Structures of Three Alkaline-Earth Metal Germanides Refined from Single-Crystal X-ray Diffraction Data
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Structure of CaGe
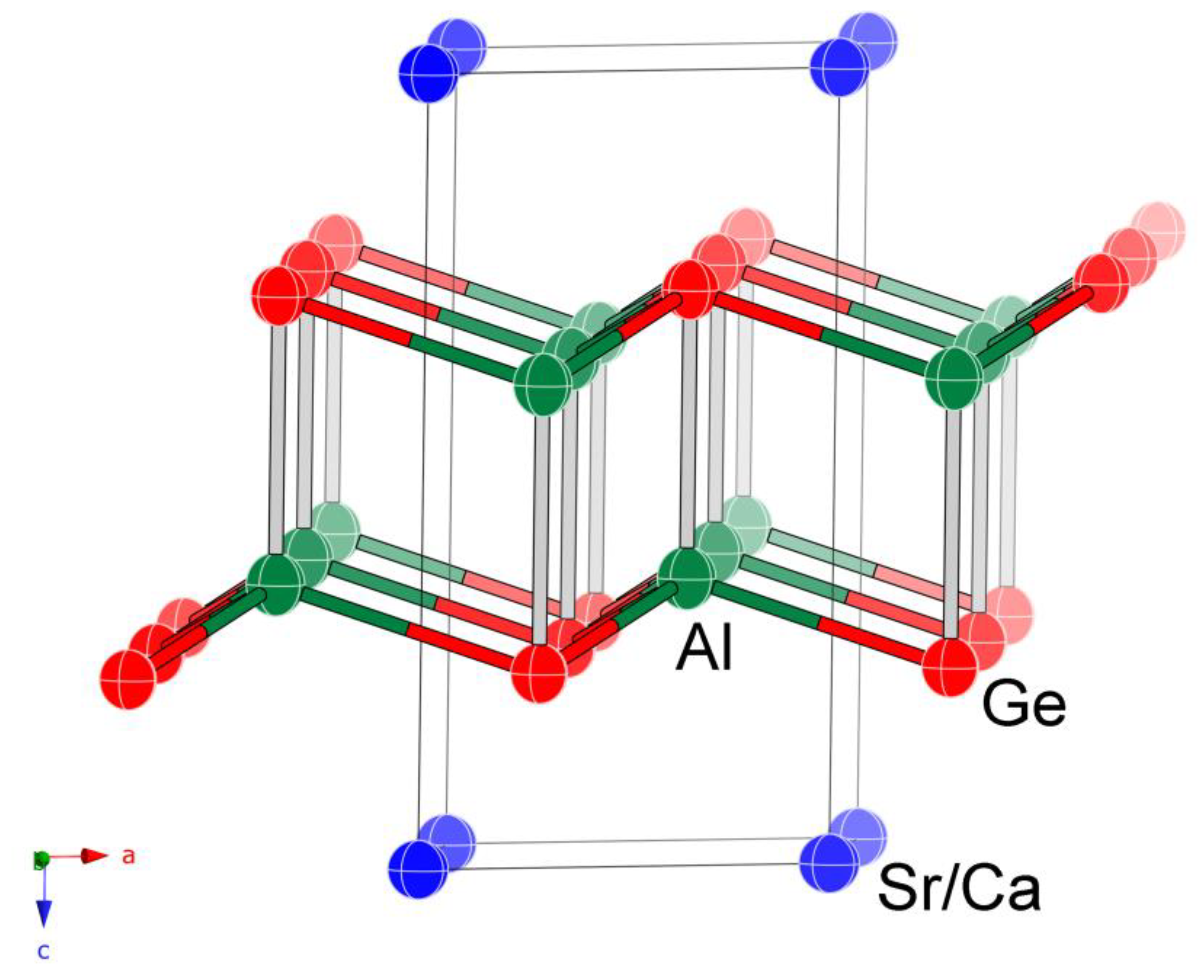
3.2. Structures of SrAl2Ge2, and SrxCa1−xAl2Ge2 (x ≈ 0.4)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tobash, P.H.; Bobev, S. Synthesis, structure and electronic structure of a new polymorph of CaGe2. J. Solid State Chem. 2007, 180, 1575–1581. [Google Scholar] [CrossRef]
- Tobash, P.H.; Lins, D.; Bobev, S.; Hur, N.; Thompson, J.D.; Sarrao, J.L. Vacancy ordering in SmGe2–x and GdGe2–x (x = 0.33): Properties of two Sm3Ge5 polymorphs and of Gd3Ge5. Inorg. Chem. 2006, 45, 7286–7294. [Google Scholar] [CrossRef] [PubMed]
- You, T.-S.; Jung, Y.; Bobev, S. Experimental and theoretical investigations of the novel ternary compound Ca4InGe4. Dalton Trans. 2012, 41, 12446–12451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bobev, S. Synthesis, Structural Characterization and Properties of SrAl4–xGex, BaAl4–xGex, and EuAl4–xGex (x ≈ 0.3–0.4)—Rare examples of electron-rich phases with the BaAl4 structure type. J. Solid State Chem. 2013, 205, 21–28. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Shek, C.H.; Wang, Y.; Bobev, S. On the structures of the rare-earth metal germanides from the series REAl1–xGe3 (RE = Nd, Sm, Gd, Tb, Dy, Ho; 0.6 <x <0.9). A tale of vacancies at the Al Sites and the concomitant structural modulations. Dalton Trans. 2017, 46, 9253–9265. [Google Scholar] [PubMed]
- Tobash, P.H.; Lins, D.; Bobev, S.; Lima, A.; Hundley, M.F.; Thompson, J.D.; Sarrao, J.L. Crystal growth, structural, and property studies on a family of ternary rare-earth phases RE2InGe2 (RE = Sm, Gd, Tb, Dy, Ho, Yb). Chem. Mater. 2005, 17, 5567–5573. [Google Scholar] [CrossRef]
- Zhang, J.; Bobev, S. Correlations between chemical bonding and magnetic exchange interactions: Synthesis, crystal structures, and magnetic properties of the new family RE2AlGe2 (RE = Tb–Tm, Lu). Inorg. Chem. 2013, 52, 5307–5315. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Bobev, S. Structural modulations in the rare-earth metal digermanides REAl1–xGe2 (RE = Gd−Tm, Lu, Y; 0.8 <x <0.9). Correlations between long- and short-range vacancy ordering. Inorg. Chem. 2015, 54, 722–732. [Google Scholar]
- You, T.-S.; Bobev, S. Synthesis and structural characterization of A3In2Ge4 and A5In3Ge6 (A = Ca, Sr, Eu, Yb)—New intermetallic compounds with complex structures, exhibiting Ge–Ge and In–In bonding. J. Solid State Chem. 2010, 183, 1258–1265. [Google Scholar] [CrossRef]
- You, T.-S.; Tobash, P.H.; Bobev, S. Mixed cations and structural complexity in (Eu1–xCax)4In3Ge4 and (Eu1–xCax)3In2Ge3—The first two members of the homologous series A2[n+m]In2n+mGe2[n+m] (n, m = 1,2…∞; A = Ca, Sr, Ba, Eu, or Yb). Inorg. Chem. 2010, 49, 1773–1783. [Google Scholar] [CrossRef]
- Suen, N.-T.; Hooper, J.; Zurek, E.; Bobev, S. On the nature of Ge−Pb bonding in the solid state. Synthesis, structural characterization, and electronic structures of two unprecedented germanide-plumbides. J. Am. Chem. Soc. 2012, 134, 12708–12716. [Google Scholar] [CrossRef] [PubMed]
- Tobash, P.H.; Bobev, S.; Ronning, F.; Thompson, J.D.; Sarrao, J.L. Structural chemistry and magnetic properties of RE2[SnxGe1–x]5 (RE = Nd, Sm) and RE[SnxGe1–x]2 (RE = Gd, Tb): Four new rare-earth metal intermetallic compounds with germanium zig-zag chains and tin square-nets. J. Alloy. Compd. 2009, 488, 511. [Google Scholar] [CrossRef]
- Suen, N.-T.; Bobev, S. Calcium substitution in rare-earth metal germanides with the Gd5Si4 type structure. Z. Anorg. Allg. Chem. 2022, 648, e202200016. [Google Scholar] [CrossRef]
- Suen, N.-T.; Bobev, S. Calcium substitution in rare-earth metal germanides with the Cr5B3 type structure. Z. Anorg. Allg. Chem. 2022; in print. [Google Scholar]
- Suen, N.-T.; Broda, M.; Bobev, S. Calcium substitution in rare-earth metal germanides with the hexagonal Mn5Si3 structure type. Structural characterization of the extended series RE5–xCaxGe3 (RE = rare-earth metal). J. Solid State Chem. 2014, 217, 142–149. [Google Scholar] [CrossRef]
- Pauling, L. The Nature of the Chemical Bond, 3rd ed.; Cornell University Press: Ithaca, NY, USA, 1960. [Google Scholar]
- Gladishevskij, E.J.; Kripjakevic, P.I.; Bodak, O.I. The crystal structures of the compound CaAl2Si2 and its analogues. Ukr. Fiz. Zh. (Russ. Ed.) 1967, 12, 447–453. [Google Scholar]
- Shi, X.-M.; Chen, L.-Q.; He, H.; Sun, Y.-X.; Zeng, Z.; Tang, J. Investigation on the preparation and thermoelectric properties of layered SrAl2Ge2. J. Sichuan Univ. (Nat. Sc. Ed) 2019, 56, 940–943. [Google Scholar]
- Schob, O.; Parthé, E. AB Compounds with Sc, Y and rare earth metals. I. Scandium and Yttrium Compounds with CrB and CsCl Structure. Acta Crystallogr. 1965, 19, 214–224. [Google Scholar] [CrossRef]
- Merlo, F.; Europa, C. The Pseudobinary systems SrAg1–xZnx, CaCu1–xGax and CaCu1–xGex and their use for testing structural maps. J. Less Common Met. 1986, 119, 45–61. [Google Scholar] [CrossRef]
- Iandelli, A. Legami covalenti nei composti intermetallici. I composti PrGe e CaGe. Atti Della Accad. Naz. Dei Lincei Cl. Di Sci. Fis. Mat. E Nat. Rend. 1955, 19, 307–313. [Google Scholar]
- Palezona, A.; Manifretti, P.; Fornasini, M.L. The phase diagram of the Ca–Ge system. J. Alloy. Compd. 2002, 345, 144–147. [Google Scholar] [CrossRef]
- SMART, version 2.10; Bruker Analytical X-ray Systems, Inc.: Madison, WI, USA, 2003.
- SAINT, version 6.45; Bruker Analytical X-ray Systems, Inc.: Madison, WI, USA, 2003.
- SADABS, version 2.10; Bruker Analytical X-ray Systems, Inc.: Madison, WI, USA, 2003.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villars, P.; Calvert, L.D. (Eds.) Pearson’s Handbook of Crystallographic Data for Intermetallic Compounds, 2nd ed.; American Society for Metals: Materials Park, OH, USA, 1991. [Google Scholar]
- Wu, L.-M.; Kim, S.-H.; De, D.-K.S. Electron-precise/deficient La5–xCaxGe4 (3.4 < x < 3.8) and Ce5–xCaxGe4 (3.0 < x < 3.3): Probing low-valence electron concentrations in metal-rich Gd5Si4-type germanides . J. Am. Chem. Soc. 2015, 127, 15682–15683. [Google Scholar]
- Suen, N.-T.; You, T.-S.; Bobev, S. Synthesis, crystal structures and chemical bonding of RE5–xLixGe4 (RE = Nd, Sm and Gd; x ≃ 1) with the orthorhombic Gd5Si4 Type. Acta Crystallogr. C 2013, 69, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Tobash, P.H.; Bobev, S.; Thompson, J.D.; Sarrao, J.L. Magnesium substitutions in rare-earth metal germanides with the orthorhombic Gd5Si4-type structure. Synthesis, crystal chemistry, and magnetic properties of RE5–xMgxGe4 [RE = Gd–Tm, Lu, and Y]. Inorg. Chem. 2009, 48, 6641–6651. [Google Scholar] [CrossRef] [PubMed]
- Suen, N.-T.; Tobash, P.H.; Bobev, S. Synthesis, structural characterization and magnetic properties of RE2MgGe2 (RE = rare-earth metal). J. Solid State Chem. 2011, 184, 2941–2947. [Google Scholar] [CrossRef]
- Guo, S.-P.; Meyers, J.J.; Tobash, P.H.; Bobev, S. Eleven new compounds in the RE-Cd-Ge systems (RE = Pr, Nd, Sm, Gd–Yb; Y): Crystal chemistry of the RE2CdGe2 series. J. Solid State Chem. 2012, 192, 16–22. [Google Scholar] [CrossRef]
- Suen, N.-T.; Huang, L.; Meyers, J.J.; Bobev, S. An Unusual triple-decker variant of the tetragonal BaAl4-structure type: Synthesis, structural characterization, and chemical bonding of Sr3Cd8Ge4 and Eu3Cd8Ge4. Inorg. Chem. 2018, 57, 833–842. [Google Scholar] [CrossRef]
- Guo, S.-P.; You, T.-S.; Bobev, S. Closely related rare-earth metal germanides RE2Li2Ge3 and RE3Li4Ge4 (RE = La–Nd, Sm): Synthesis, crystal chemistry, and magnetic properties. Inorg. Chem. 2012, 51, 3119–3129. [Google Scholar] [CrossRef]
- Guo, S.-P.; You, T.-S.; Jung, Y.; Bobev, S. Synthesis, crystal chemistry, and magnetic properties of RE7Li8Ge10 and RE11Li12Ge16 (RE = La−Nd, Sm): New members of the [REGe2]n[RELi2Ge]m homologous series. Inorg. Chem. 2012, 51, 6821–6829. [Google Scholar] [CrossRef]
- Eisenmann, B.; Schäfer, H. The crystal structures of Ca5Si3 and Ca5Ge3. Z. Naturforsch. B 1974, 29, 460–463. [Google Scholar] [CrossRef]
- Mudring, A.V.; Corbett, J.D. Unusual electronic and bonding properties of the Zintl phase Ca5Ge3 and related compounds. A theoretical analysis. J. Am. Chem. Soc. 2004, 126, 5277–5281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nesper, R. The Zintl-Klemm concept—A historical survey. Z. Anorg. Allg. Chem. 2014, 640, 2639–2648. [Google Scholar] [CrossRef]
- The Materials Project. Materials Data on CaGe by Materials Project; USA, 2020; N. p. Web. [Google Scholar] [CrossRef]
- Kranenberg, C.; Johrendt, D.; Mewis, A. The stability range of the CaAl2Si2-type structure in case of LnAl2Ge2 compounds. Solid State Sci. 2002, 4, 261–265. [Google Scholar] [CrossRef]
- Carrillo-Cabrera, W.; Gil, R.C.; Grin, Y. Refinement of the crystal structure of monocalcium dialuminide digermanide, Ca[Al2Ge2]. Z. Krist.—New Cryst. Struct. 2001, 216, 535–536. [Google Scholar] [CrossRef]
- Zheng, C.; Hoffmann, R. Complementary local and extended views of bonding in the ThCr2Si2 and CaAl2Si2 structures. J. Solid State Chem. 1988, 72, 58–71. [Google Scholar] [CrossRef]
- Zheng, C.; Hoffmann, R.; Nesper, R.; von Schnering, H.-G. Site preferences and bond length differences in CaAl2Si2 type Zintl compounds. J. Am. Chem. Soc. 1986, 108, 1876–1884. [Google Scholar] [CrossRef]
- Peng, W.; Chanakian, S.; Zevalkink, A. Crystal chemistry and thermoelectric transport of layered AM2X2 compounds. Inorg. Chem. Front. 2018, 5, 1744–1759. [Google Scholar] [CrossRef]
- Burdett, J.K.; Miller, G.J. Fragment formalism in main-group solids: Applications to AlB2, CaAl2Si2, BaAl4 and related materials. Chem. Mater. 1990, 2, 12–26. [Google Scholar] [CrossRef]
- Schäfer, M.C.; Suen, N.-T.; Raglione, M.; Bobev, S. The layered antimonides RELi3Sb2 (RE = Ce–Nd, Sm, Gd–Ho). Filled derivatives of the CaAl2Si2 structure type. J. Solid State Chem. 2014, 210, 89–95. [Google Scholar] [CrossRef]
- The Materials Project. Materials Data on Ca(AlGe)2 by Materials Project; USA, 2020; N. p. Web. [Google Scholar] [CrossRef]
- Strikos, S.; Joseph, B.; Alabarse, F.G.; Valadares, G.; Costa, D.G.; Capaz, R.B.; ElMassalami, M. Structural metastability and Fermi surface topology of SrAl2Si2. Inorg. Chem. 2021, 60, 18652–18661. [Google Scholar] [CrossRef]
- Kauzlarich, S.M.; Condron, C.L.; Wassei, J.K.; Ikeda, T.; Snyder, G.J. Structure and high-temperature thermoelectric properties of SrAl2Si2. J. Solid State Chem. 2009, 182, 240–245. [Google Scholar] [CrossRef]
- Zevalkink, A.; Bobnar, M.; Schwarz, U.; Grin, Y. Making and braking bonds in superconducting SrAl4-xSix (0 <x <2). Chem. Mater 2017, 29, 1236–1244. [Google Scholar]
- Imai, M.; Abe, H.; Yamada, K. Electrical properties of single-crystalline CaAl2Si2. Inorg. Chem. 2004, 43, 5186–5188. [Google Scholar] [CrossRef] [PubMed]

| Empirical Formula | CaGe | SrAl2Ge2 | Sr0.36(1)Ca0.64Al2Ge2 |
|---|---|---|---|
| Formula weight | 112.67 | 286.76 | 256.33 |
| Temperature (K) | 200(2) | 200(2) | 200(2) |
| Radiation, λ | Mo Kα, 0.71073 Å | Mo Kα, 0.71073 Å | Mo Kα, 0.71073 Å |
| Space group, Z | Cmcm, 4 | Pm1, 1 | Pm1, 1 |
| a (Å) | 4.5698(8) | 4.2157(13) | 4.1929(3) |
| b (Å) | 10.832(2) | - | - |
| c (Å) | 3.9979(8) | 7.443 (3) | 7.2810(12) |
| V (Å3) | 197.90(6) | 114.55(7) | 110.85(2) |
| ρcal (g/cm3) | 3.78 | 4.16 | 3.84 |
| μ (cm−1) | 175.2 | 248.1 | 187.6 |
| Goodness-of-fit on F2 | 1.084 | 1.135 | 1.282 |
| Unique reflections | 157 | 136 | 151 |
| Refined parameters | 10 | 9 | 11 |
| R1 (I > 2σI) a | 0.0222 | 0.0304 | 0.0155 |
| wR2 (I > 2σI) a | 0.0501 | 0.0675 | 0.0367 |
| R1 (all data) a | 0.0233 | 0.0432 | 0.0159 |
| wR2 (all data) a | 0.0507 | 0.0722 | 0.0369 |
| Largest diff. peak andhole (e−/Å3) | 0.57 and −0.87 | 1.83 and −0.86 | 0.46 and −0.69 |
| Atom | Site | x | y | z | Ueq (Å2) |
|---|---|---|---|---|---|
| CaGe | |||||
| Ca | 4c | 0 | 0.0763(1) | 1/4 | 0.010(1) |
| Ge | 4c | 0 | 0.3622(1) | 1/4 | 0.010(1) |
| SrAl2Ge2 | |||||
| Sr | 1a | 0 | 0 | 0 | 0.014(1) |
| Al | 2d | 2/3 | 1/3 | 0.3738(5) | 0.013(1) |
| Ge | 2d | 1/3 | 2/3 | 0.2724(2) | 0.014(1) |
| Sr0.36(1)Ca0.64Al2Ge2 | |||||
| Ca/Sr b | 1a | 0 | 0 | 0 | 0.010(1) |
| Al | 2d | 1/3 | 2/3 | 0.3719(1) | 0.010(1) |
| Ge | 2d | 2/3 | 1/3 | 0.2656(2) | 0.009(1) |
| Atom Pair | Distance |
|---|---|
| Ge–Ge (×2) | 2.5934(9) |
| Ge–Ca | 3.098(2) |
| Ge–Ca (×4) | 3.1082(5) |
| Ge–Ca (×2) | 3.255(1) |
| Ca–Ca (×2) | 3.593(2) |
| Atom Pair | Distance | Atom Pair | Distance |
|---|---|---|---|
| SrAl2Ge2 | Sr0.36(1)Ca0.64Al2Ge2 | ||
| Ge–Al (×3) | 2.548(2) | Ge–Al (×3) | 2.5415(5) |
| Ge–Al | 2.633(4) | Ge–Al | 2.639(1) |
| Sr–Ge (×6) | 3.168(1) | Sr/Ca–Ge (×6) | 3.0984(4) |
| Sr–Al (×6) | 3.697(3) | Sr/Ca–Al (×6) | 3.632(1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suen, N.-T.; Bobev, S. Structures of Three Alkaline-Earth Metal Germanides Refined from Single-Crystal X-ray Diffraction Data. Chemistry 2022, 4, 1429-1438. https://doi.org/10.3390/chemistry4040094
Suen N-T, Bobev S. Structures of Three Alkaline-Earth Metal Germanides Refined from Single-Crystal X-ray Diffraction Data. Chemistry. 2022; 4(4):1429-1438. https://doi.org/10.3390/chemistry4040094
Chicago/Turabian StyleSuen, Nian-Tzu, and Svilen Bobev. 2022. "Structures of Three Alkaline-Earth Metal Germanides Refined from Single-Crystal X-ray Diffraction Data" Chemistry 4, no. 4: 1429-1438. https://doi.org/10.3390/chemistry4040094





