Cationic Polystyrene Resin Bound Silver Nanocomposites Assisted Fourier Transform Infrared Spectroscopy for Enhanced Catalytic Reduction of 4-Nitrophenol in Aqueous Medium
Abstract
1. Introduction
2. Materials and Method
2.1. Reagents and Solutions
2.2. Instruments
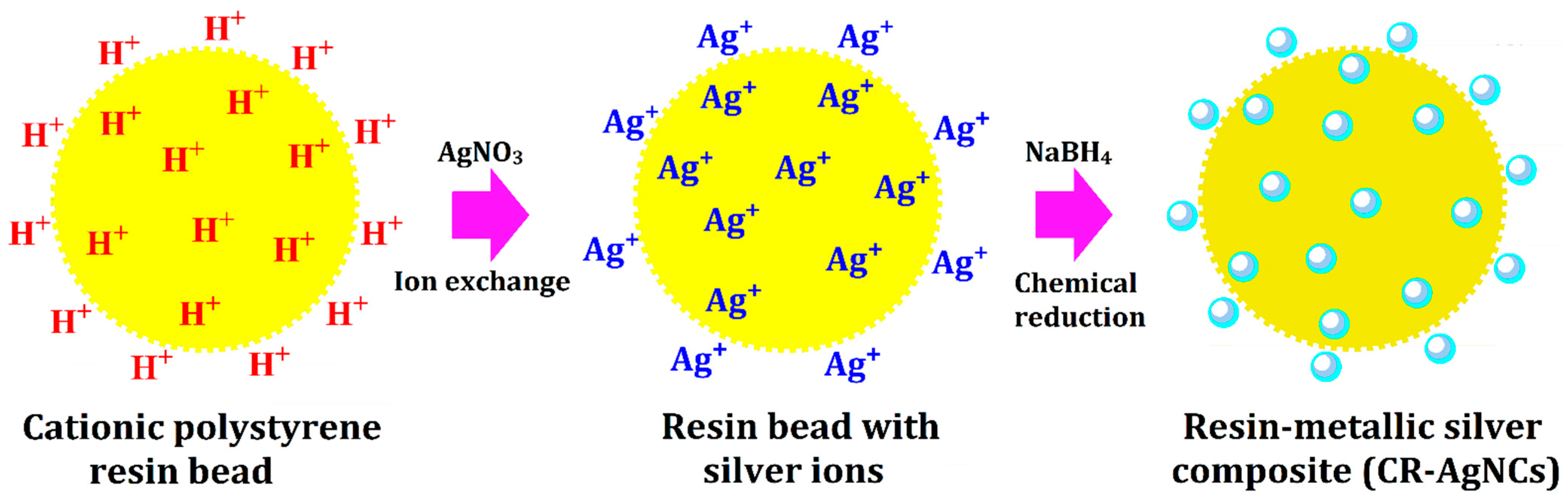
2.3. Preparation of Cationic Polystyrene Resin Bound Silver Nanocomposites (CR-AgNCs)
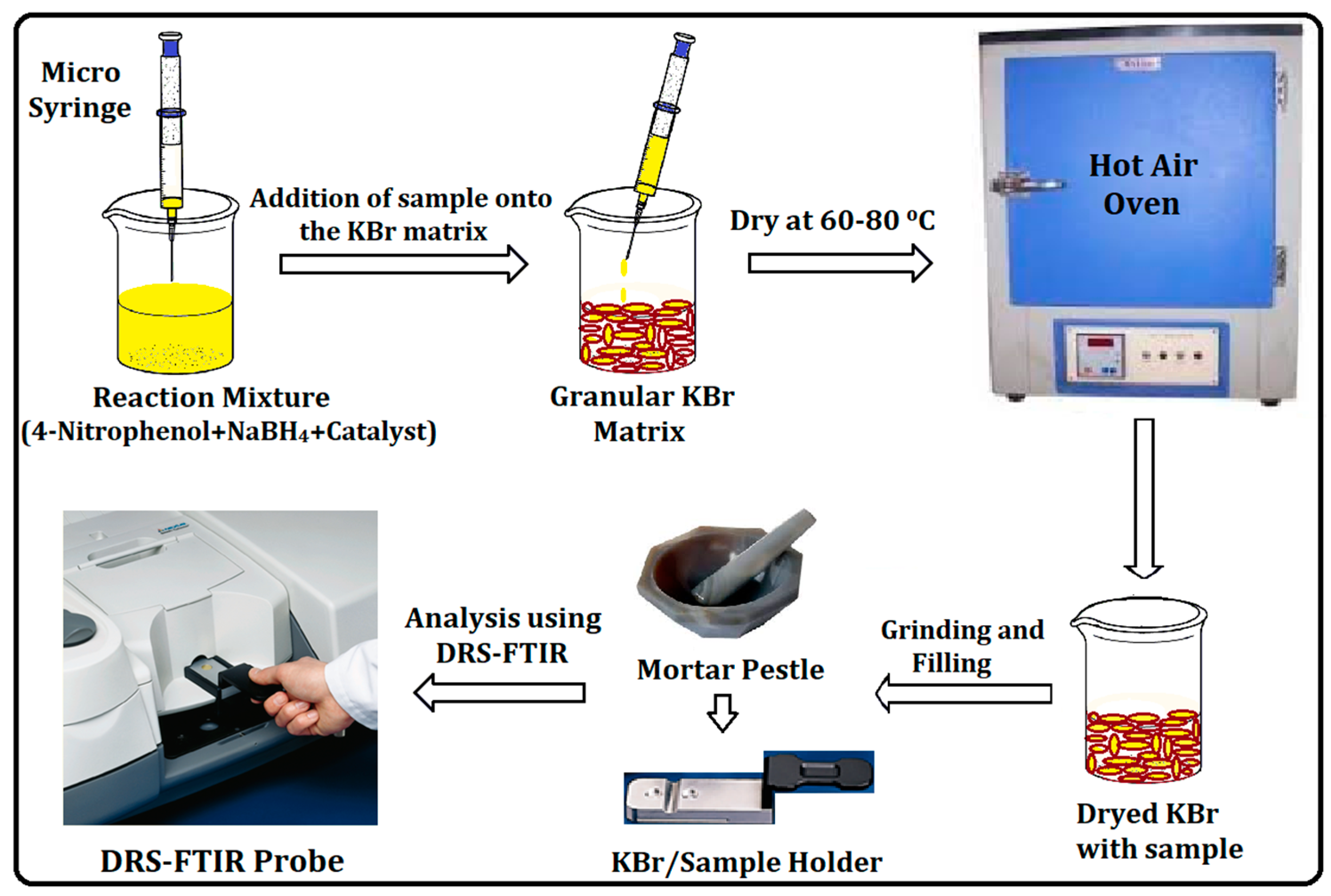
2.4. Catalytic Reduction Study Employing DRS-FTIR
3. Result and Discussion
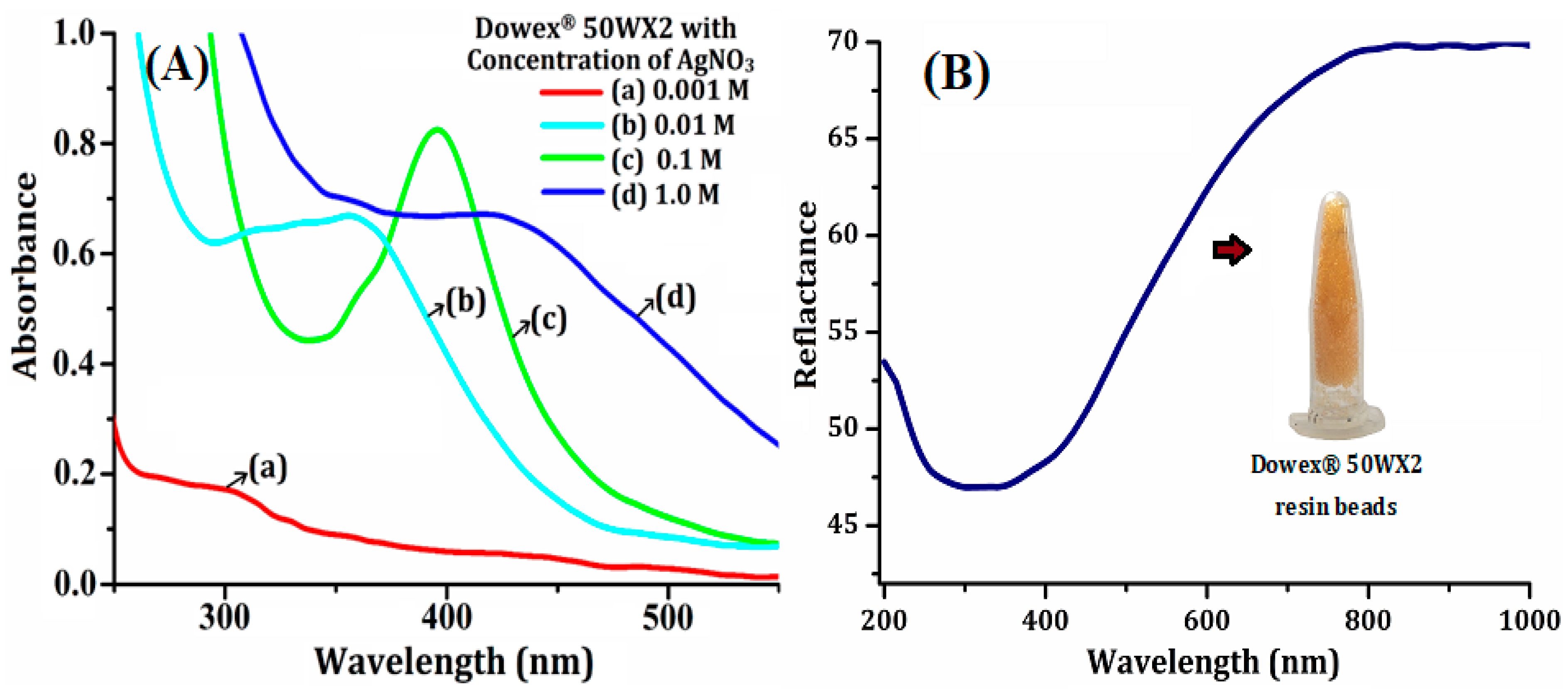
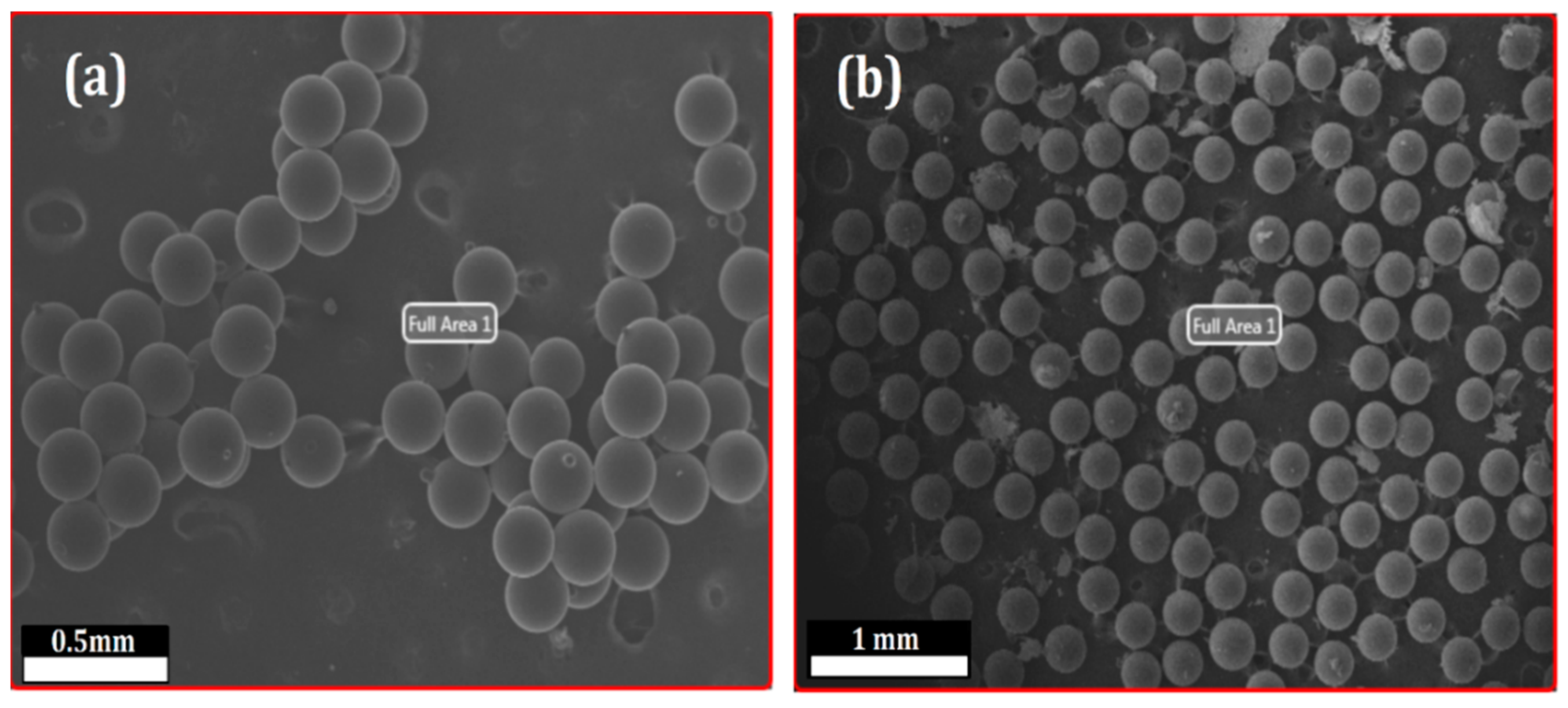
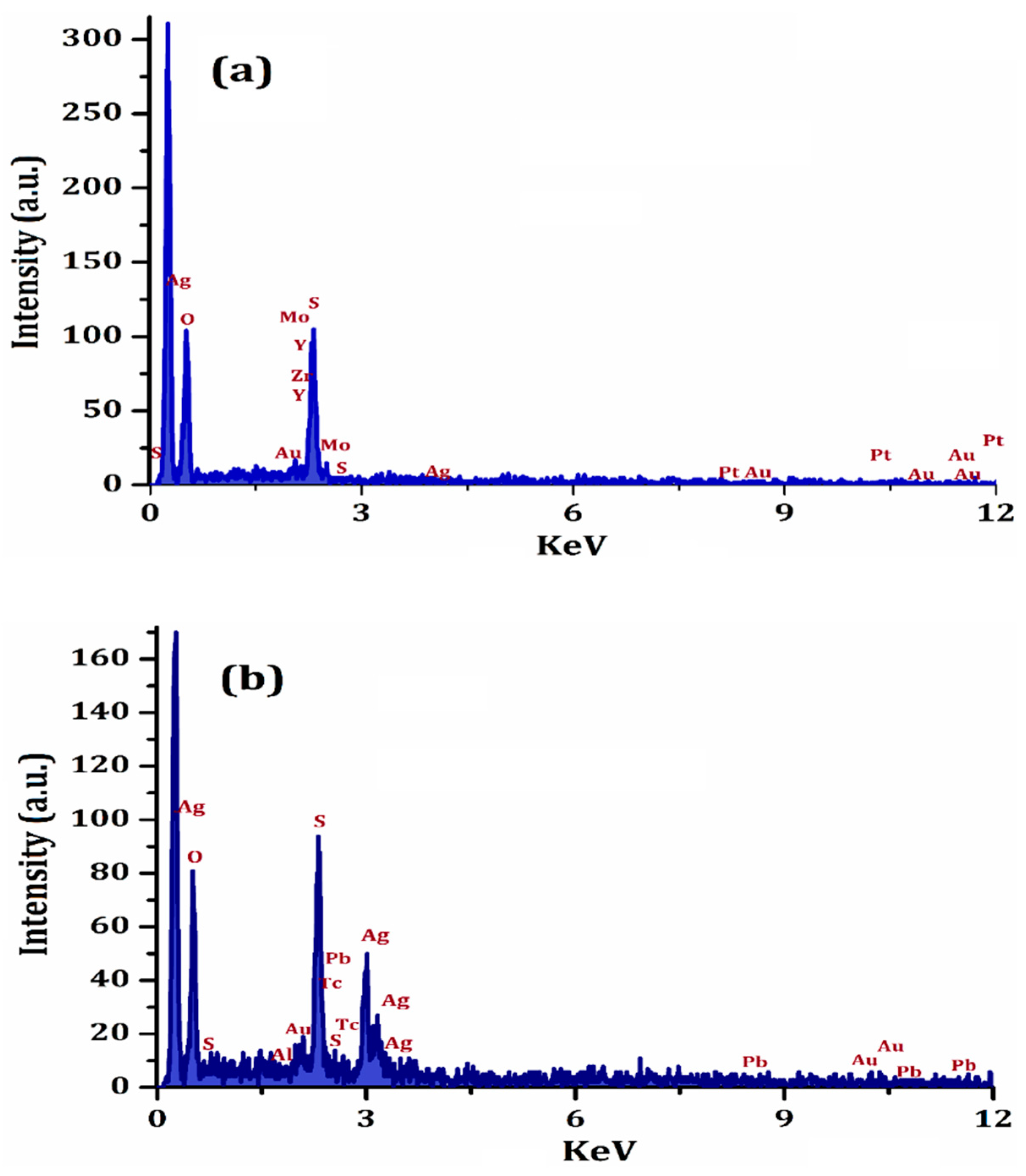
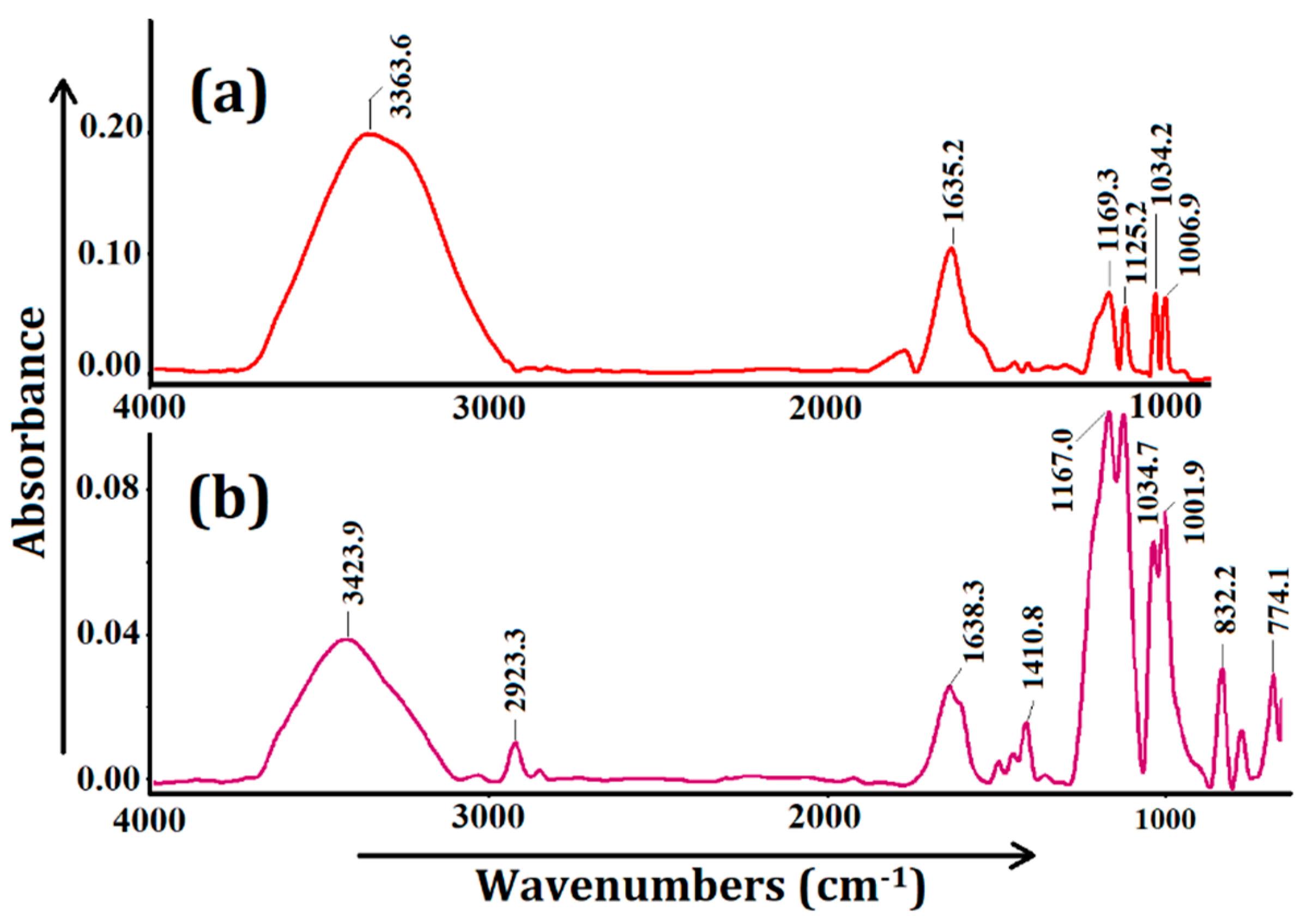
3.1. Selection and Characterization of CR-AgNCs
3.2. DRS-FTIR Spectral Assignment of Catalytic Reduction of 4-Nitrophenol Employing CR-AgNCs as a Catalyst
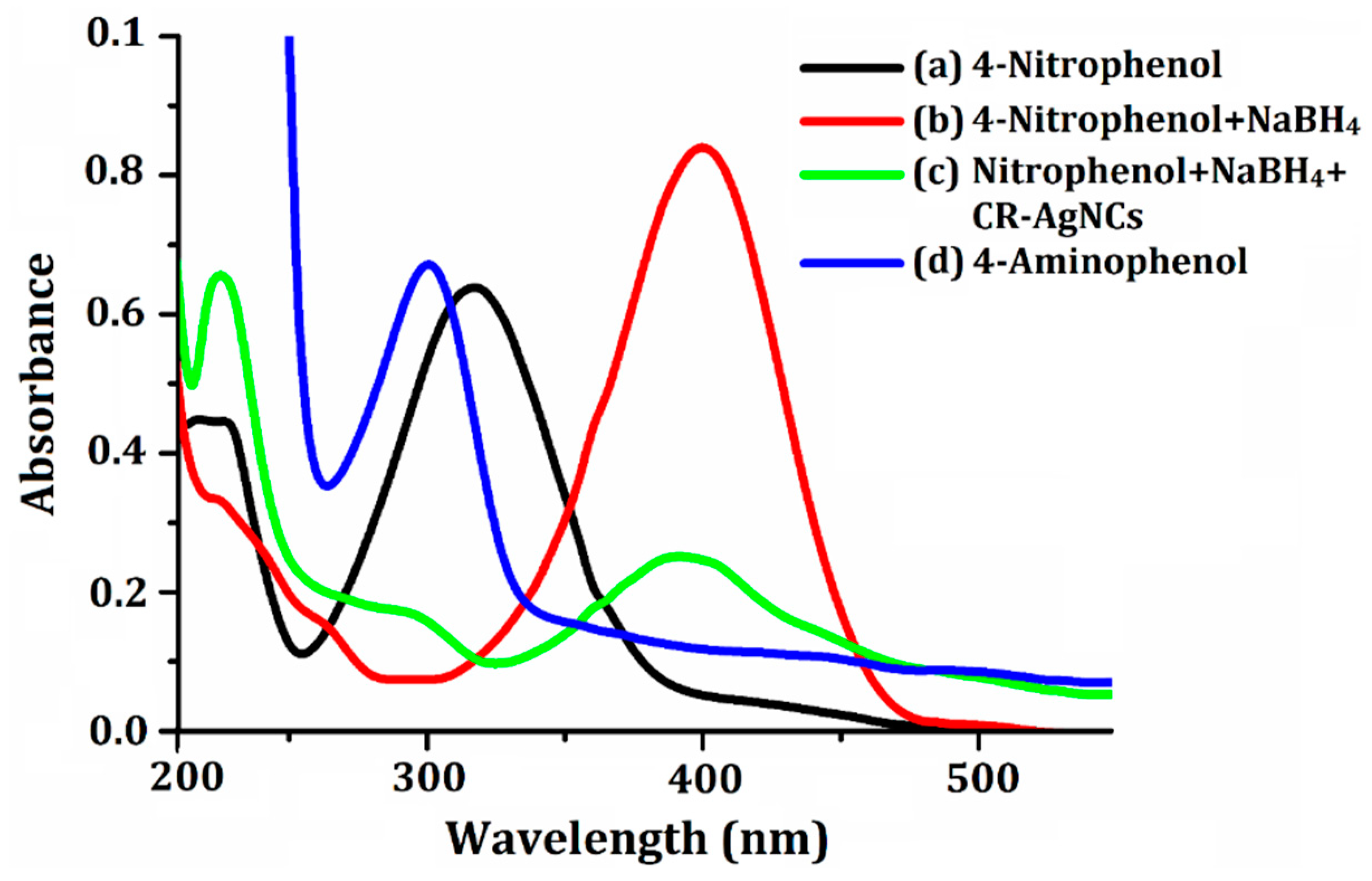
UV-Vis Study of 4-Nitrophenol Reduction
3.3. Mechanism Involved in the Preparation of CR-AgNCs and Their Catalytic Performance in DRS-FTIR Spectroscopic Study
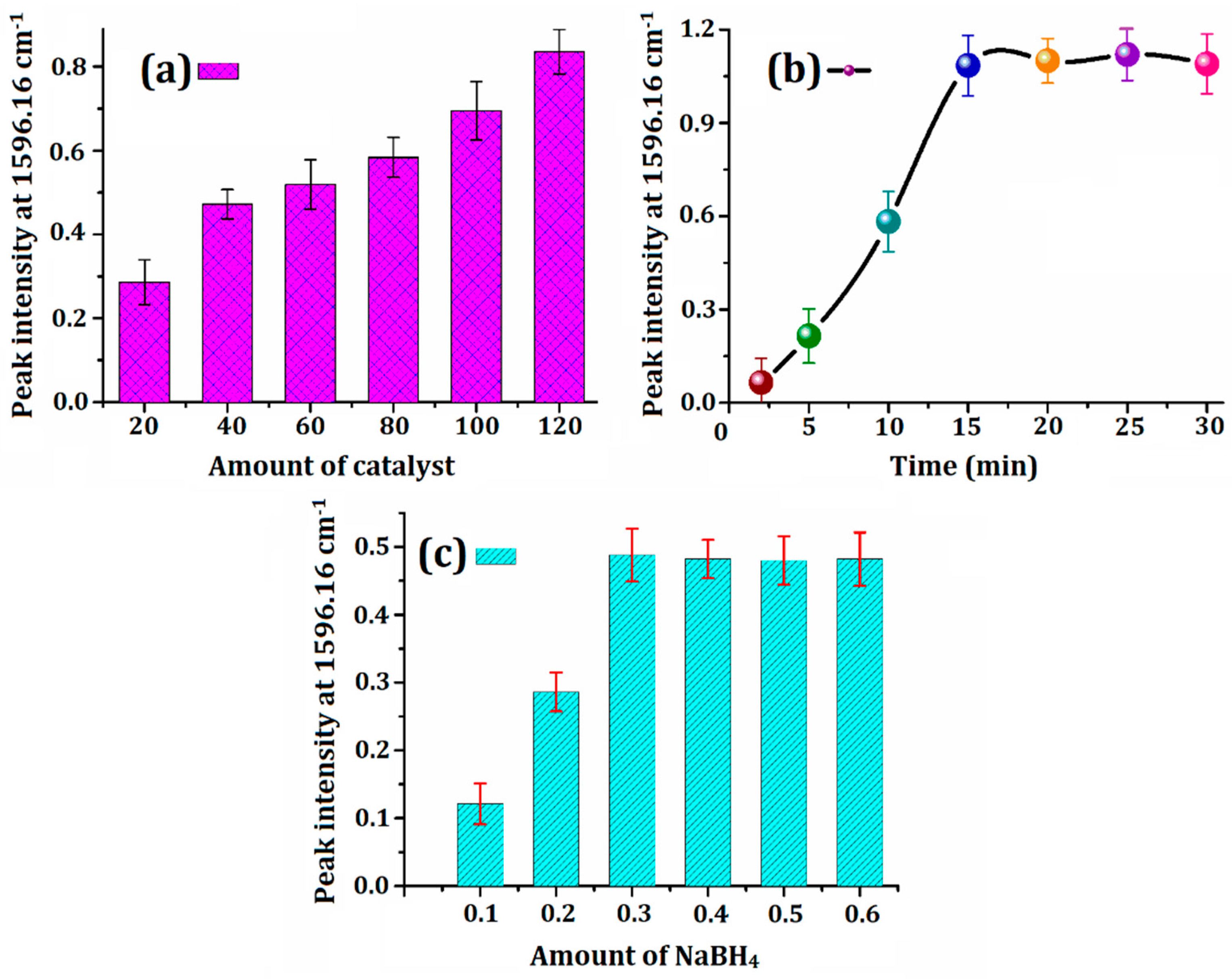
3.4. Factors Affecting the Catalysis Process
3.4.1. Effect of Catalyst Dosage
3.4.2. Effect of Time
3.4.3. Effect of Amount of NaBH4
3.5. Comparison of Present CR-AgNCs/FTIR Method with Other Reported Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Emmanuel, R.; Karuppiah, C.; Chen, S.-M.; Palanisamy, S.; Padmavathy, S.; Prakash, P. Green synthesis of gold nanoparticles for trace level detection of a hazardous pollutant (nitrobenzene) causing Methemoglobinaemia. J. Hazard. Mater. 2014, 279, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kuang, D.; Feng, Y.; Zhang, F.; Xu, Z.; Liu, M. A graphene oxide-based electrochemical sensor for sensitive determination of 4-nitrophenol. J. Hazard. Mater. 2012, 201–202, 250–259. [Google Scholar] [CrossRef]
- Guo, P.; Tang, L.; Tang, J.; Zeng, G.; Huang, B.; Dong, H.; Zhang, Y.; Zhou, Y.; Deng, Y.; Ma, L.; et al. Catalytic reduction–adsorption for removal of p-nitrophenol and its conversion p-aminophenol from water by gold nanoparticles supported on oxidized mesoporous carbon. J. Colloid Interface Sci. 2016, 469, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Chiou, J.-R.; Lai, B.-H.; Hsu, K.-C.; Chen, D.-H. One-pot green synthesis of silver/iron oxide composite nanoparticles for 4-nitrophenol reduction. J. Hazard. Mater. 2013, 248–249, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Chen, D.-H. Catalytic reduction of 4-nitrophenol by magnetically recoverable Au nanocatalyst. J. Hazard. Mater. 2009, 165, 664–669. [Google Scholar] [CrossRef]
- Sun, J.; Fu, Y.; He, G.; Sun, X.; Wang, X. Catalytic hydrogenation of nitrophenols and nitrotoluenes over a palladi-um/graphene nanocomposite. Catal. Sci. Technol. 2014, 4, 1742–1748. [Google Scholar] [CrossRef]
- Wang, C.-B.; Zhang, W.-X. Synthesizing Nanoscale Iron Particles for Rapid and Complete Dechlorination of TCE and PCBs. Environ. Sci. Technol. 1997, 31, 2154–2156. [Google Scholar] [CrossRef]
- Jana, S.; Ghosh, S.K.; Nath, S.; Pande, S.; Praharaj, S.; Panigrahi, S.; Basu, S.; Endo, T.; Pal, T. Synthesis of silver nanoshell-coated cationic polystyrene beads: A solid phase catalyst for the reduction of 4-nitrophenol. Appl. Catal. A Gen. 2006, 313, 41–48. [Google Scholar] [CrossRef]
- Dong, A.G.; Wang, Y.J.; Tang, Y.; Ren, N.; Yang, W.L.; Gao, Z. Fabrication of compact silver nanoshells on polystyrene spheres through electrostatic attraction. Chem. Commun. 2002, 4, 350–351. [Google Scholar] [CrossRef]
- Haberecht, J.; Krumeich, F.; Stalder, M.; Nesper, R. Carbon nanostructures on high-temperature ceramics—A novel composite material and its functionalization. Catal. Today 2005, 102–103, 40–44. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, L.; Duan, Y.; An, J.; Luo, Q.; Zhang, Y.; Tang, Y.; Huang, J.; Zhang, B.; Liu, J.; et al. A novel strategy to construct supported silver nanocomposite as an ultra-high efficient catalyst. Appl. Catal. B Environ. 2021, 283, 119592. [Google Scholar] [CrossRef]
- Manjare, S.B.; Chaudhari, R.A.; Thopate, S.R.; Risbud, K.P.; Badade, S.M. Resin loaded palladium nanoparticle catalyst, characterization and application in–C–C–coupling reaction. SN Appl. Sci. 2020, 2, 988. [Google Scholar] [CrossRef]
- Yee, M.S.-L.; Khiew, P.S.; Tan, Y.F.; Kok, Y.-Y.; Cheong, K.W.; Chiu, W.S.; Leong, C.-O. Potent antifouling silver-polymer nanocomposite microspheres using ion-exchange resin as templating matrix. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 382–391. [Google Scholar] [CrossRef]
- Barbaro, P.; Liguori, F. Ion Exchange Resins: Catalyst Recovery and Recycle. Chem. Rev. 2009, 109, 515–529. [Google Scholar] [CrossRef]
- Meier, J.; Friedrich, K.A.; Stimming, U. Novel method for the investigation of single nanoparticle reactivity. Faraday Discuss. 2002, 121, 365–372. [Google Scholar] [CrossRef]
- Van Hardeveld, R.; Hartog, F. The statistics of surface atoms and surface sites on metal crystals. Surf. Sci. 1969, 15, 189–230. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Sharma, M. Cationic ion exchange resins as catalyst. React. Polym. 1993, 20, 1–45. [Google Scholar] [CrossRef]
- Jiang, Z.; Lv, L.; Zhang, W.; Du, Q.; Pan, B.; Yang, L.; Zhang, Q. Nitrate reduction using nanosized zero-valent iron supported by polystyrene resins: Role of surface functional groups. Water Res. 2011, 45, 2191–2198. [Google Scholar] [CrossRef]
- Pande, S.; Saha, A.; Jana, S.; Sarkar, S.; Basu, M.; Pradhan, M.; Sinha, A.K.; Saha, S.; Pal, A.; Pal, T. Alcohol Oxidation with Resin-Immoblized Cu Nanocomposites. Synfacts 2009, 2009, 0229. [Google Scholar] [CrossRef]
- Pande, S.; Saha, A.; Jana, S.; Sarkar, S.; Basu, M.; Pradhan, M.; Sinha, A.K.; Saha, S.; Pal, A.; Pal, T. Resin-Immobilized CuO and Cu Nanocomposites for Alcohol Oxidation. Org. Lett. 2008, 10, 5179–5181. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, S.; Basu, S.; Praharaj, S.; Pande, S.; Jana, S.; Pal, A.; Ghosh, S.K.; Pal, T. Synthesis and Size-Selective Catalysis by Supported Gold Nanoparticles: Study on Heterogeneous and Homogeneous Catalytic Process. J. Phys. Chem. C 2007, 111, 4596–4605. [Google Scholar] [CrossRef]
- Praharaj, S.; Nath, S.; Ghosh, S.K.; Kundu, A.S.; Pal, T. Immobilization and Recovery of Au Nanoparticles from Anion Exchange Resin: Resin-Bound Nanoparticle Matrix as a Catalyst for the Reduction of 4-Nitrophenol. Langmuir 2004, 20, 9889–9892. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Kurrey, R.; Deb, M.K.; Verma, S.K. Resin immobilized gold nanocomposites assisted surface enhanced infrared absorption (SEIRA) spectroscopy for improved surface assimilation of methylene blue from aqueous solution. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 262, 120144. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Deb, M.K. Direct and rapid determination of sulphate in environmental samples with diffuse reflectance Fourier transform infrared spectroscopy using KBr substrate. Talanta 2007, 71, 1546–1552. [Google Scholar] [CrossRef] [PubMed]
- Kurrey, R.; Mahilang, M.; Deb, M.K.; Nirmalkar, J.; Shrivas, K.; Pervez, S.; Rai, M.K.; Rai, J. A direct DRS-FTIR probe for rapid detection and quantification of fluoroquinolone antibiotics in poultry egg-yolk. Food Chem. 2019, 270, 459–466. [Google Scholar] [CrossRef]
- Shrivas, K.; Kant, T.M.; Karbhal, I.; Kurrey, R.; Sahu, B.; Sinha, D.; Patra, G.K.; Deb, M.K.; Pervez, S. Smartphone coupled with paper-based chemical sensor for on-site determination of iron(III) in environmental and biological samples. Anal. Bioanal. Chem. 2020, 412, 1573–1583. [Google Scholar] [CrossRef]
- Khalkho, B.R.; Kurrey, R.; Deb, M.K.; Karbhal, I.; Sahu, B.; Sinha, S.; Sahu, Y.K.; Jain, V.K. A simple and convenient dry-state SEIRS method for glutathione detection based on citrate functionalized silver nanoparticles in human biological fluids. New J. Chem. 2021, 45, 1339–1354. [Google Scholar] [CrossRef]
- Frens, G. Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Jana, S.; Pande, S.; Panigrahi, S.; Praharaj, S.; Basu, S.; Pal, A.; Pal, T. Exploitation of electrostatic field force for immobilization and catalytic reduction of o-nitrobenzoic acid to anthranilic acid on resin-bound silver nanocomposites. Langmuir 2006, 22, 7091–7095. [Google Scholar] [CrossRef]
- Mulfinger, L.; Solomon, S.D.; Bahadory, M.; Jeyarajasingam, A.V.; Rutkowsky, S.A.; Boritz, C. Synthesis and Study of Silver Nanoparticles. J. Chem. Educ. 2007, 84, 322. [Google Scholar] [CrossRef]
- Stiufiuc, R.; Iacovita, C.; Lucaciu, C.M.; Stiufiuc, G.; Dutu, A.G.; Braescu, C.; Leopold, N. SERS-active silver colloids prepared by reduction of silver nitrate with short-chain polyethylene glycol. Nanoscale Res. Lett. 2013, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Sharma, M.; Kumar, A.; Basu, S. Gold Nanoparticles Grafted Mesoporous Silica: A Highly Efficient and Recyclable Heterogeneous Catalyst for Reduction of 4-Nitrophenol. Nano 2016, 11, 1650104. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, R.; Han, L.; Tu, B.; Zhao, D. One-pot synthesis of thermally stable gold@mesoporous silica core-shell nanospheres with catalytic activity. Nano Res. 2013, 6, 871–879. [Google Scholar] [CrossRef]
- Ayodhya, D.; Veerabhadram, G. Microwave-assisted fabrication of g-C3N4 nanosheets sustained Bi2S3 heterojunction composites for the catalytic reduction of 4-nitrophenol. Environ. Technol. 2021, 42, 826–841. [Google Scholar] [CrossRef]
- Kurrey, R.; Deb, M.K.; Shrivas, K. Methyl Orange Paired Microextraction and Diffuse Reflectance-Fourier Transform Infrared Spectral Monitoring for Improved Signal Strength of Total Mixed Cationic Surfactants. J. Surfactants Deterg. 2018, 21, 197–208. [Google Scholar] [CrossRef]
- Kurrey, R.; Deb, M.K.; Shrivas, K.; Khalkho, B.R.; Nirmalkar, J.; Sinha, D.; Jha, S. Citrate-capped gold nanoparticles as a sensing probe for determination of cetyltrimethylammonium surfactant using FTIR spectroscopy and colorimetry. Anal. Bioanal. Chem. 2019, 411, 6943–6957. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Deb, M.K. Nondestructive and rapid determination of nitrate in soil, dry deposits and aerosol samples using KBr-matrix with diffuse reflectance Fourier transform infrared spectroscopy (DRIFTS). Anal. Chim. Acta 2007, 582, 382–389. [Google Scholar] [CrossRef]
- Eid, S.M.; Kelani, K.M.; Badran, O.M.; Rezk, M.R.; Elghobashy, M.R. Surface enhanced infrared absorption spectroscopy (SEIRA) as a green analytical chemistry approach: Coating of recycled aluminum TLC sheets with citrate capped silver na-noparticles for chemometric quantitative analysis of ternary mixtures as a green alternative to the traditional methods. Anal. Chim. Acta 2020, 1117, 60–73. [Google Scholar]
- Arora, N.; Mehta, A.; Mishra, A.; Basu, S. 4-Nitrophenol reduction catalysed by Au-Ag bimetallic nanoparticles supported on LDH: Homogeneous vs. heterogeneous catalysis. Appl. Clay Sci. 2018, 151, 1–9. [Google Scholar] [CrossRef]
- Zhang, P.; Shao, C.; Zhang, Z.; Zhang, M.; Mu, J.; Guo, Z.; Liu, Y. In situ assembly of well-dispersed Ag nanoparticles (AgNPs) on electrospun carbon nanofibers (CNFs) for catalytic reduction of 4-nitrophenol. Nanoscale 2011, 3, 3357–3363. [Google Scholar] [CrossRef]
- Saha, A.; Deb, M.K.; Mahilang, M.; Kurrey, R.; Sinha, S. Polymeric resins as nano-catalysts: A brief review. J. Indian Chem. Soc. 2020, 97, 1–13. [Google Scholar]
- Saha, A.; Deb, M.K.; Mahilang, M.; Sinha, S. Intriguing Clinical and Pharmaceutical Applications of IERs: A Mini Review. J. Ravishankar Univ. 2020, 33, 47–57. [Google Scholar] [CrossRef]
- Hubicki, Z.; Kołodyńska, D. Selective removal of heavy metal ions from waters and waste waters using ion exchange methods. Ion Exch. Technol. 2012, 7, 193–240. [Google Scholar]
- Alonso, A.; Macanás, J.; Shafir, A.; Muñoz, M.; Vallribera, A.; Prodius, D.; Melnic, S.; Turta, C.; Muraviev, D.N. Don-nan-exclusion-driven distribution of catalytic ferromagnetic nanoparticles synthesized in polymeric fibers. Dalton Trans. 2010, 39, 2579–2586. [Google Scholar] [CrossRef] [PubMed]






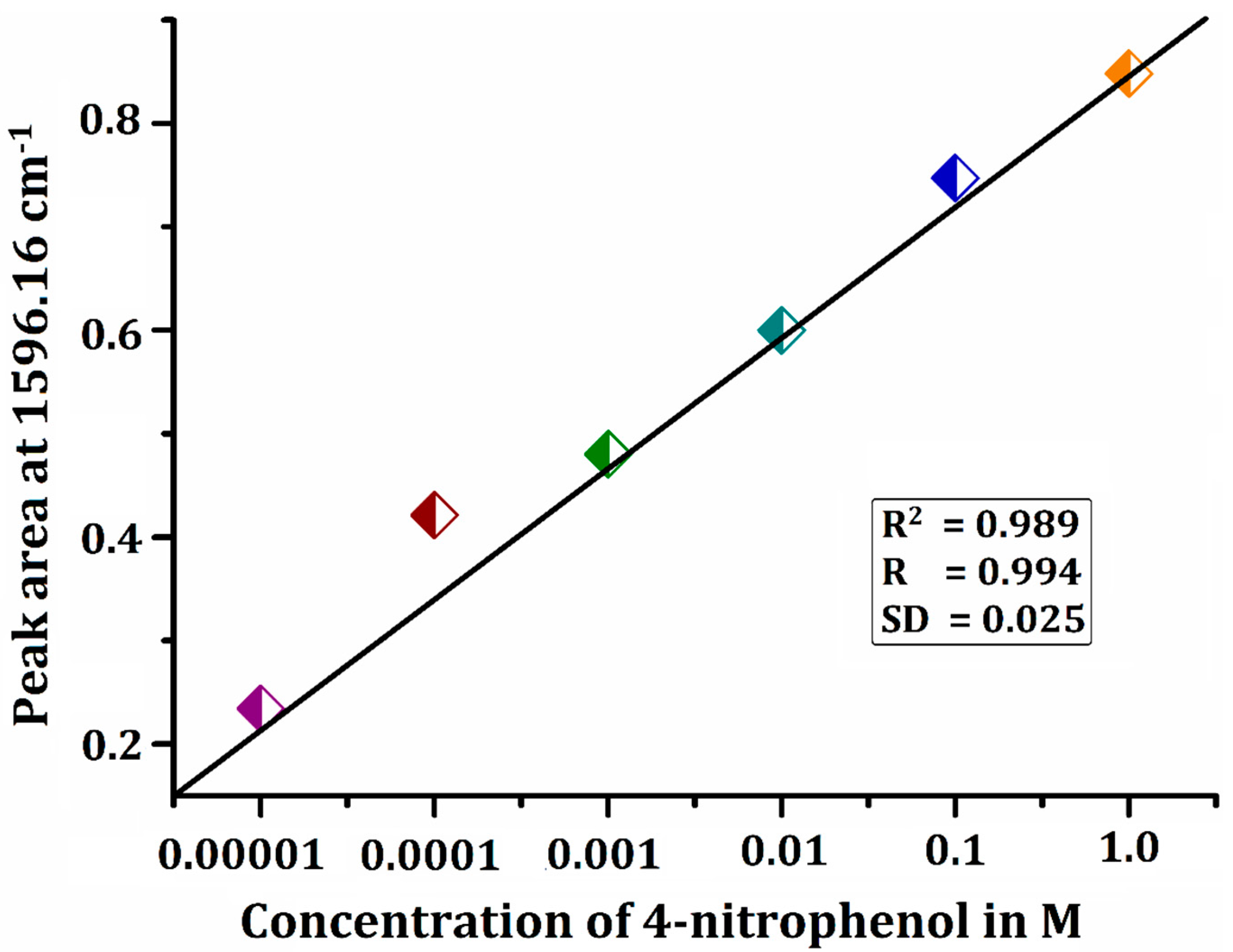
| S. No. | Statistical Parameters | Values Obtained |
|---|---|---|
| 1. | Linear range (M) | 0.1 × 10−4 to 1.0 |
| 2. | LOD (M) | 0.6 |
| 3. | LOQ (M) | 2.1 |
| 4. | RSD (%) | 4.5 |
| 5. | Correlation coefficient (R2) | 0.989 |
| 6. | Catalytic efficiency (%) | 96.8 |
| 7. | Reduction reaction rate (min−1) | 8.0 × 10−3 |
| S. No. | Catalyst | Catalysis System | Concentration of 4-Nitrophenol (M) | Catalytic Dose (mg) | Catalyst Efficiency (%)/ Reaction Rate (min−1) | Reaction Time (min) | Reference |
|---|---|---|---|---|---|---|---|
| 1. | a AgNPs | UV-Vis | 0.1 × 10−3 | 3.527 × 10−5 g L−1 | 1.024 | 8.33 | [40] |
| 2. | b Au-Ag BNPs@LDH | UV-Vis | 0.1 × 10−3 | 20 µL/1 mg | c 3.75 × 10−2 and d 2.85 × 10−2 | 30 | [39] |
| 3. | e Au@MSNs | UV-Vis | 0.5 × 10−2 | ~2 | 0.18 | 25 | [33] |
| 4. | f Au/SiO2 | UV-Vis and HPLC | 0.5 × 10−2 | 5 | 98.0 | 20 | [32] |
| 5. | CR-AgNCs | DRS-FTIR | 0.1 × 10−3 | 40 | 96.8/8.0 × 10−3 | 10 | Present method |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saha, A.; Kurrey, R.; Verma, S.K.; Deb, M.K. Cationic Polystyrene Resin Bound Silver Nanocomposites Assisted Fourier Transform Infrared Spectroscopy for Enhanced Catalytic Reduction of 4-Nitrophenol in Aqueous Medium. Chemistry 2022, 4, 1757-1774. https://doi.org/10.3390/chemistry4040114
Saha A, Kurrey R, Verma SK, Deb MK. Cationic Polystyrene Resin Bound Silver Nanocomposites Assisted Fourier Transform Infrared Spectroscopy for Enhanced Catalytic Reduction of 4-Nitrophenol in Aqueous Medium. Chemistry. 2022; 4(4):1757-1774. https://doi.org/10.3390/chemistry4040114
Chicago/Turabian StyleSaha, Anushree, Ramsingh Kurrey, Santosh Kumar Verma, and Manas Kanti Deb. 2022. "Cationic Polystyrene Resin Bound Silver Nanocomposites Assisted Fourier Transform Infrared Spectroscopy for Enhanced Catalytic Reduction of 4-Nitrophenol in Aqueous Medium" Chemistry 4, no. 4: 1757-1774. https://doi.org/10.3390/chemistry4040114
APA StyleSaha, A., Kurrey, R., Verma, S. K., & Deb, M. K. (2022). Cationic Polystyrene Resin Bound Silver Nanocomposites Assisted Fourier Transform Infrared Spectroscopy for Enhanced Catalytic Reduction of 4-Nitrophenol in Aqueous Medium. Chemistry, 4(4), 1757-1774. https://doi.org/10.3390/chemistry4040114








