Imidazolium-Modified Silica Gel for Highly Selective Preconcentration of Ag(I) from the Nitric Acid Medium
Abstract
:1. Introduction
- -
- Covalent immobilization of a cation or an anion on the surface of silica gel;
- -
- The design of adsorption layers of ionic liquids on the surface of silica gel pores;
- -
- The hydrolytic polycondensation of silicon-containing precursors and in situ encapsulation of ionic liquids in the resulting porous framework of the matrix.
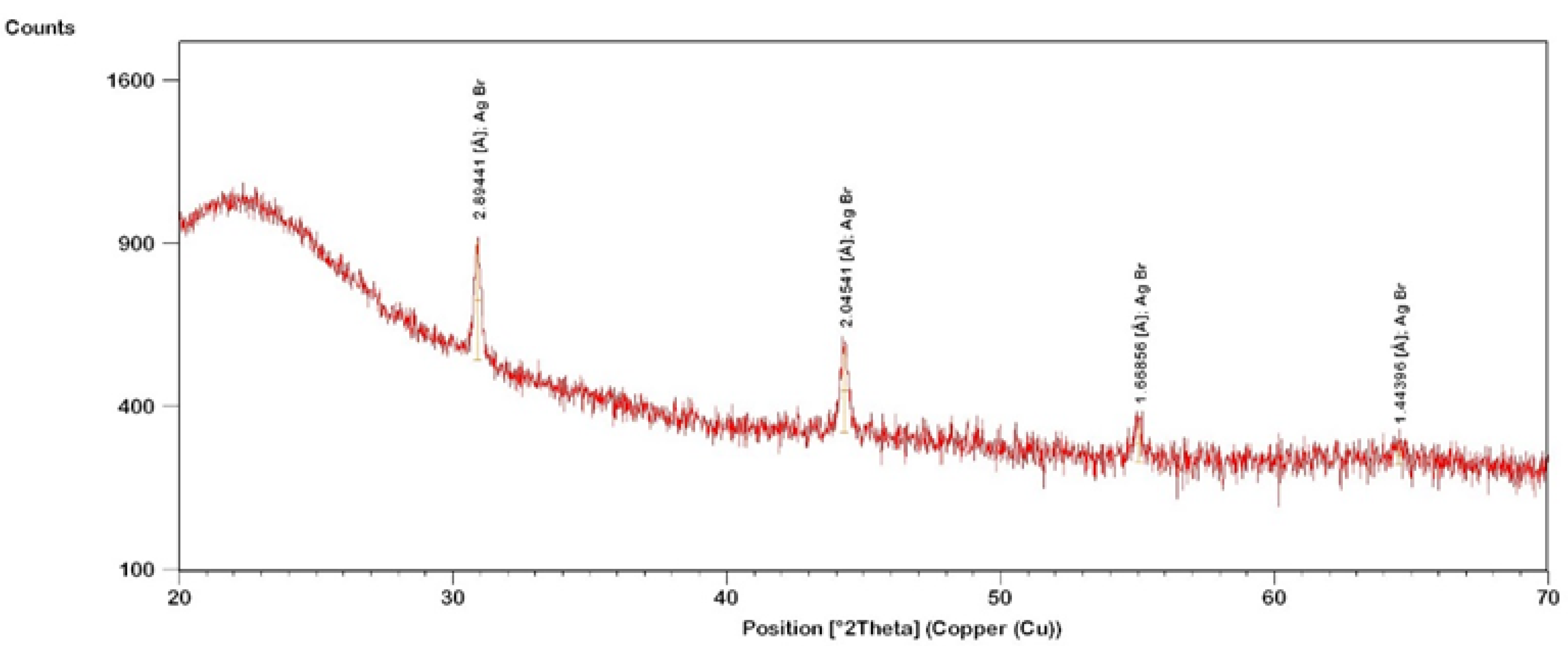
2. Materials and Methods
3. Results
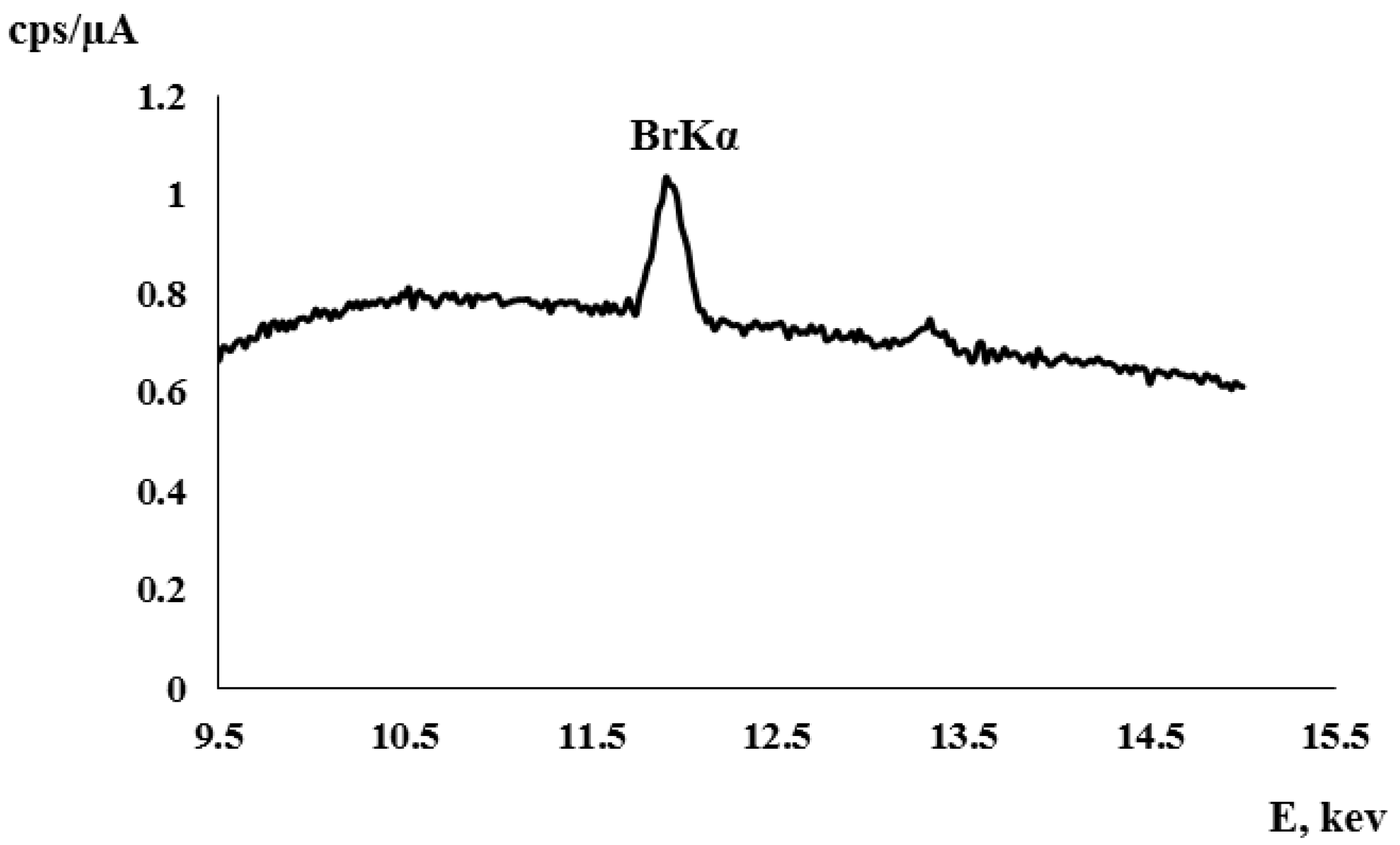
3.1. Distribution Coefficient
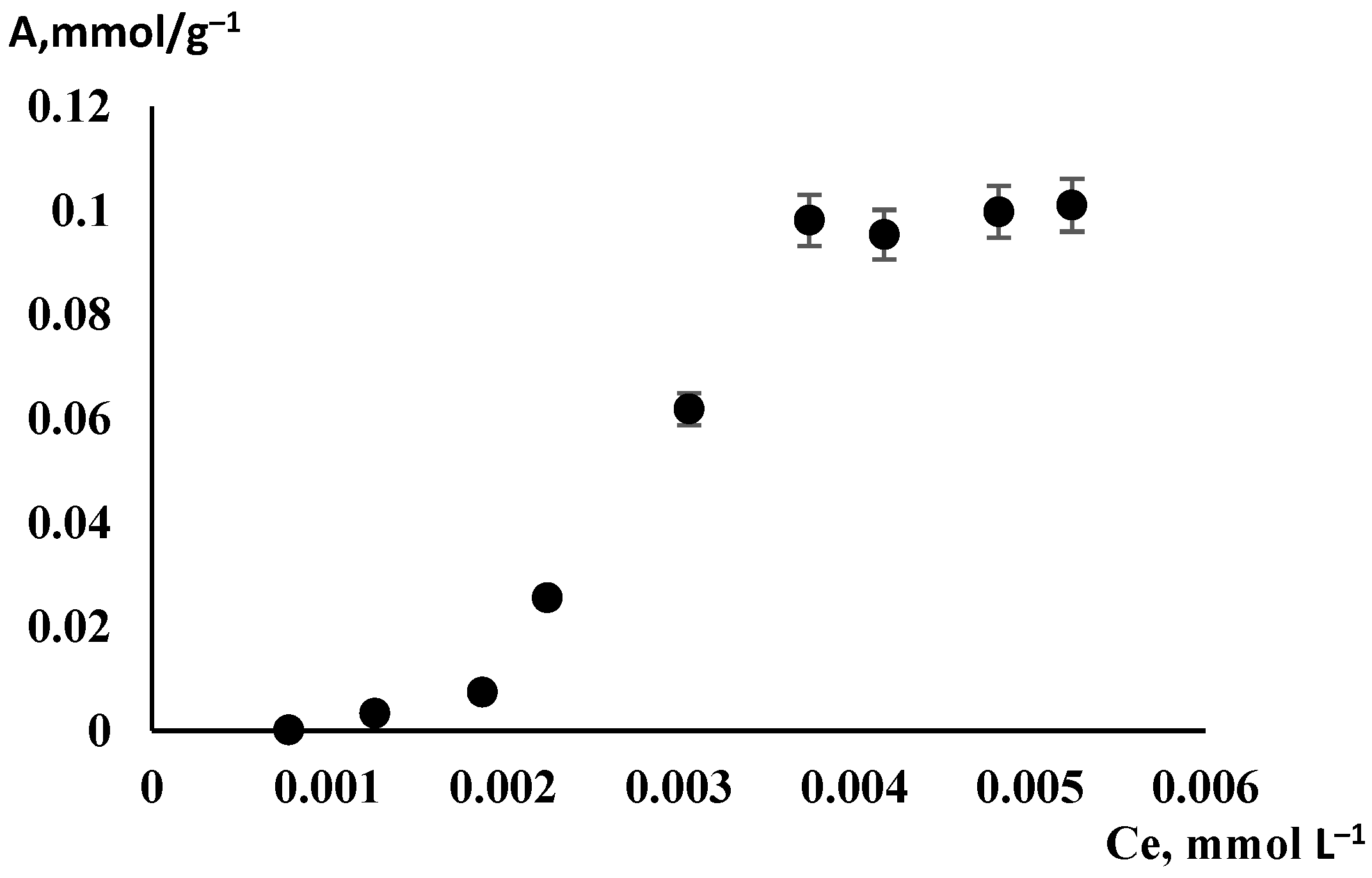
3.2. Adsorption Isotherm
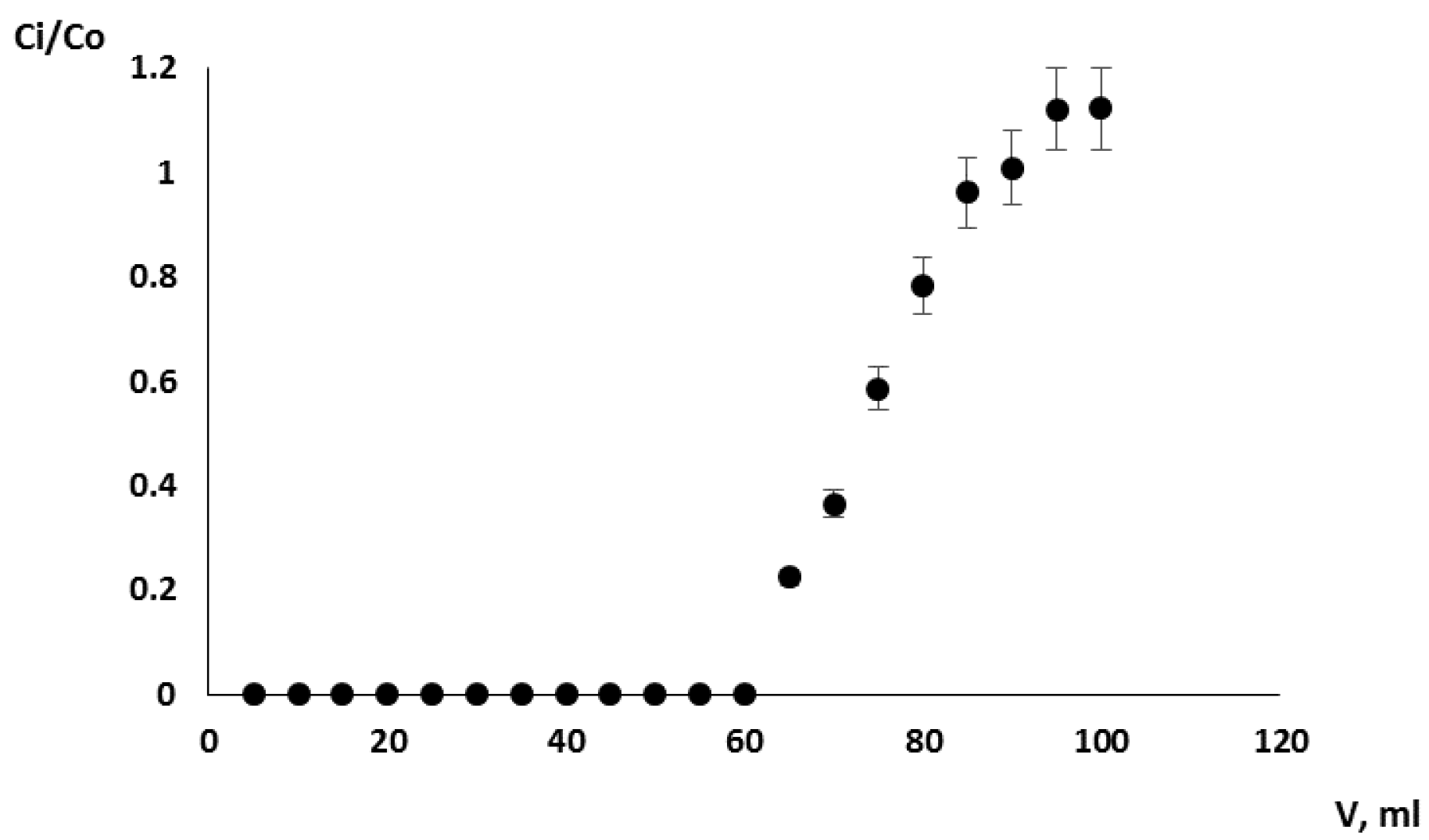
3.3. Selection of Dynamic Ion-Exchange Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huddleston, J.G.; Willauer, H.D.; Swatloski, R.P.; Visser, A.E.; Rogers, R.D. Room temperature ionic liquids as novel media for ‘clean’ liquid-liquid extraction. Chem. Commun. 1998, 16, 1765–1766. [Google Scholar] [CrossRef]
- Dai, S.; Ju, Y.H.; Barnes, C.E. Solvent extraction of strontium nitrate by a crown ether using room-temperature ionic liquids. J. Chem. Soc.-Dalton Trans. 1999, 8, 1201–1202. [Google Scholar] [CrossRef]
- Visser, A.E.; Swatloski, R.P.; Reichert, W.M.; Griffin, S.T.; Rogers, R.D. Traditional extractants in nontraditional solvents: Groups 1 and 2 extraction by crown ethers in room-temperature ionic liquids. Ind. Eng. Chem. Res. 2000, 39, 3596–3604. [Google Scholar] [CrossRef]
- Welton, T. Room-temperature ionic liquidssolvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2083. [Google Scholar] [CrossRef] [PubMed]
- Hallett, J.P.; Welton, T. Room-temperature ionic liquids: Solvents for synthesis and catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef]
- Zazybin, A.G.; Rafikova, K.; Yu, V.; Zolotareva, D.; Dembitsky, V.M.; Sasaki, T. Metal-containing ionic liquids: Current paradigm and applications. Russ. Chem. Rev. 2017, 86, 1254–1270. [Google Scholar] [CrossRef]
- Pletnev, I.V.; Smirnova, S.V.; Shvedene, N.V. New directions in using ionic liquids in analytical chemistry. 2: Electrochemical methods. J. Anal. Chem. 2019, 74, 1–10. [Google Scholar] [CrossRef]
- Pletnev, I.V.; Smirnova, S.V.; Shvedene, N.V. New directions in using ionic liquids in analytical chemistry. 1: Liquid–Liquid extraction. J. Anal. Chem. 2019, 74, 625–658. [Google Scholar] [CrossRef]
- Pletnev, I.V.; Smirnova, S.V.; Sharov, A.V.; Zolotov, Y.A. New generation extraction solvents: From ionic liquids and aqueous biphasic systems to deep eutectic solvents. Russ. Chem. Rev. 2021, 90, 1109–1141. [Google Scholar] [CrossRef]
- Smirnova, S.V.; Pletnev, I.V. New ionic liquids for extraction preconcentration. J. Anal. Chem. 2019, 74, 1–11. [Google Scholar] [CrossRef]
- Visser, A.E.; Swatloski, R.P.; Griffin, S.T.; Hartman, D.H.; Rogers, R.D. Liquid/liquid extraction of metal ions in room temperature ionic liquids. Sep. Sci. Technol. 2001, 36, 785–804. [Google Scholar] [CrossRef]
- Wei, G.; Yang, Z.; Chen, C. Room temperature ionic liquid as a novel medium for liquid/liquid extraction of metal ions. Anal. Chim. Acta 2003, 488, 183–192. [Google Scholar] [CrossRef]
- Domańska, U.; Rękawek, A. Extraction of metal ions from aqueous solutions using imidazolium based ionic liquids. J. Solution Chem. 2009, 38, 739–751. [Google Scholar] [CrossRef]
- Nakashima, K.; Kubota, F.; Maruyama, T.; Goto, M. Feasibility of ionic liquids as alternative separation media for industrial solvent extraction processes. Ind. Eng. Chem. Res. 2005, 44, 4368–4372. [Google Scholar] [CrossRef]
- Hirayama, N.; Deguchi, M.; Kawasumi, H.; Honjo, T. Use of 1-alkyl-3-methylimidazolium hexafluorophosphate room temperature ionic liquids as chelate extraction solvent with 4,4,4-trifluoro-1-(2-thienyl)-1,3-butanedione. Talanta 2005, 65, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Berton, P.; Martinis, E.M.; Martinez, L.D.; Wuilloud, R.G. Room temperature ionic liquid-based microextraction for vanadium species separation and determination in water samples by electrothermal atomic absorption spectrometry. Anal. Chim. Acta 2009, 640, 40–46. [Google Scholar] [CrossRef]
- Çağlar, Y.; Saka, E.T. Ionic liquid based dispersive liquid–liquid microextraction procedure for the spectrophotometric determination of copper using 3-dimethylamino rhodanine as a chelating agent in natural waters. Karbala Int. J. Mod. Sci. 2017, 3, 185–190. [Google Scholar] [CrossRef]
- Harjani, J.R.; Friščić, T.; MacGillivray, L.R.; Singer, R.D. Removal of metal ions from aqueous solutions using chelating task-specific ionic liquids. Dalton Trans. 2008, 34, 4595–4601. [Google Scholar] [CrossRef]
- Luo, H.; Dai, S.; Bonnesen, P.V.; Buchanan, A.C., III. Separation of fission products based on ionic liquids: Task-specific ionic liquids containing an aza-crown ether fragment. J. Alloys Compd. 2006, 418, 195–199. [Google Scholar] [CrossRef]
- Sang, H.P.; Dorjnamjin, D.; Seung, H.J.; Myung, W.B. Ionic liquid-type crown ether as a novel medium for a liquid/liquid extraction of radioactive metal ion 85 Sr2+. Chem. Lett. 2006, 35, 1024–1025. [Google Scholar] [CrossRef]
- Germani, R.; Mancini, M.V.; Savelli, G.; Spreti, N. Mercury extraction by ionic liquids: Temperature and alkyl chain length effect. Tetrahedron Lett. 2007, 48, 1767–1769. [Google Scholar] [CrossRef]
- Papaiconomou, N.; Lee, J.-M.; Salminen, J.; Von Stosch, M.; Prausnitz, J.M. Selective extraction of copper, mercury, silver, and palladium ions from water using hydrophobic ionic liquids. Ind. Eng. Chem. Res. 2008, 47, 5080–5086. [Google Scholar] [CrossRef] [Green Version]
- Kozonoi, N.; Ikeda, Y. Extraction mechanism of metal ion from aqueous solution to the hydrophobic ionic liquid, 1-butyl-3-methylimidazolium nonafluorobutanesulfonate. Mon. Chem. 2007, 138, 1145–1151. [Google Scholar] [CrossRef]
- De Los Ríos, A.P.; Hernández-Fernández, F.J.; Sánchez-Segado, S.; Lozano, L.J.; Moreno, J.I.; Godínez, C. Selective separation of zn(II) over fe(III) from acidic media using ionic liquids as sole extraction agents. Chem. Eng. Trans. 2010, 21, 625–630. [Google Scholar] [CrossRef]
- Dietz, M.L.; Stepinski, D.C. A ternary mechanism for the facilitated transfer of metal ions into room-temperature ionic liquids (RTILs): Implications for the “greenness” of RTILs as extraction solvents. Green Chem. 2005, 7, 747–750. [Google Scholar] [CrossRef]
- Kim, E.; Jang, J.; Chung, J.S. Synthesis and characteristics of silica-supported N-heterocyclic carbene catalyst for ring-opening polymerization of D-lactide to produce polylactide. Macromol. Res. 2014, 22, 864–869. [Google Scholar] [CrossRef]
- Wang, L.; Shylesh, S.; Dehe, D.; Philippi, T.; Dörr, G.; Seifert, A.; Zhou, Z.; Hartmann, M.; Taylor, R.K.; Jia, M.; et al. Covalent immobilization of imidazolium cations inside a silica support: Palladium-catalyzed olefin hydrogenation. ChemCatChem 2012, 4, 395–400. [Google Scholar] [CrossRef]
- Zarezadeh-Mehrizi, M.; Badiei, A.; Mehrabadi, A.R. Ionic liquid functionalized nanoporous silica for removal of anionic dye. J. Mol. Liq. 2013, 180, 95–100. [Google Scholar] [CrossRef]
- Khaniani, Y.; Badiei, A.; Ziarani, G.M. Application of clickable nanoporous silica surface for immobilization of ionic liquids. J. Mater. Res. 2012, 27, 932–938. [Google Scholar] [CrossRef]
- Campisciano, V.; Giacalone, F.; Gruttadauria, M. Supported ionic liquids: A versatile and useful class of materials. Chem. Rec. 2017, 17, 918–938. [Google Scholar] [CrossRef]
- Giacalone, F.; Gruttadauria, M. Covalently supported ionic liquid phases: An advanced class of recyclable catalytic systems. ChemCatChem 2016, 8, 664–684. [Google Scholar] [CrossRef]
- Gharehbaghi, M.; Shemirani, F. Ionic liquid modified silica sorbent for simultaneous separation and preconcentration of heavy metals from water and tobacco samples prior to their determination by flame atomic absorption spectrometry. Anal. Methods 2012, 4, 2879–2886. [Google Scholar] [CrossRef]
- Nie, L.; Lu, J.; Zhang, W.; He, A.; Yao, S. Ionic liquid-modified silica gel as adsorbents for adsorption and separation of water-soluble phenolic acids from salvia militiorrhizabunge. Sep. Purif. Technol. 2015, 155, 2–12. [Google Scholar] [CrossRef]
- Song, H.; Yang, C.; Yohannes, A.; Yao, S. Acidic ionic liquid modified silica gel for adsorption and separation of bovine serum albumin (BSA). RSC Adv. 2016, 109, 107452–107462. [Google Scholar] [CrossRef]
- Qiu, H.; Mallik, A.K.; Takafuji, M.; Jiang, S.; Ihara, H. Ionic liquids in liquid chromatography. In Handbook of Ionic Liquids; Nova Science Publishers, Inc.: New York, NY, USA, 2012; pp. 325–329. [Google Scholar]
- Konshin, V.V.; Chuprynina, D.A.; Lupanova, I.A.; Konshina, D.N. Silica gel with covalently immobilized imidazolium salt as a new stationary phase for ion chromatography. AIP Conf. Proc. 2020, 2280, 050028. [Google Scholar] [CrossRef]
- Chuprynina, D.A.; Lupanova, I.A.; Konshin, V.V.; Konshina, D.N. Silica gel functionalized with imidazolium group via click chemistry—New stationary phase for ion chromatography. Chim. Techno Acta 2021, 8, 4. [Google Scholar] [CrossRef]
- Burylin, M.; Kopeyko, E.; Bokiy, E.; Konshin, V.; Konshina, D. Determination of indium in water samples using solid-phase extraction with modified silica gel and slurry sampling with electrothermal atomic absorption spectrometry. Chem. Pap. 2022, 76, 5893–5899. [Google Scholar] [CrossRef]
- Fehrmann, R.; Riisager, A.; Haumann, M. Supported Ionic Liquids: Fundamentals and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014; pp. 1–474. [Google Scholar] [CrossRef]
- Skwarek, E.; Matysek-Nawrocka, M.; Janusz, W.; Zarko, V.I.; Gun’ko, V.M. Adsorption of heavy metal ions at the Al2O3-SiO2/NaClO4 electrolyte interface. Physicochem. Probl. Miner. Process. 2008, 42, 153–164. [Google Scholar]
- Janusz, W.; Skwarek, E.; Sydorchuk, V.; Khalameida, S. Adsorption of Ag (I) ions at the zirconium Phosphate/KNO3 aqueous solution interface. Materials 2022, 15, 5050. [Google Scholar] [CrossRef]
- Samara, C.; Kouimtzis, T.A. Preconcentration of silver(I), gold(III) and palladium(II) in water samples with 2,2′-dipyridyl-3-[(4-aimno-5-mercapto)-1,2,4-triazolyl]hydrazone supported on silica gel. Fresenius’ Z. Anal. Chem. 1987, 327, 509–512. [Google Scholar] [CrossRef]
- Liu, P.; Pu, Q.; Su, Z. Synthesis of silica gel immobilized thiourea and its application to the on-line preconcentration and separation of silver, gold and palladium. Analyst 2000, 125, 147–150. [Google Scholar] [CrossRef]
- Madrakian, T.; Afkhami, A.; Zolfigol, M.A.; Solgi, M. Separation, preconcentration and determination of silver ion from water samples using silica gel modified with 2,4,6-trimorpholino-1,3,5-triazin. J. Hazard. Mater. 2006, 128, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Quang, D.V.; Lee, J.E.; Kim, J.; Kim, Y.N.; Shao, G.N.; Kim, H.T. A gentle method to graft thiol-functional groups onto silica gel for adsorption of silver ions and immobilization of silver nanoparticles. Powder Technol. 2013, 235, 221–227. [Google Scholar] [CrossRef]
- Absalan, G.; Mehrdjardi, M.A. Separation and preconcentration of silver ion using 2-mercaptobenzothiazole immobilized on surfactant-coated alumina. Sep. Purif. Technol. 2003, 33, 95–101. [Google Scholar] [CrossRef]
- Akhond, M.; Absalan, G.; Sheikhian, L.; Eskandari, M.M.; Sharghi, H. Di (n-propyl) thiuram disulfide bonded on silica gel as a new sorbent for separation, preconcentration, and measurement of silver ion from aqueous samples. Sep. Purif. Technol. 2006, 52, 53–59. [Google Scholar] [CrossRef]
- Yildirim, G.; Tokalioǧlu, S.; Şahan, H.; Patat, S. Preconcentration of ag and Pd ions using graphite oxide and 2,6-diaminopyridyne from water, anode slime and catalytic converter samples. RSC Adv. 2014, 4, 18108–18116. [Google Scholar] [CrossRef]
- Peng, Y.; Li, Z.; Zeng, Y.; Xie, X.; Wang, H.; Li, L.; Liu, X. Solid phase extractors derived by functionalising sub-micro silica gel with chelating agents and their pH-tunable adsorbing capability towards Pb(II) and Ag(I). Microchim. Acta 2010, 170, 17–26. [Google Scholar] [CrossRef]
- Nikolov, M.; Simenova, P.; Mitev, V. Spectrophotometric Determination of Silver with Brilliant Green and Its Application in Photographic Fixing Solutions. Ecol. Chem. Eng. A 2009, 16, 399–403. [Google Scholar]
- Konshina, D.N.; Lupanova, I.A.; Mazur, A.S.; Konshin, V.V. Ion-exchange extraction of palladium(II) from chloride solutions using a silica gel-immobilized imidazolium salt. Solvent Extr. Ion Exch. 2019, 37, 461–472. [Google Scholar] [CrossRef]
- Iamamoto, M.S.; Gushikem, Y. Adsorption of metal ions from aqueous and ethanol solutions by silica gel functionalized with pyridinium ions. J. Colloid Interface Sci. 1989, 129, 162–165. [Google Scholar] [CrossRef]
- Dasthaiah, K.; Robert Selvan, B.; Suneesh, A.S.; Venkatesan, K.A.; Antony, M.P.; Gardas, R.L. Ionic liquid modified silica gel for the sorption of americium(III) and europium(III) from dilute nitric acid medium. J. Radioanal. Nucl. Chem. 2017, 313, 515–521. [Google Scholar] [CrossRef]
- Lide, D.R.; Weast, R.C. CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical; Data Chemical Rubber Company CRC Press: Cleveland, OH, USA, 1984. [Google Scholar]

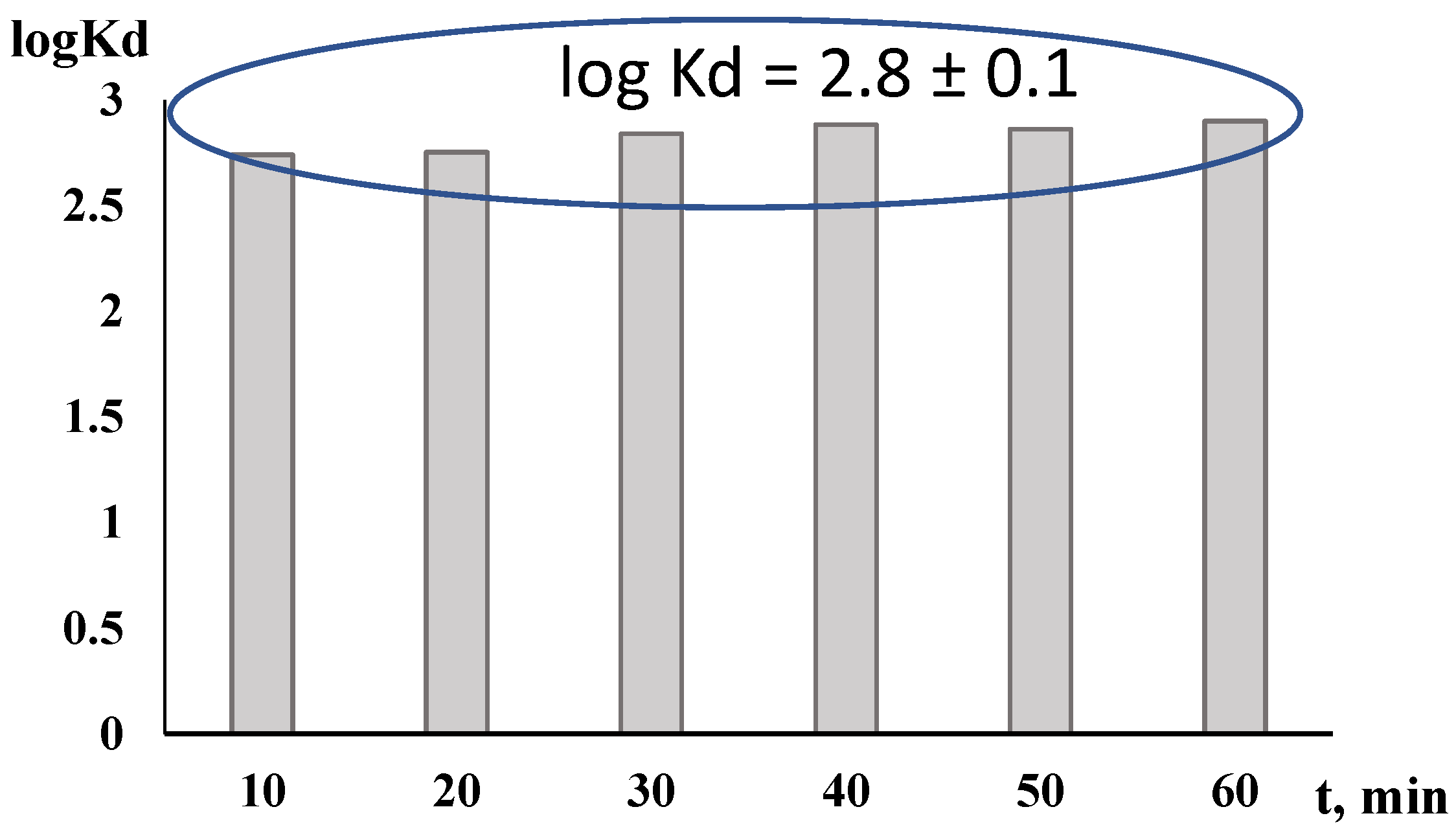
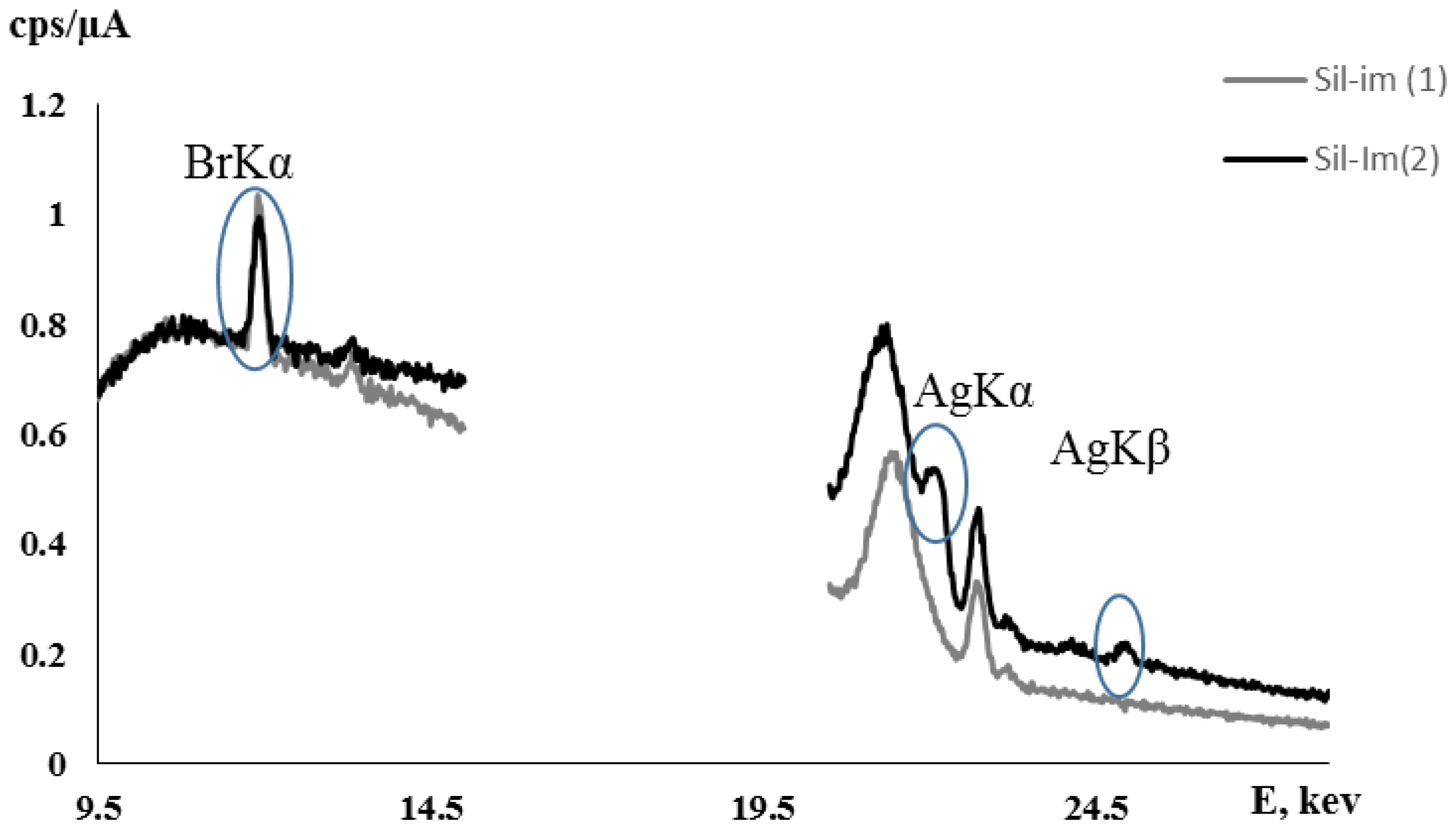
| Concentration of Nitric Acid (M) | log(Distribution Coefficient of Ag(I)), logKd |
|---|---|
| 0.01 | 2.7 |
| 0.1 | 2.8 |
| 0.5 | 1.8 |
| 1 | 1.7 |
| 3 | 1.5 |
| 5 | 1.3 |
| n(Ag(I)): n(Me) | |||||||
|---|---|---|---|---|---|---|---|
| 1:100 | 2855 ± 51 | 62 ± 3 | 58 ± 4 | 67 ± 3 | 49 ± 2 | 53 ± 3 | 67 ± 3 |
| 1:1000 | 3088 ± 43 | 57 ± 2 | 58 ± 3 | 64 ± 2 | 52 ± 2 | 58 ± 3 | 69 ± 3 |
| n(Ag(I)): n (Me) | |||||||
|---|---|---|---|---|---|---|---|
| 1:100 | 2015 ± 38 | 64 ± 2 | 52 ± 3 | 57 ± 3 | 51 ± 2 | 51 ± 3 | 46 ± 3 |
| 1:1000 | 1995 ± 35 | 57 ± 2 | 48 ± 2 | 52 ± 3 | 52 ± 1 | 48 ± 3 | 49 ± 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konshina, D.N.; Lupanova, I.A.; Konshin, V.V. Imidazolium-Modified Silica Gel for Highly Selective Preconcentration of Ag(I) from the Nitric Acid Medium. Chemistry 2022, 4, 1702-1713. https://doi.org/10.3390/chemistry4040111
Konshina DN, Lupanova IA, Konshin VV. Imidazolium-Modified Silica Gel for Highly Selective Preconcentration of Ag(I) from the Nitric Acid Medium. Chemistry. 2022; 4(4):1702-1713. https://doi.org/10.3390/chemistry4040111
Chicago/Turabian StyleKonshina, Dzhamilay N., Ida A. Lupanova, and Valery V. Konshin. 2022. "Imidazolium-Modified Silica Gel for Highly Selective Preconcentration of Ag(I) from the Nitric Acid Medium" Chemistry 4, no. 4: 1702-1713. https://doi.org/10.3390/chemistry4040111





