Towards a Real Knotaxane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Methods
2.2. General Synthetic Procedure
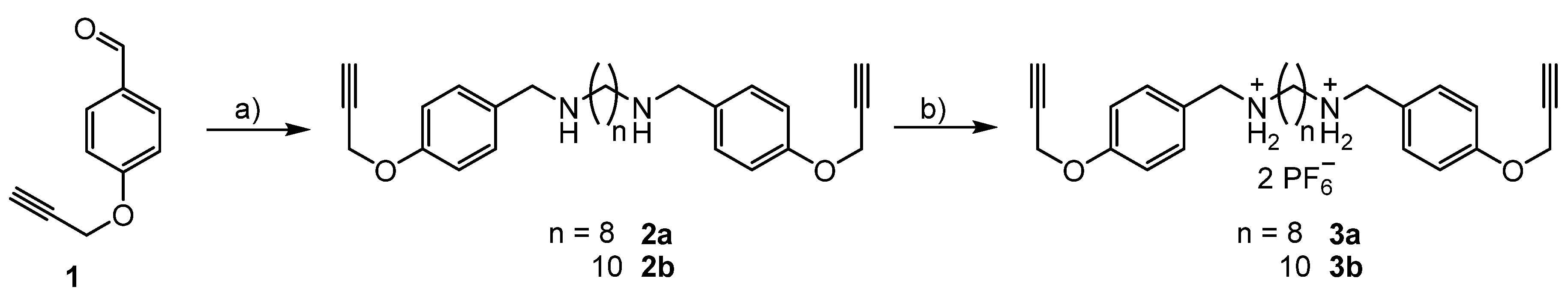
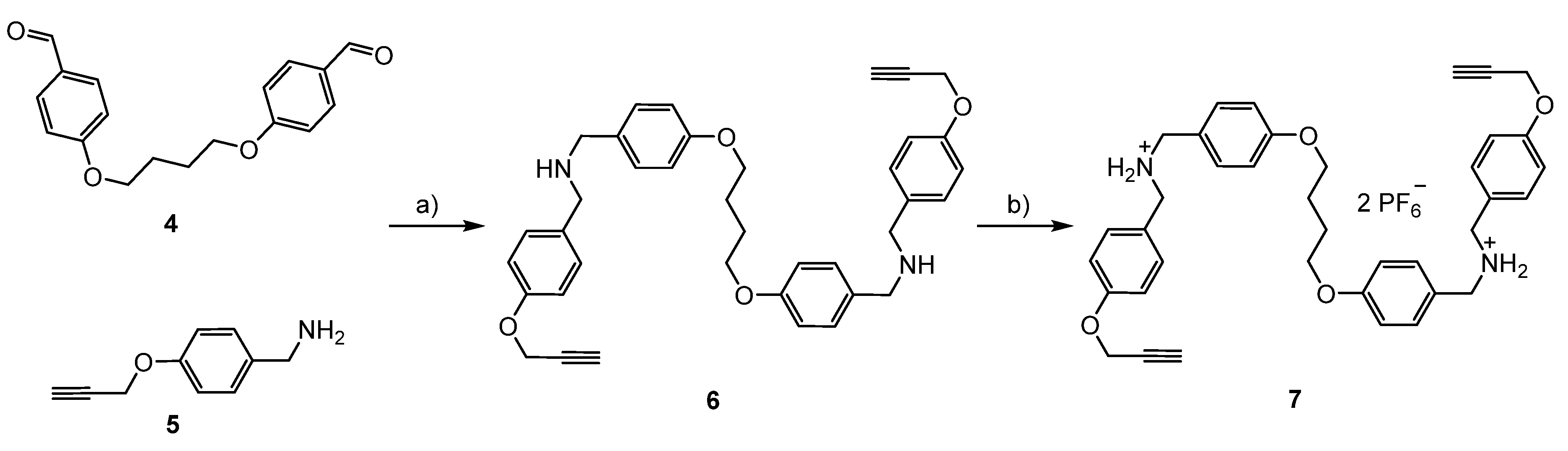
2.2.1. Reductive Amination of Aldehydes
2.2.2. Protonation and Ion Exchange
2.2.3. Click Reaction to Form [3]rotaxanes
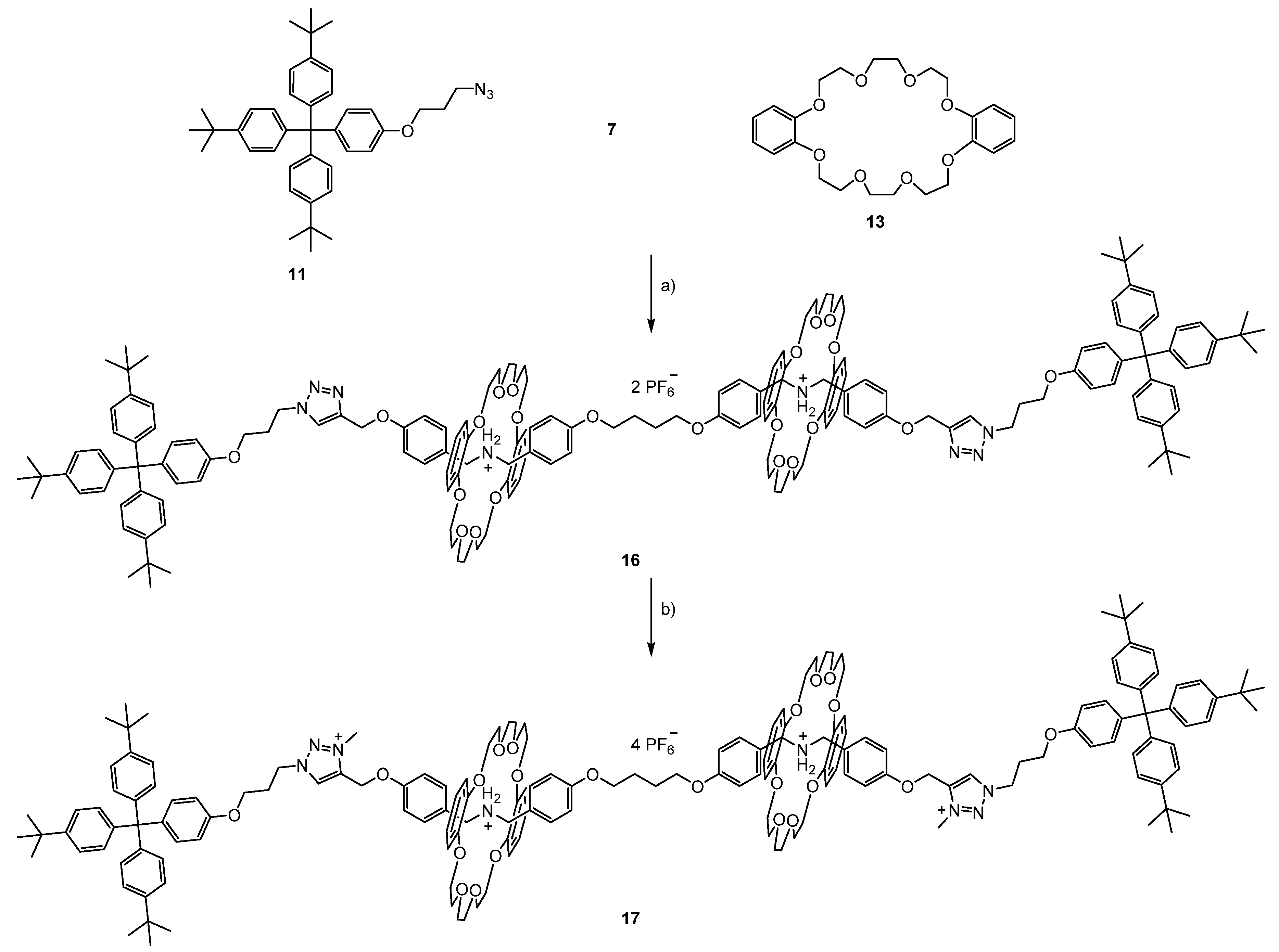
2.2.4. Methylation of the Triazole
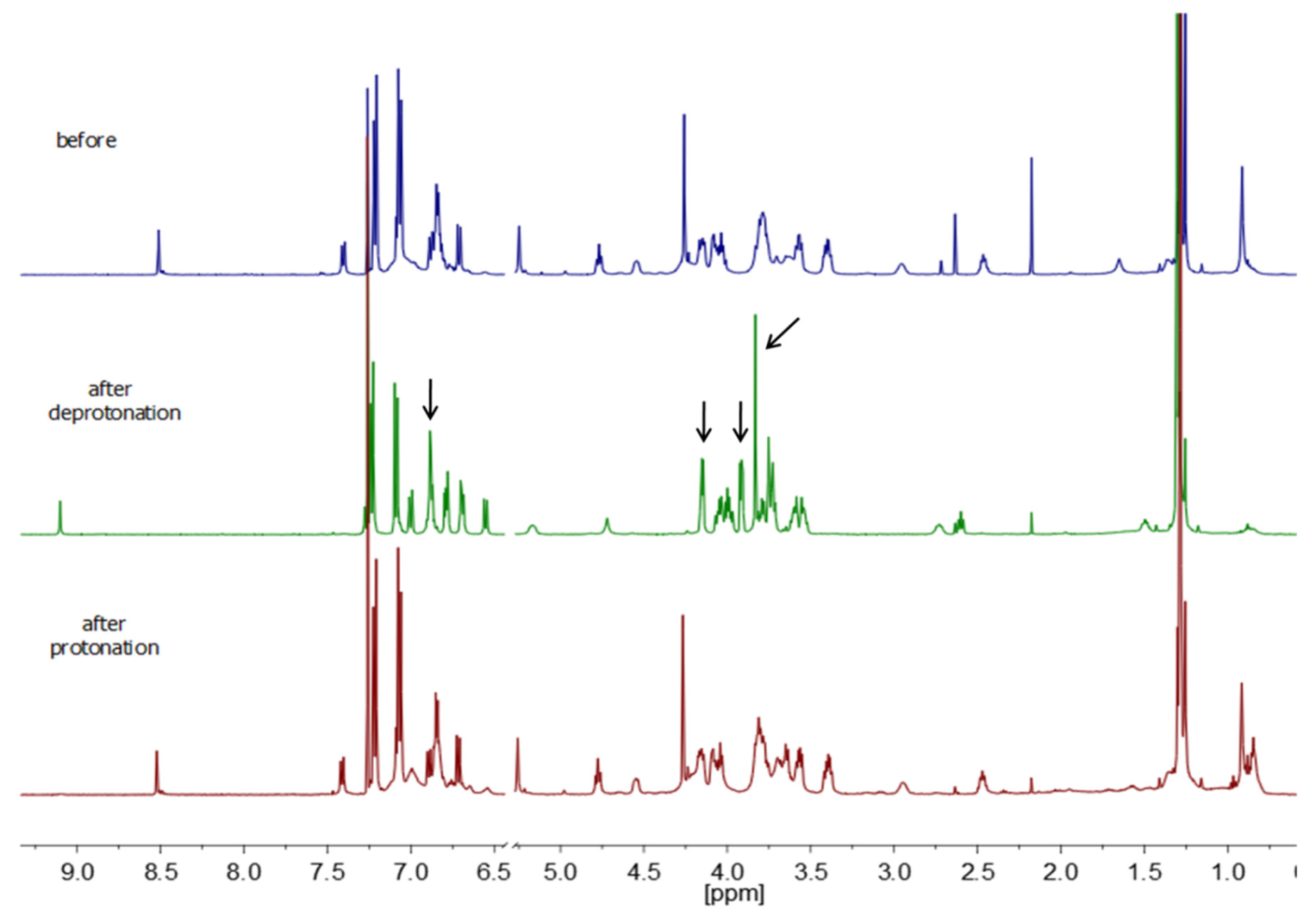
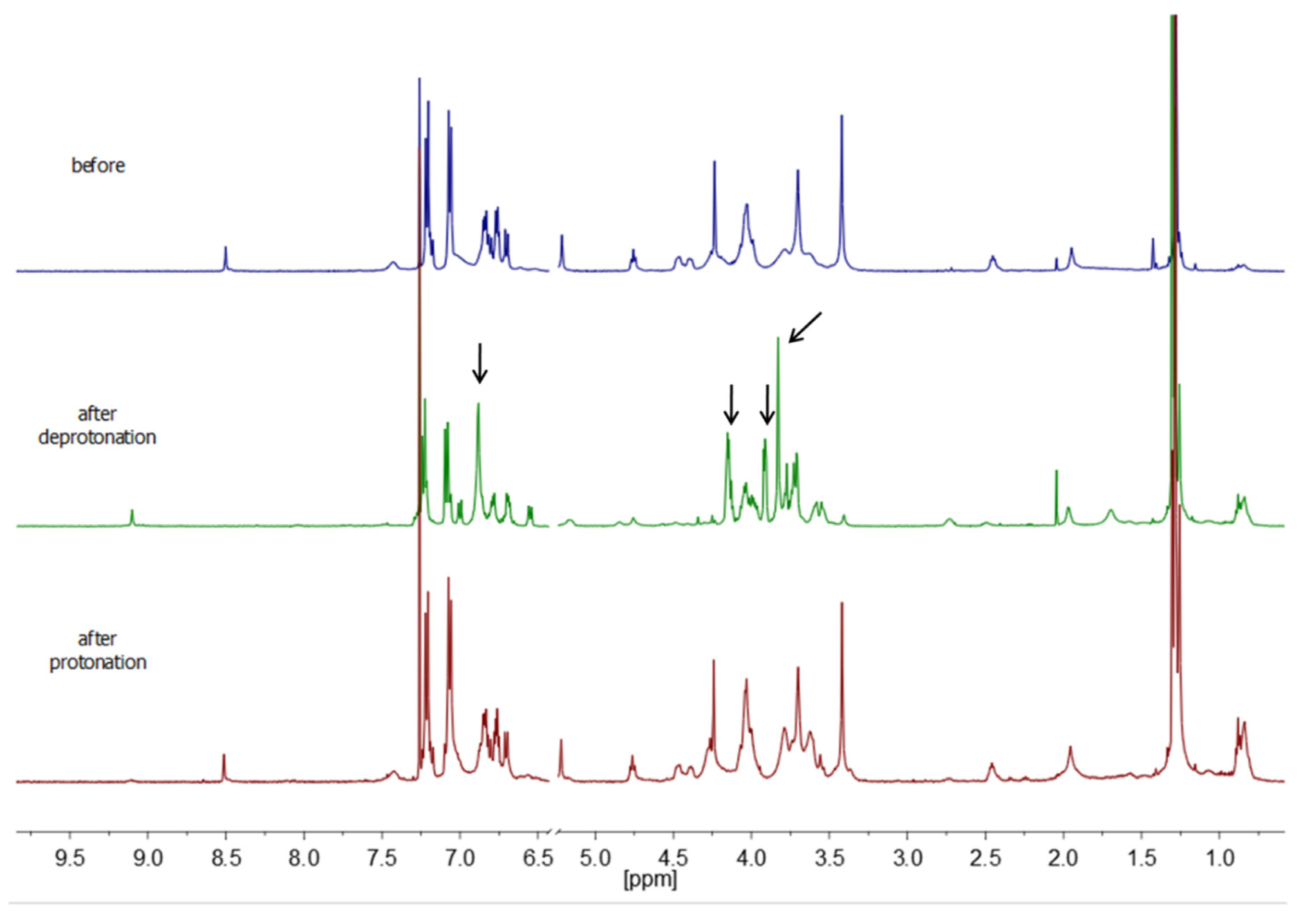
2.2.5. Switching of the Rotaxanes (Deprotonation and Protonation)
2.2.6. Etherification of 4-Benzyloxyphenol (25)
2.2.7. Reductive Deprotection of the Benzyl Protecting Group
2.2.8. Substitution of Bromide by Azide
3. Results and Discussion
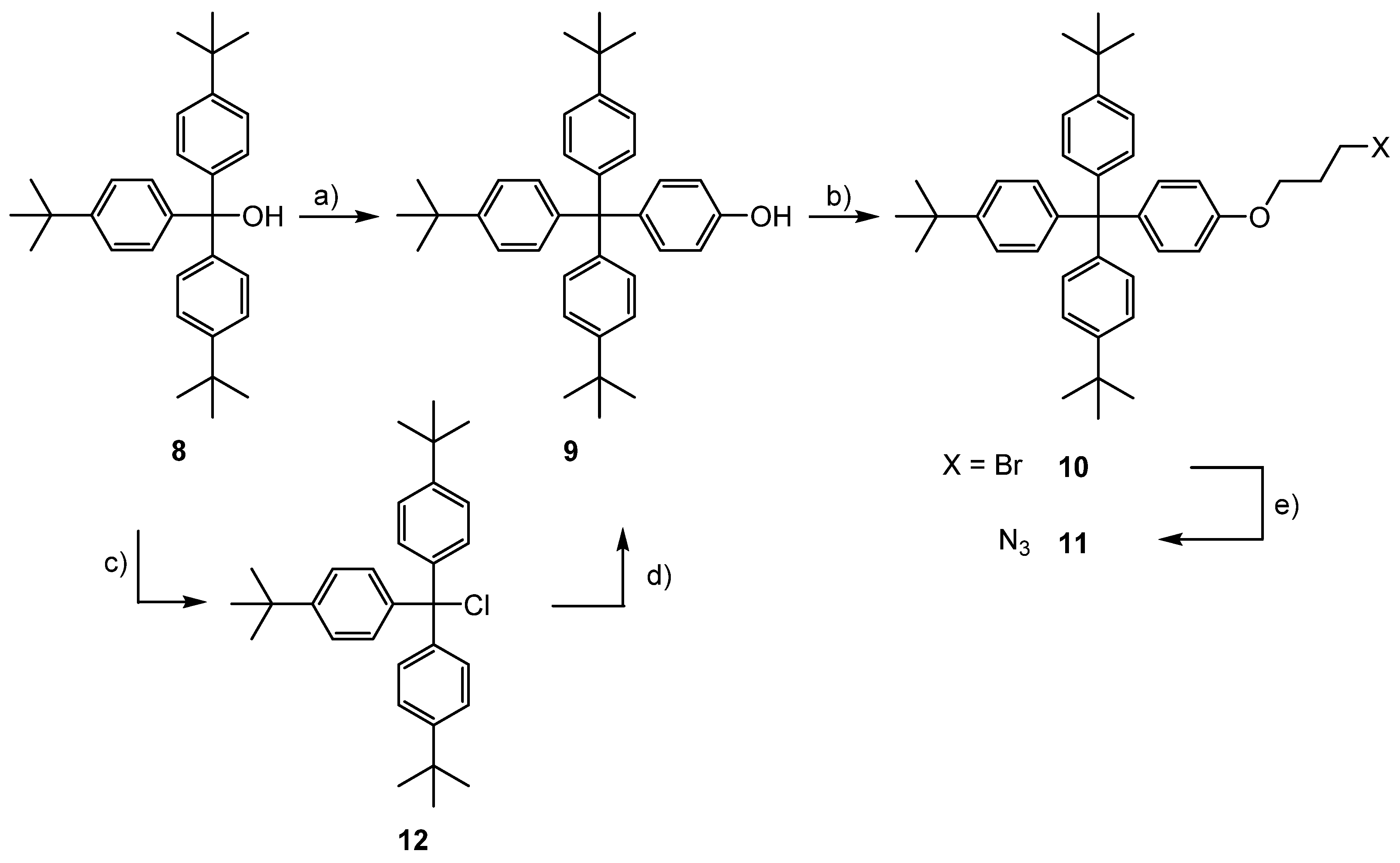
3.1. Syntheses of the Axles
3.2. Syntheses of the [3]rotaxanes
3.3. Switching of the [3]rotaxanes
3.3.1. Switching of [3]rotaxane 15a
3.3.2. Switching of [3]rotaxane 15b
3.3.3. Switching of [3]rotaxane 17
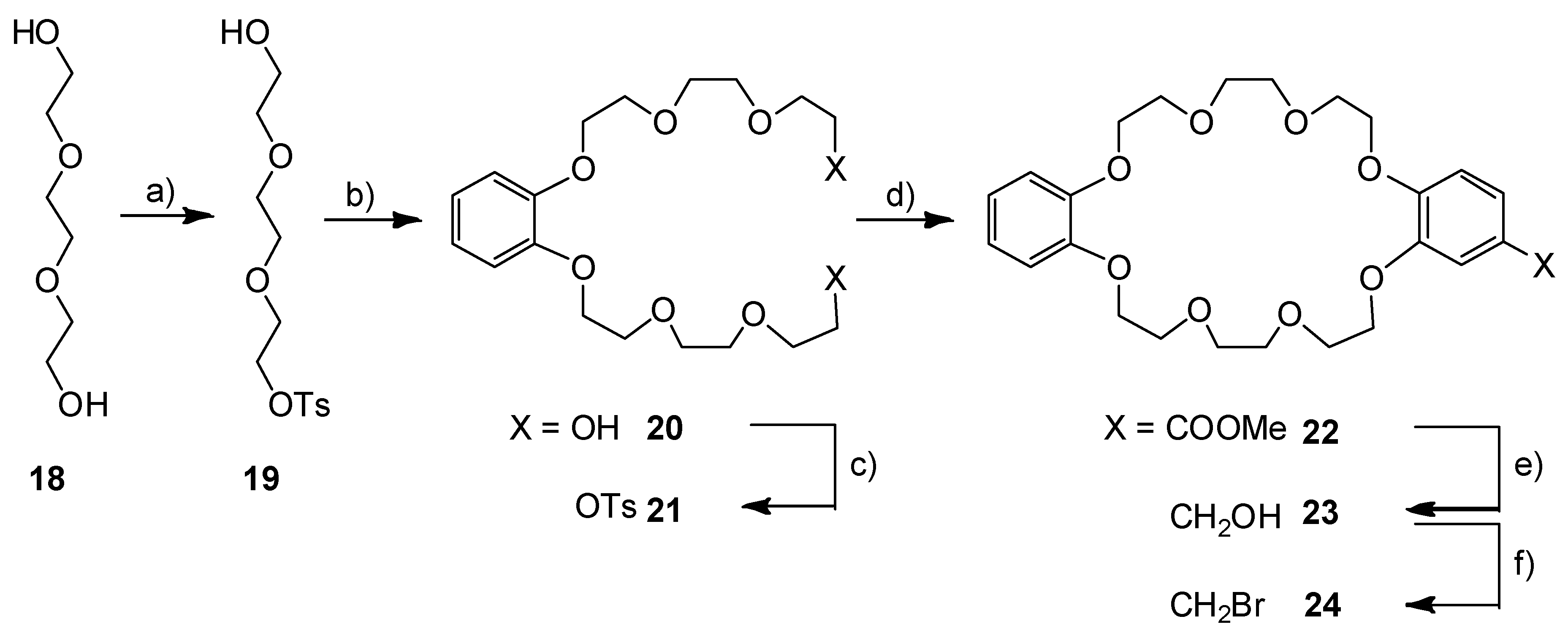
3.4. Synthesis of Crown Ether DB24C8-CH2Br (24)
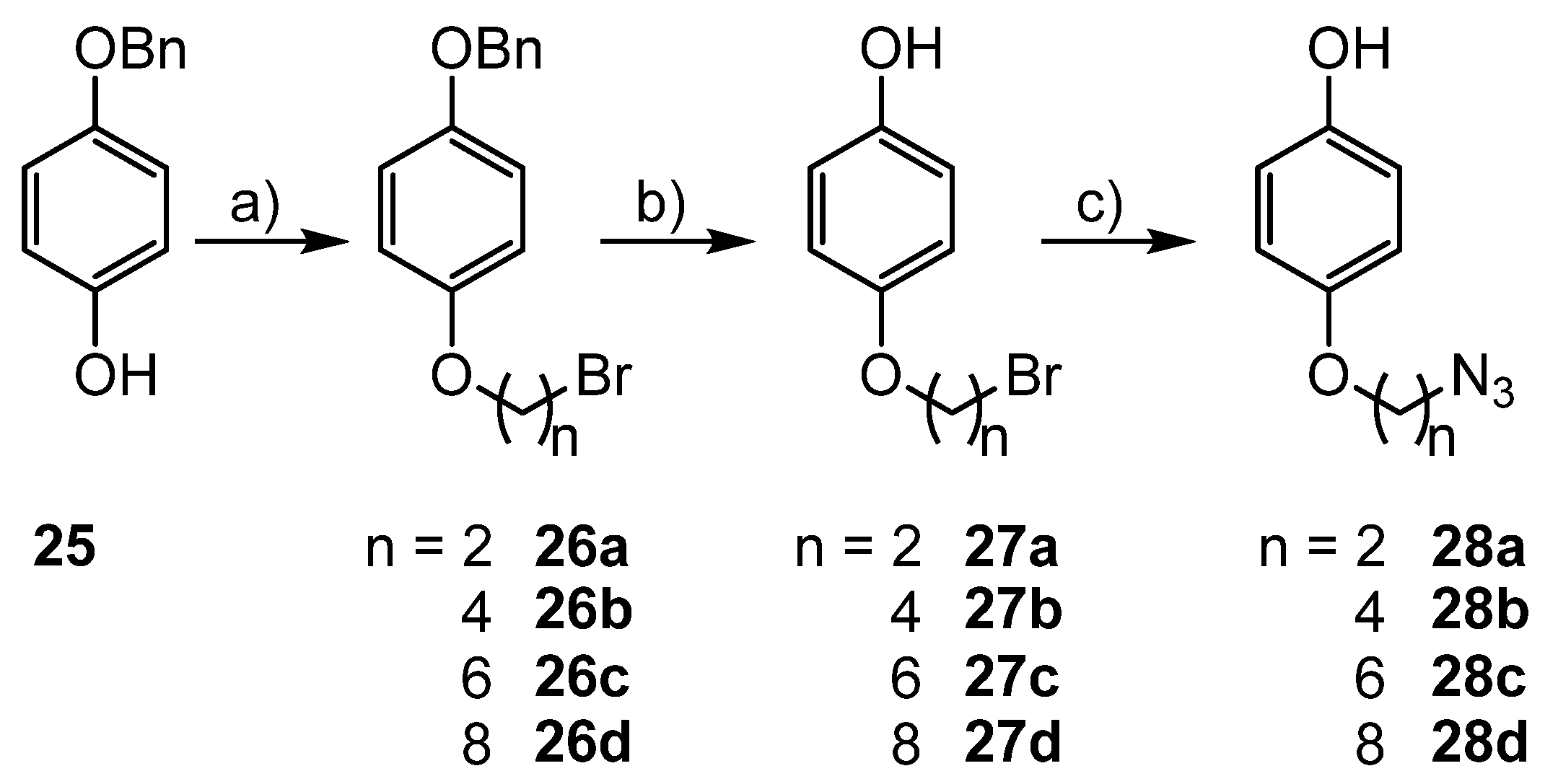
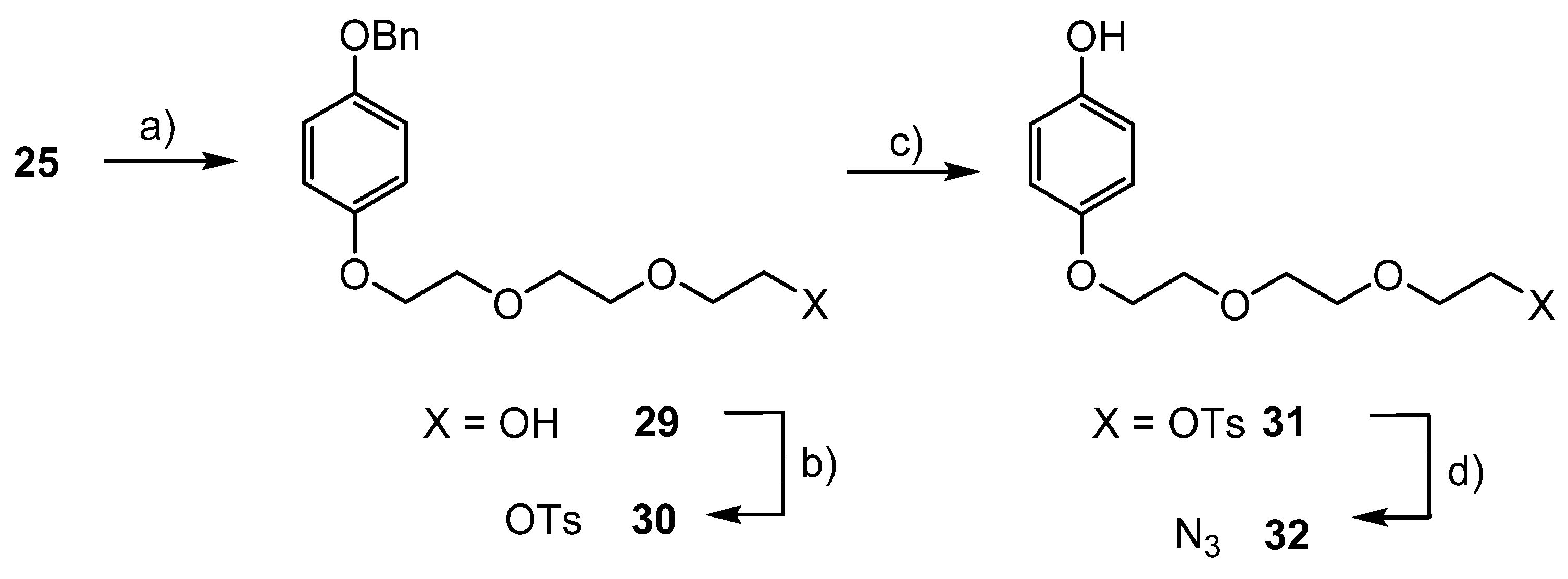
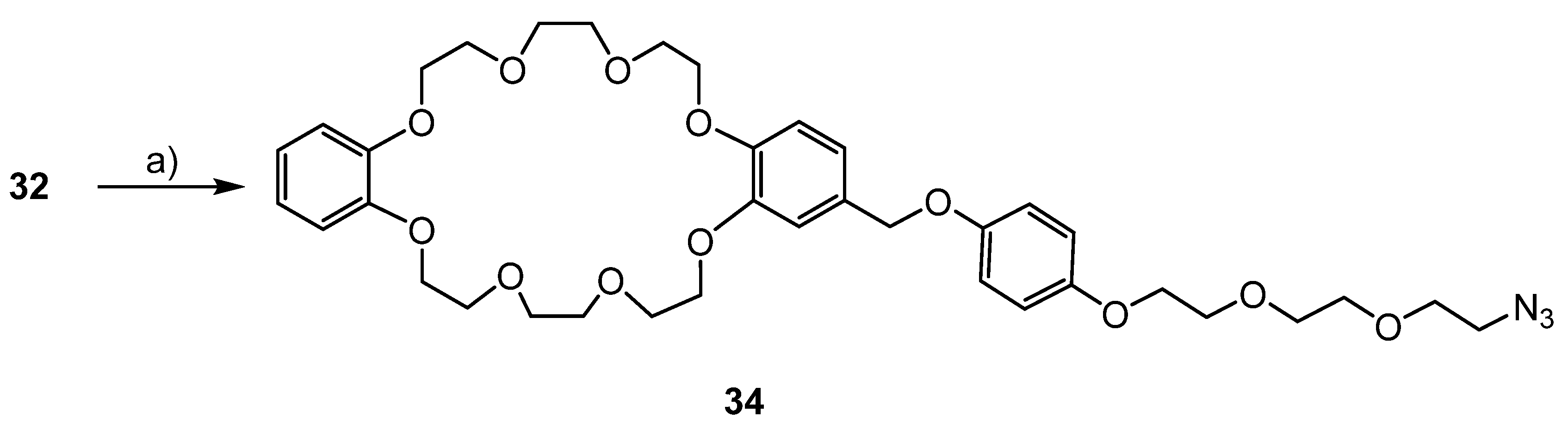
3.5. Syntheses of Azide Substituted Phenols 28a–d
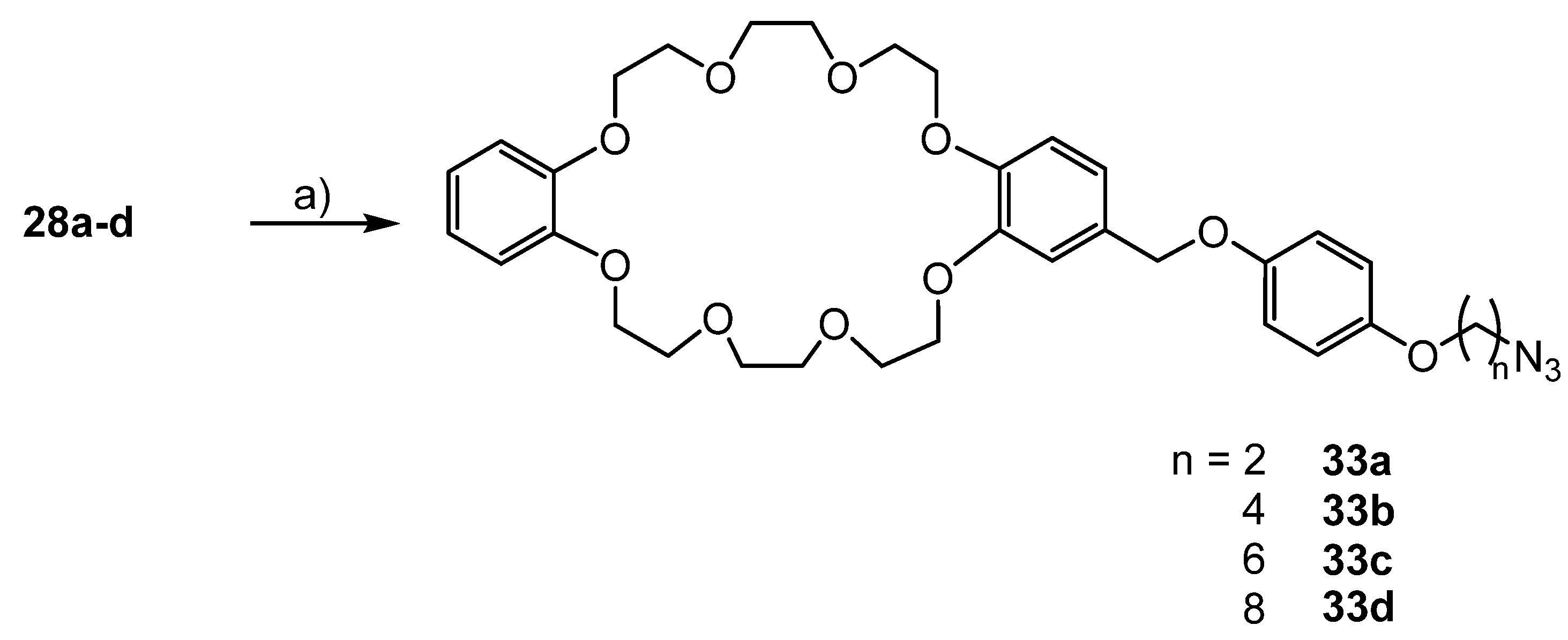
3.6. Syntheses of the Azide Containing Crown Ethers
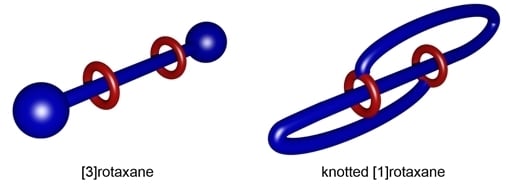
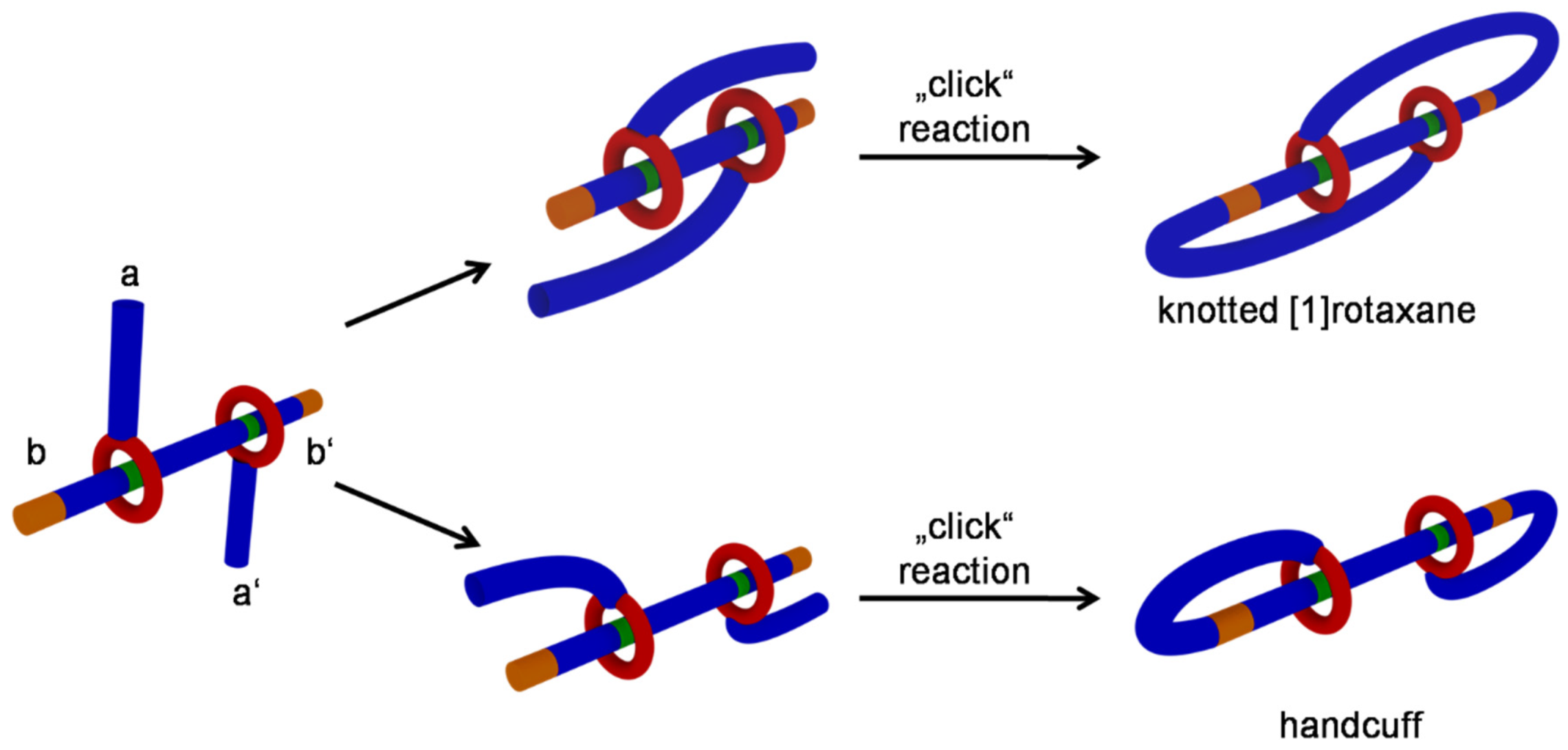
3.7. Knotting Attempts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Note
- Bruns, C.J.; Stoddart, J.F. The Nature of the Mechanical Bond: From Molecules to Machines; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Schill, G.; Zollenkopf, H. Rotaxan-Verbindungen. Liebigs Ann. Chem. 1969, 721, 53–74. [Google Scholar] [CrossRef]
- Wasserman, E. The preparation of interlocking rings: A catenane. J. Am. Chem. Soc. 1960, 82, 4433–4434. [Google Scholar] [CrossRef]
- Schill, G.; Lüttringhaus, A. The preparation of catena compounds by directed synthesis. Angew. Chem. 1964, 76, 567–568, Internation version in Angew. Chem. Int. Ed. Engl. 1964, 3, 546–547. [Google Scholar] [CrossRef]
- Dietrich-Buchecker, C.O.; Sauvage, J.-P. A synthetic molecular trefoil knot. Angew. Chem. 1989, 101, 192–194, Internation version in Angew. Chem. Int. Ed. Engl. 1989, 28, 189–192. [Google Scholar] [CrossRef]
- Lukin, O.; Kubota, T.; Okamoto, Y.; Schelhase, F.; Yoneva, A.; Walter, M.M.; Müller, U.; Vögtle, F. Knotaxanes—Rotaxanes with knots as stoppers. Angew. Chem. 2003, 115, 4681–4684, Internation version in Angew. Chem. Int. Ed. 2003, 42, 4542–4545. [Google Scholar] [CrossRef]
- Whether a rotaxane is stable or not depends on the relative sizes of ring and stopper but also on the temperature: [8,9] and reference cited.
- Harrison, I.T. The effect of ring size on threading reactions of macrocycles. J. Chem. Soc. Chem. Commun. 1972, 231–232. [Google Scholar] [CrossRef]
- Saito, S.; Takahashi, E.; Wakatsuki, K.; Inoue, K.; Orikasa, T.; Sakai, K.; Yamasaki, R.; Mutoh, Y.; Kasama, T. Synthesis of large [2]rotaxanes. The relationship between the size of the blocking group and the stability of the rotaxane. J. Org. Chem. 2013, 78, 3553–3560. [Google Scholar] [CrossRef]
- Bissell, R.A.; Cordova, E.; Kaifer, A.E.; Stoddart, J.F. A chemically and electrochemically switchable molecular shuttle. Nature 1994, 369, 131–137. [Google Scholar] [CrossRef]
- Coutrot, F.; Busseron, E. A new glycorotaxane molecular machine based on an anilinium and a triazolium station. Chem. Eur. J. 2008, 14, 4784–4787. [Google Scholar] [CrossRef]
- Chavez-Acevedo, L.; Miranda, L.D. Synthesis of novel tryptamine-based macrocycles using an Ugi 4-CR/microwave assisted click-cycloaddition reaction protocol. Org. Biomol. Chem. 2015, 13, 4408–4412. [Google Scholar] [CrossRef]
- Talotta, C.; Gaeta, C.; Pierro, T.; Neri, P. Sequence stereoisomerism in calixarene-based pseudo[3]rotaxanes. Org. Lett. 2011, 13, 2098–2101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lai, L.; Cai, P.; Cheng, G.-Z.; Xuc, X.-M.; Liua, Y. Synthesis, characterization and anticancer activity of dinuclear ruthenium(ii) complexes linked by an alkyl chain. New J. Chem. 2015, 39, 5805–5812. [Google Scholar] [CrossRef]
- Zhang, Z.-J.; Zhang, H.-Y.; Wang, H.; Liu, Y. A twin-axial hetero[7]rotaxane. Angew. Chem. 2011, 123, 11026–11030, Internation version in Angew. Chem. Int. Ed. 2011, 50, 10834–10838. [Google Scholar] [CrossRef]
- Aucagne, V.; Hänni, K.D.; Leigh, D.A.; Lusby, P.J.; Walke, D.B. Catalytic click rotaxanes: A substoichiometric metal-template pathway to mechanically interlocked architectures. J. Am. Chem. Soc. 2006, 128, 2186–2187. [Google Scholar] [CrossRef]
- Zheng, H.; Zhou, W.; Lv, J.; Yin, X.; Li, Y.; Liu, H.; Li, Y. A dual-response [2]rotaxane based on a 1,2,3-triazole ring as a novel recognition station. Chem. Eur. J. 2009, 15, 13253–13262. [Google Scholar] [CrossRef]
- Ashton, P.R.; Ballardini, R.; Balzani, V.; Bělohradský, M.; Gandolfi, M.T.; Philp, D.; Prodi, L.; Raymo, F.M.; Reddington, M.V.; Spencer, N.; et al. Self-assembly, spectroscopic, and electrochemical properties of [n]rotaxanes. J. Am. Chem. Soc. 1996, 118, 4931–4951. [Google Scholar] [CrossRef]
- Wang, X.; Ervithayasuporn, V.; Zhang, Y.; Kawakami, Y. Reversible self-assembly of dendrimer based on polyhedral oligomeric silsesquioxanes (POSS). Chem. Commun. 2011, 1282–1284. [Google Scholar] [CrossRef]
- Wu, L.; He, Y.-M.; Fan, Q.-H. Controlled reversible anchoring of η6-arene/TsDPEN- ruthenium(II) complex onto magnetic nanoparticles: A new strategy for catalyst separation and recycling. Adv. Synth. Catal. 2011, 353, 2915–2919. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Gibson, H.W. Formation of supramolecular polymers from homoditopic molecules containing secondary ammonium ions and crown ether moieties. Angew. Chem. 1999, 111, 195–199, Internation version in Angew. Chem. Int. Ed. 1999, 38, 143–147. [Google Scholar] [CrossRef]
- Davidson, A.B.; Guthrie, R.W.; Kierstead, R.W.; Ziering, A. Hoffmann-La Roche Inc. U.S. Patent 4471116, 11 September 1984. [Google Scholar]
- Grice, C.A.; Tays, K.L.; Savall, B.M.; Wei, J.; Butler, C.R.; Axe, F.U.; Bembenek, S.D.; Fouriem, A.M.; Dunford, P.J.; Lundeen, K.; et al. Identification of a potent, selective, and orally active leukotriene A4 hydrolase inhibitor with anti-inflammatory activity. J. Med. Chem. 2008, 51, 4150–4169. [Google Scholar] [CrossRef]
- Lim, J.Y.C.; Marques, I.; Thompson, A.L.; Christensen, K.E.; Félix, V.; Beer, P.D. Chalcogen bonding macrocycles and [2]rotaxanes for anion recognition. J. Am. Chem. Soc. 2017, 139, 3122–3133. [Google Scholar] [CrossRef] [PubMed]
- Hodyl, J.A.Z.; Lincoln, S.F.; Wainwright, K.P. Silica-attached molecular receptor complexes for benzoates and naphthoates. J. Incl. Phenom. Macrocycl. Chem. 2010, 68, 261–270. [Google Scholar] [CrossRef]
- Amabilino, D.B.; Ashton, P.R.; Boyd, S.E.; Gomez-Lopez, M.; Hayes, W.; Stoddart, J.F. Translational isomerism in some two- and three-station [2]rotaxanes. J. Org. Chem. 1997, 62, 3062–3075. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fu, H.; Cui, M.; Peng, C.; Liang, Z.; Dai, J.; Zhang, Z.; Lin, C.; Liu, B. Preliminary evaluation of fluoro-pegylated benzyloxybenzenes for quantification of β-amyloid plaques by positron emission tomography. Eur. J. Med. Chem. 2015, 104, 86–96. [Google Scholar] [CrossRef] [PubMed]



| Axle | m/z | Product |
|---|---|---|
| 3a | - | - |
| 3b | - | - |
| 7 | 2127.2 and 1981.2 | 7•2 33c − PF6− 7•2 33c − 2 PF6−− H+ |
| Axle | m/z | Molecule |
|---|---|---|
| 3a | 940.5175 | 3a•2 33d − 2 PF6− |
| 3b | 955.0352 | 3b•2 33d − 2 PF6− |
| 7 | 1019.0300 | 7•2 33d − 2 PF6− |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duden, T.; Lüning, U. Towards a Real Knotaxane. Chemistry 2020, 2, 305-321. https://doi.org/10.3390/chemistry2020020
Duden T, Lüning U. Towards a Real Knotaxane. Chemistry. 2020; 2(2):305-321. https://doi.org/10.3390/chemistry2020020
Chicago/Turabian StyleDuden, Torben, and Ulrich Lüning. 2020. "Towards a Real Knotaxane" Chemistry 2, no. 2: 305-321. https://doi.org/10.3390/chemistry2020020