G. What Controls the Magnetic Exchange and Anisotropy in a Family of Tetranuclear {Mn2IIMn2III} Single-Molecule Magnets? Inorg. Chem. 2017, 56, 1932–1949. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skordi, K.; Alexandropoulos, D.I.; Fournet, A.D.; Panagiotou, N.; Moushi, E.E.; Papatriantafyllopoulou, C.; Christou, G.; Tasiopoulos, A.J. Rare Nuclearities and Unprecedented Structural Motifs in Manganese Cluster Chemistry from the Combined Use of Di-2-Pyridyl Ketone with Selected Diols. Chemistry 2023, 5, 1681-1695. https://doi.org/10.3390/chemistry5030115
Skordi K, Alexandropoulos DI, Fournet AD, Panagiotou N, Moushi EE, Papatriantafyllopoulou C, Christou G, Tasiopoulos AJ. Rare Nuclearities and Unprecedented Structural Motifs in Manganese Cluster Chemistry from the Combined Use of Di-2-Pyridyl Ketone with Selected Diols. Chemistry. 2023; 5(3):1681-1695. https://doi.org/10.3390/chemistry5030115
Chicago/Turabian StyleSkordi, Katerina, Dimitris I. Alexandropoulos, Adeline D. Fournet, Nikos Panagiotou, Eleni E. Moushi, Constantina Papatriantafyllopoulou, George Christou, and Anastasios J. Tasiopoulos. 2023. "Rare Nuclearities and Unprecedented Structural Motifs in Manganese Cluster Chemistry from the Combined Use of Di-2-Pyridyl Ketone with Selected Diols" Chemistry 5, no. 3: 1681-1695. https://doi.org/10.3390/chemistry5030115