Flow Dynamics and Acoustics from Glottal Vibrations at Different Frequencies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
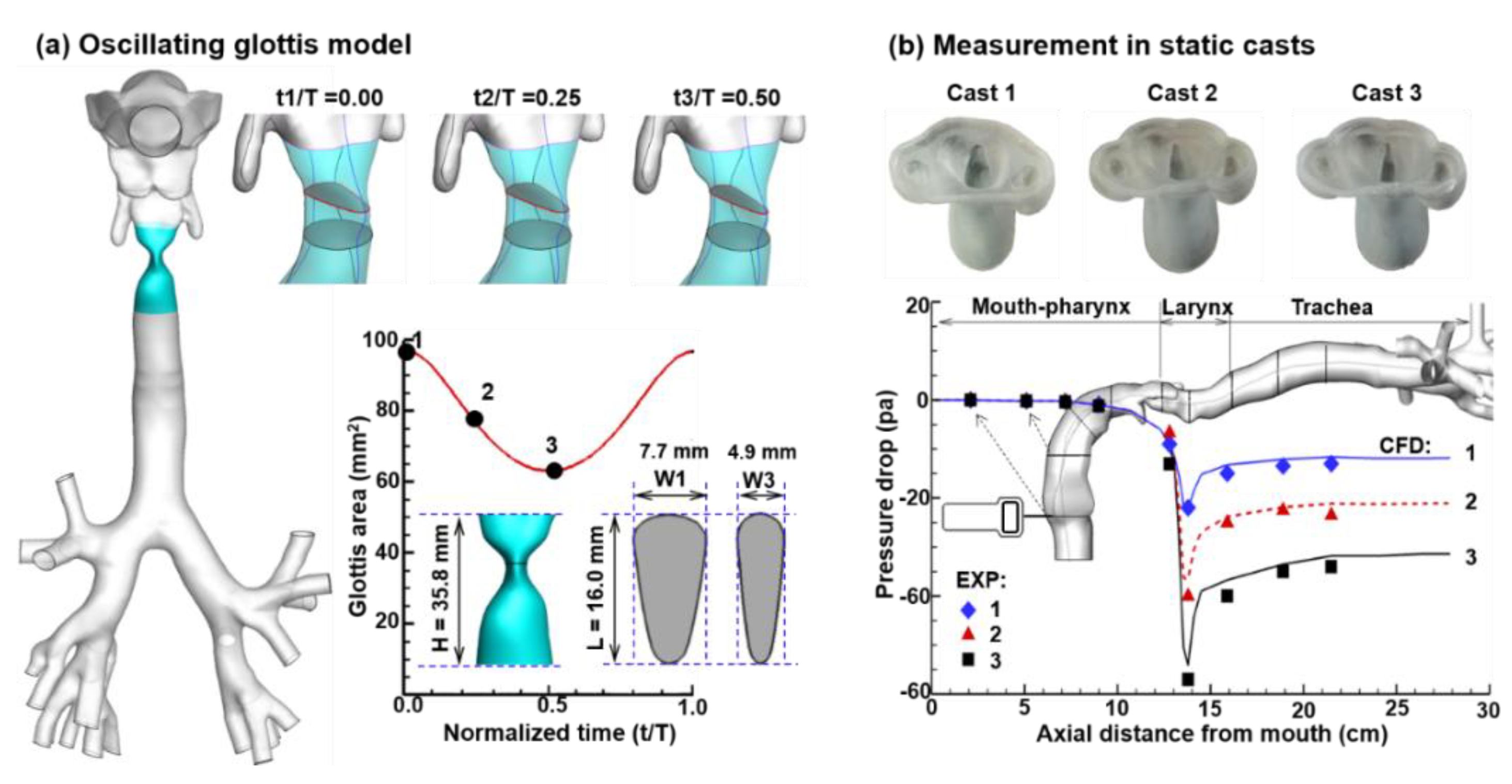
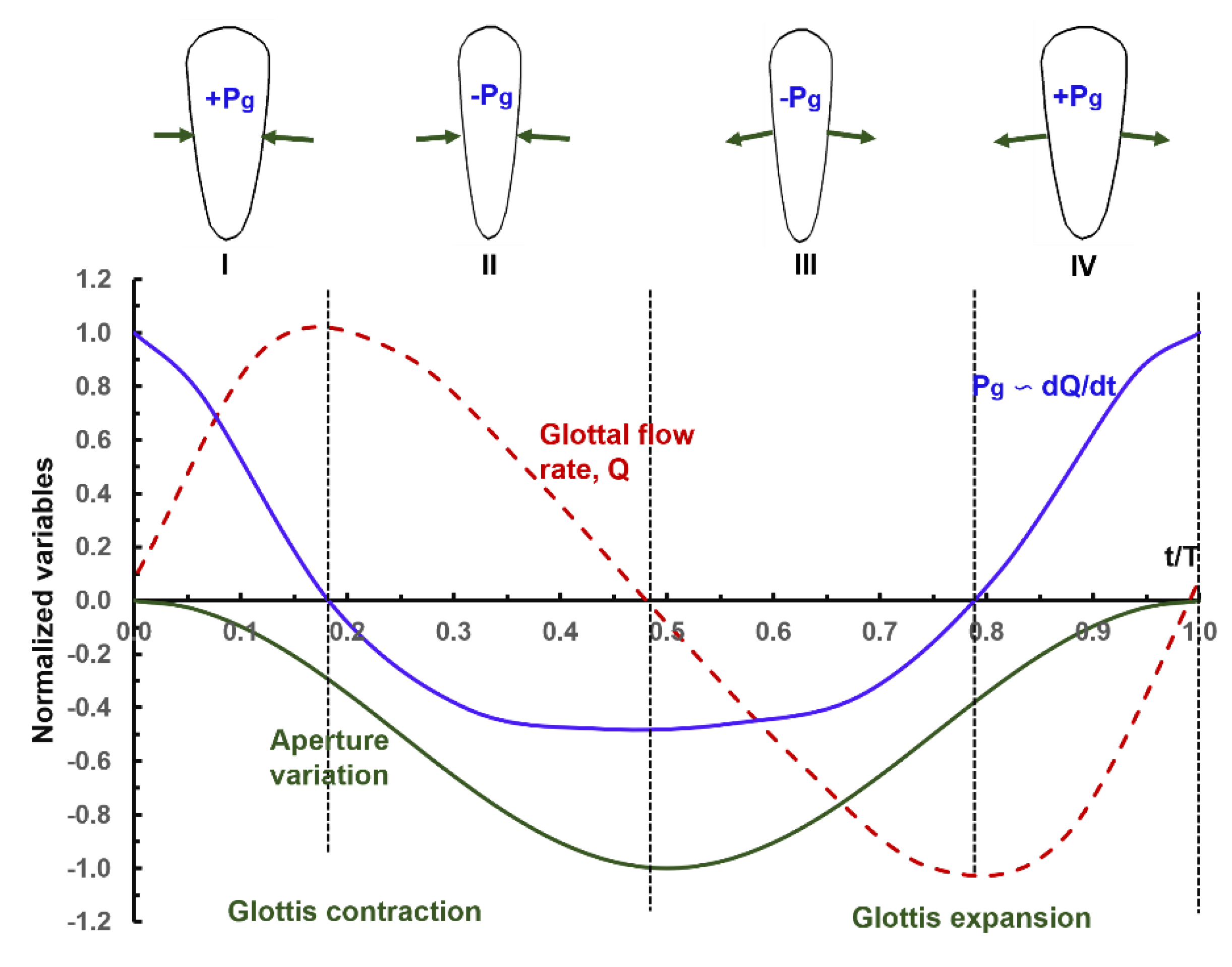
2.2. Dynamic Glottal Model
2.3. Numerical Methods
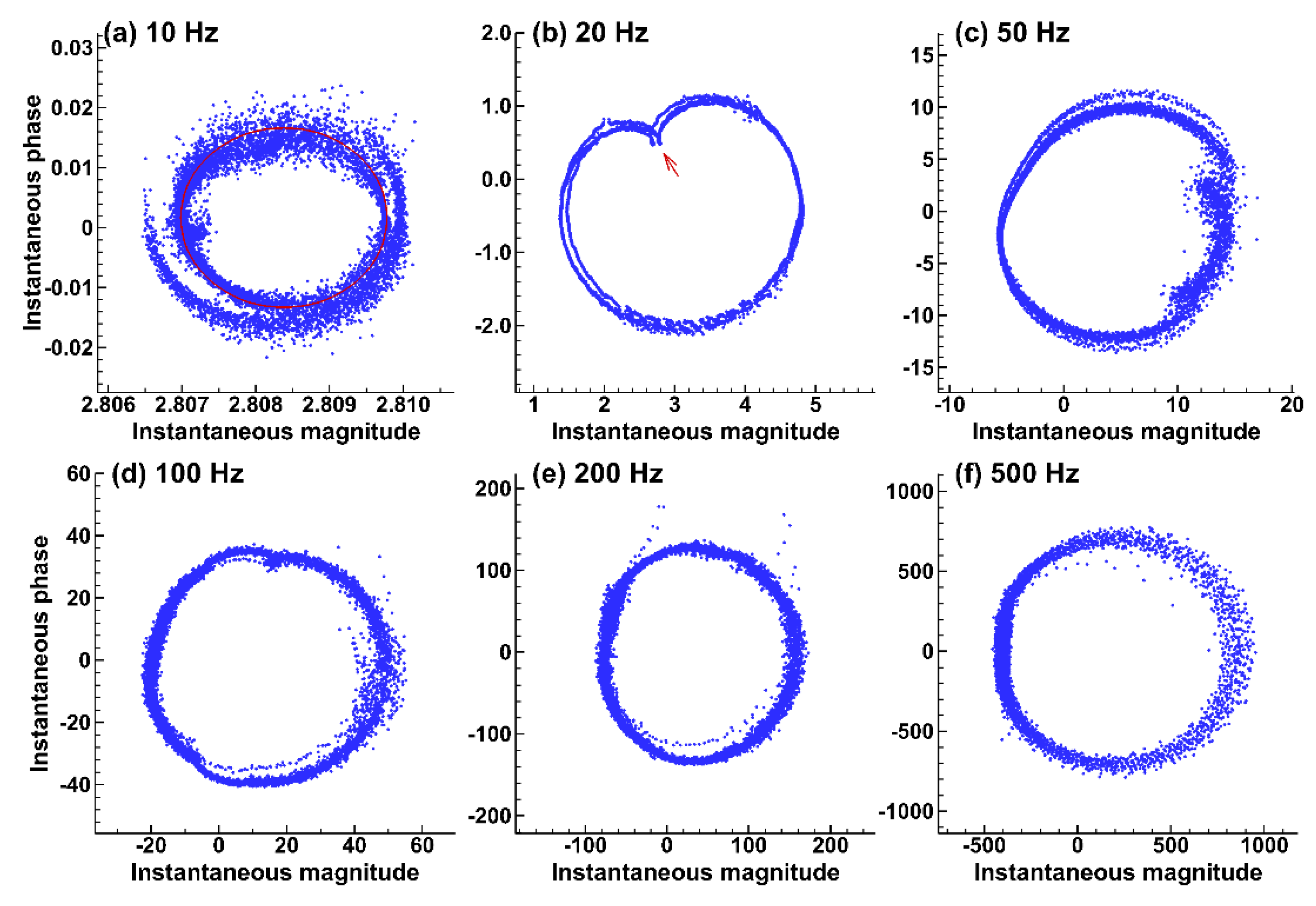
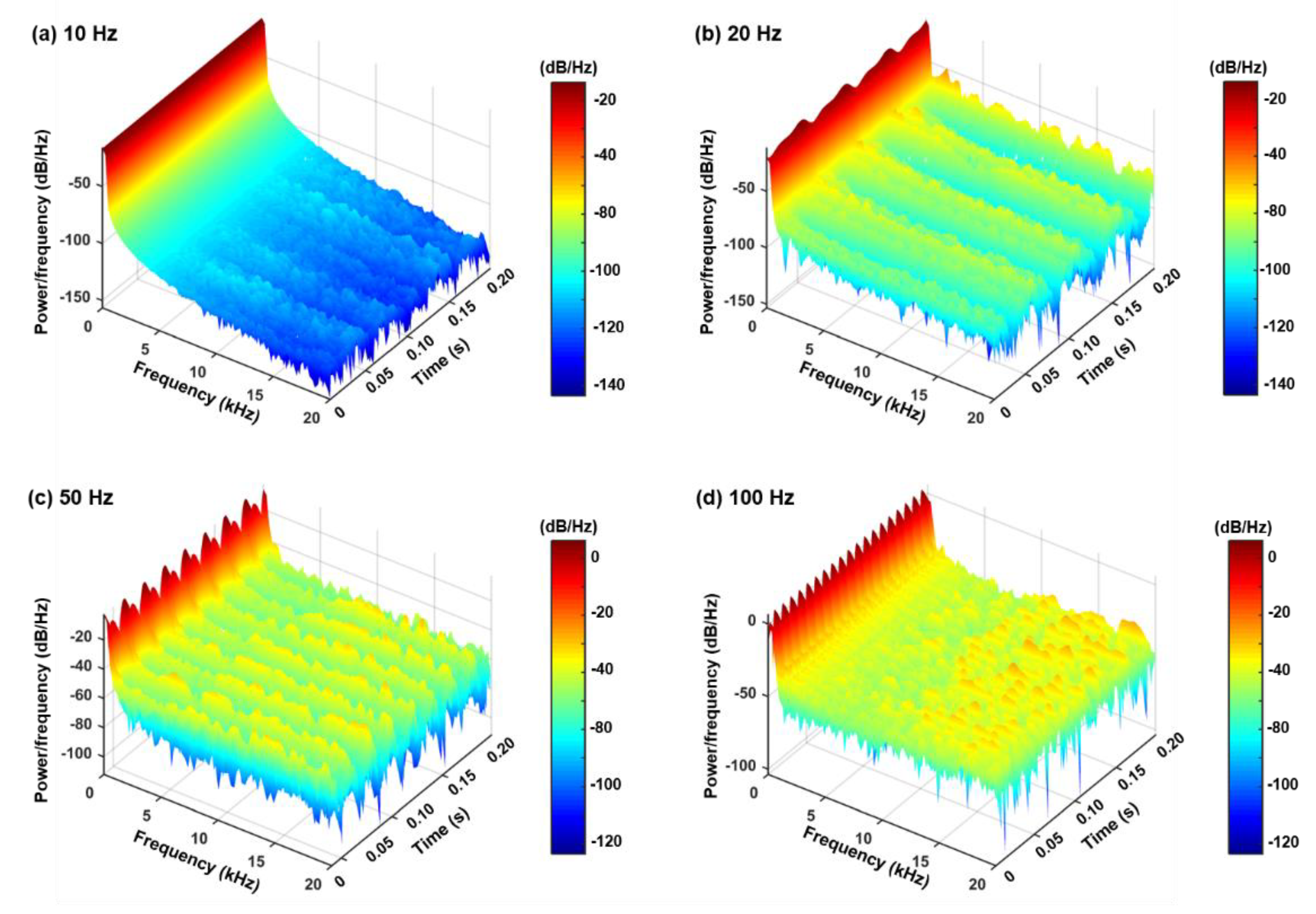
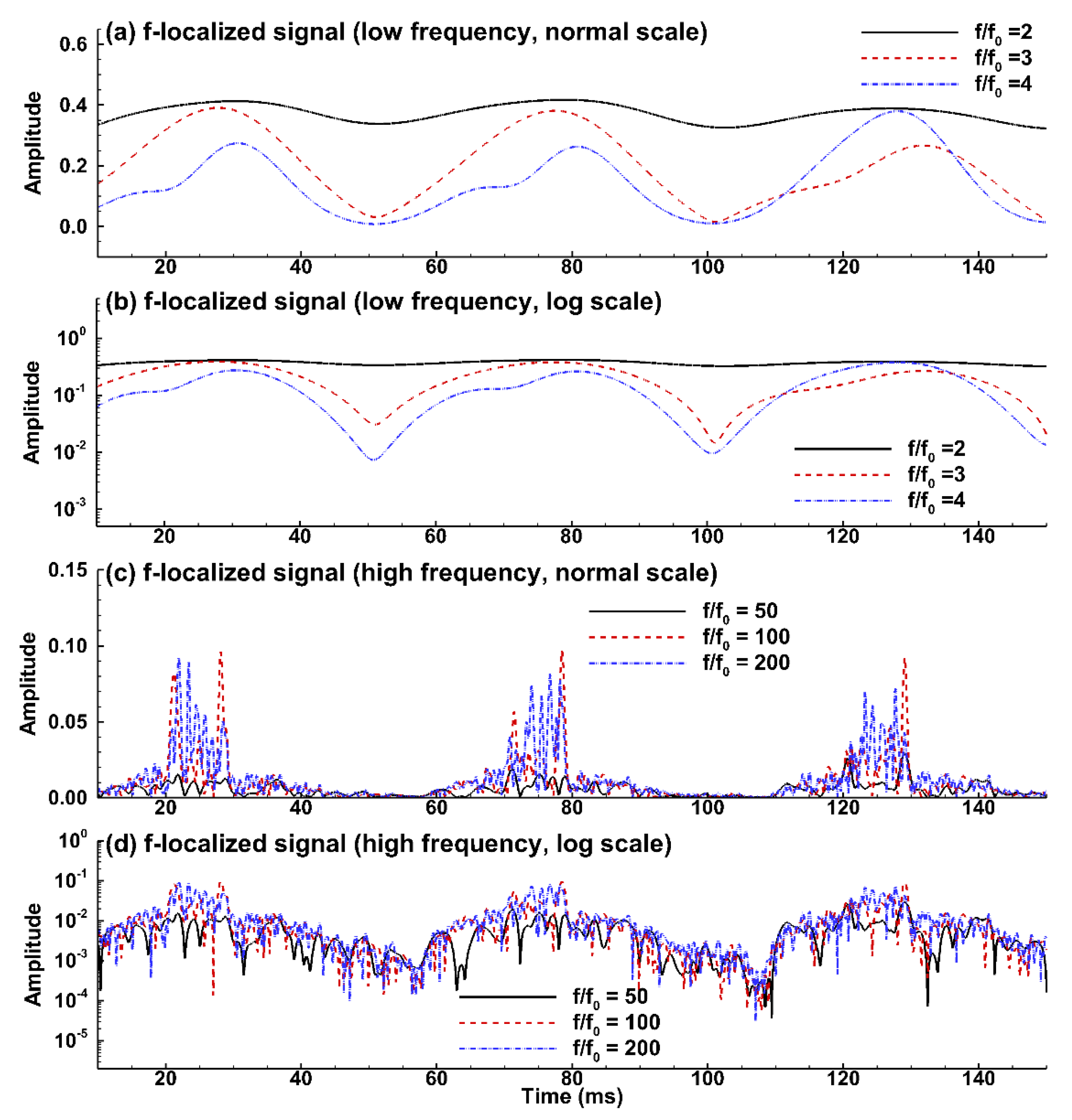
2.4. Hilbert and Wavelet Transform Analyses
3. Results
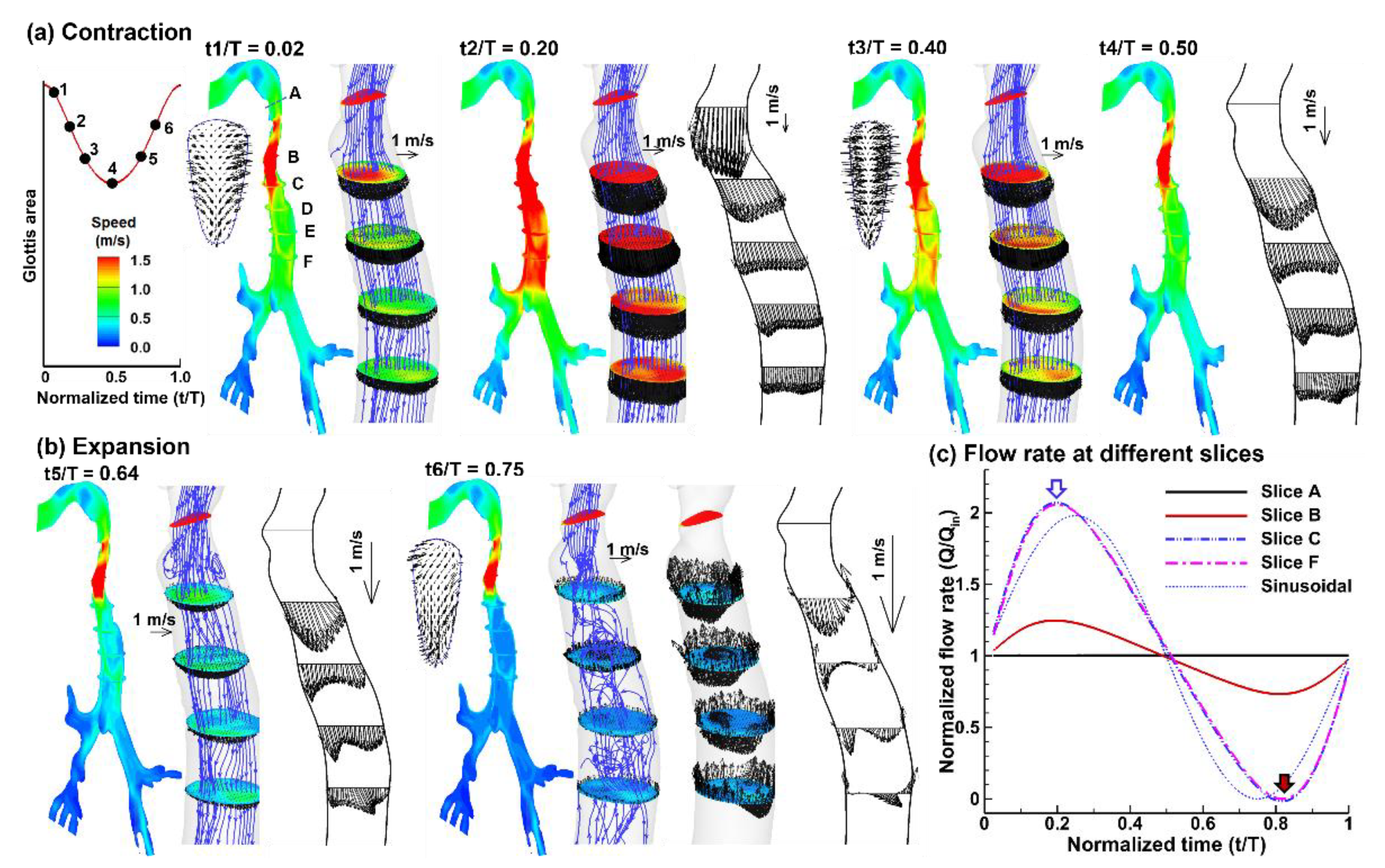
3.1. Airflow Dynamics
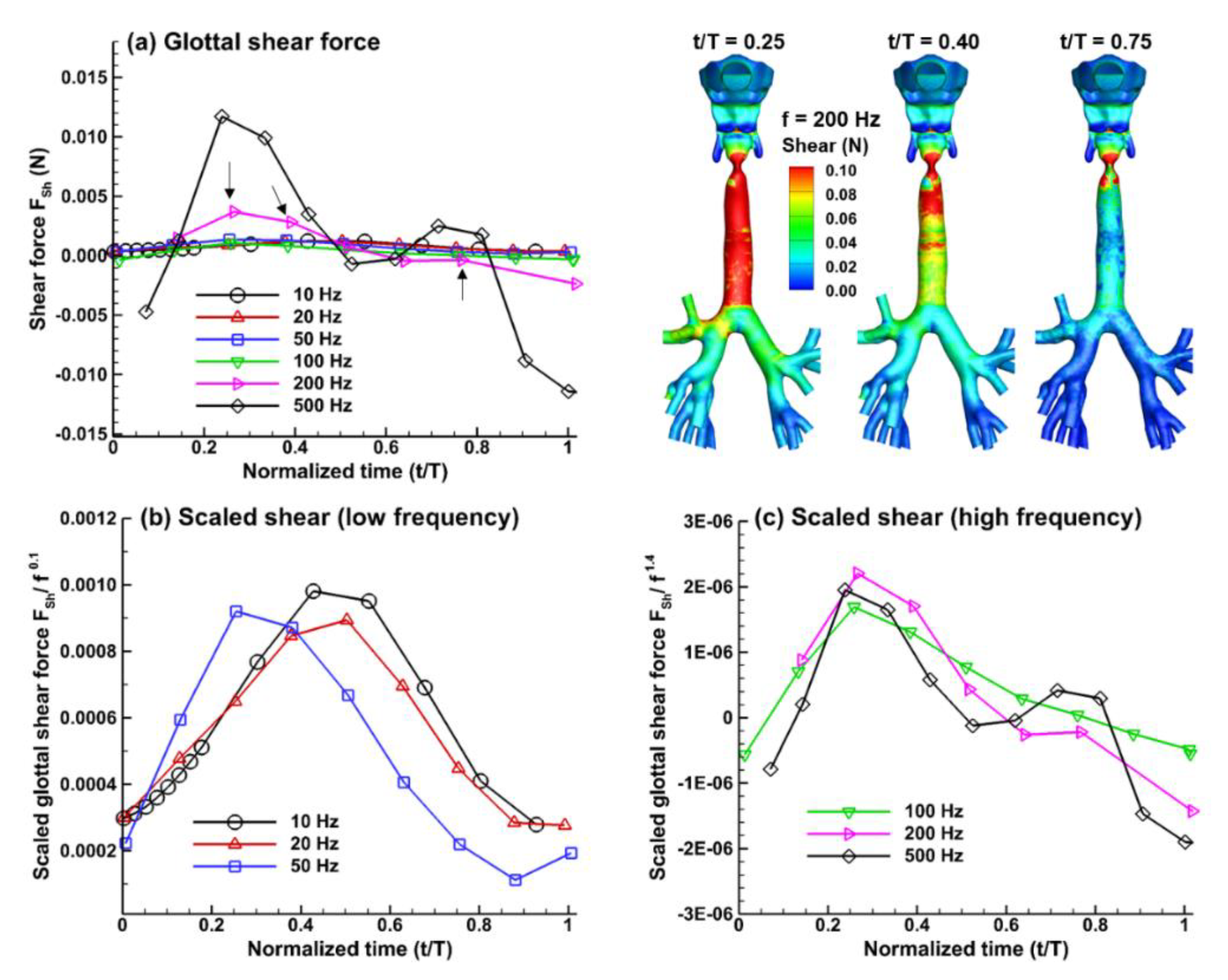
3.2. Forces on the Glottal Walls
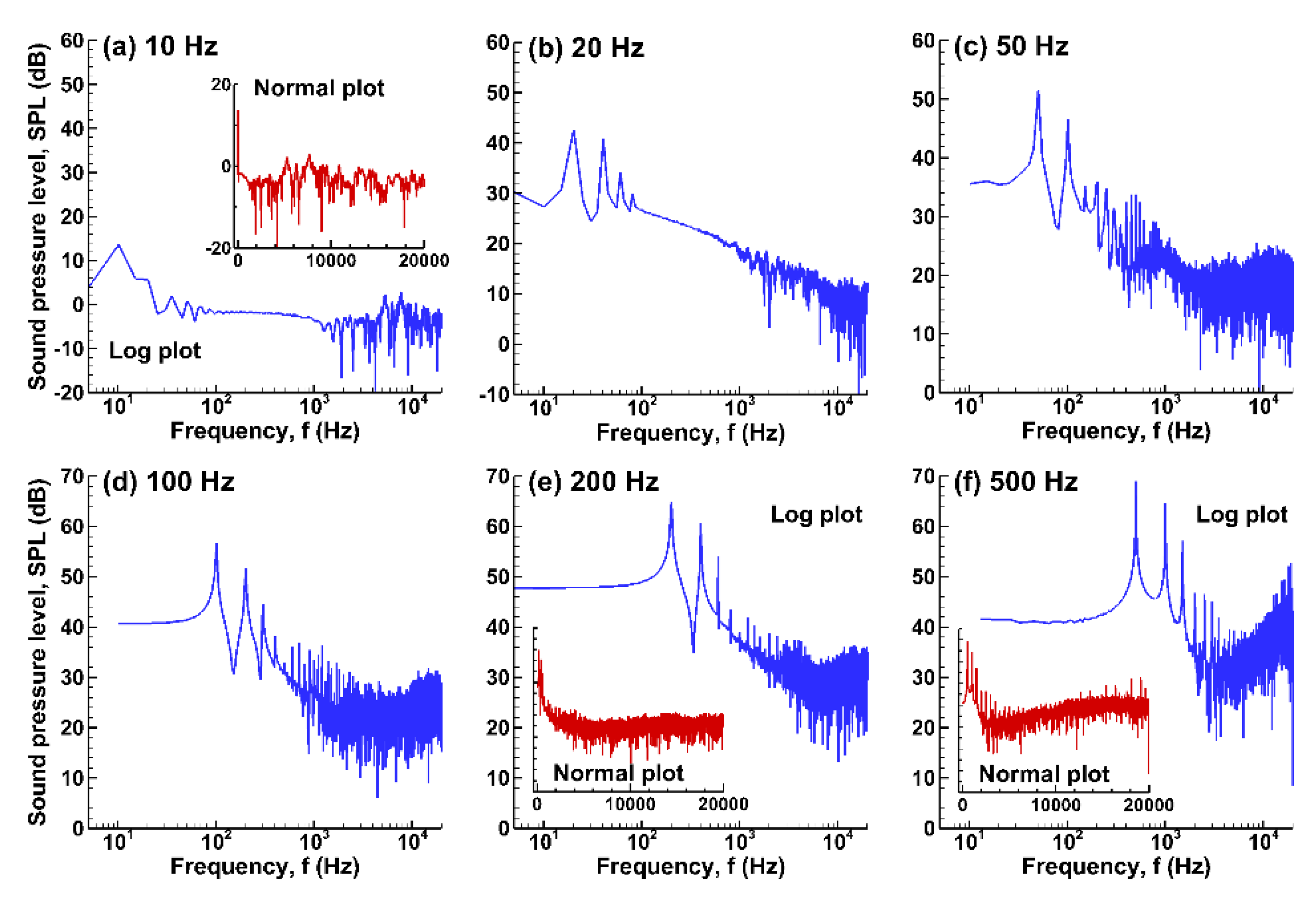
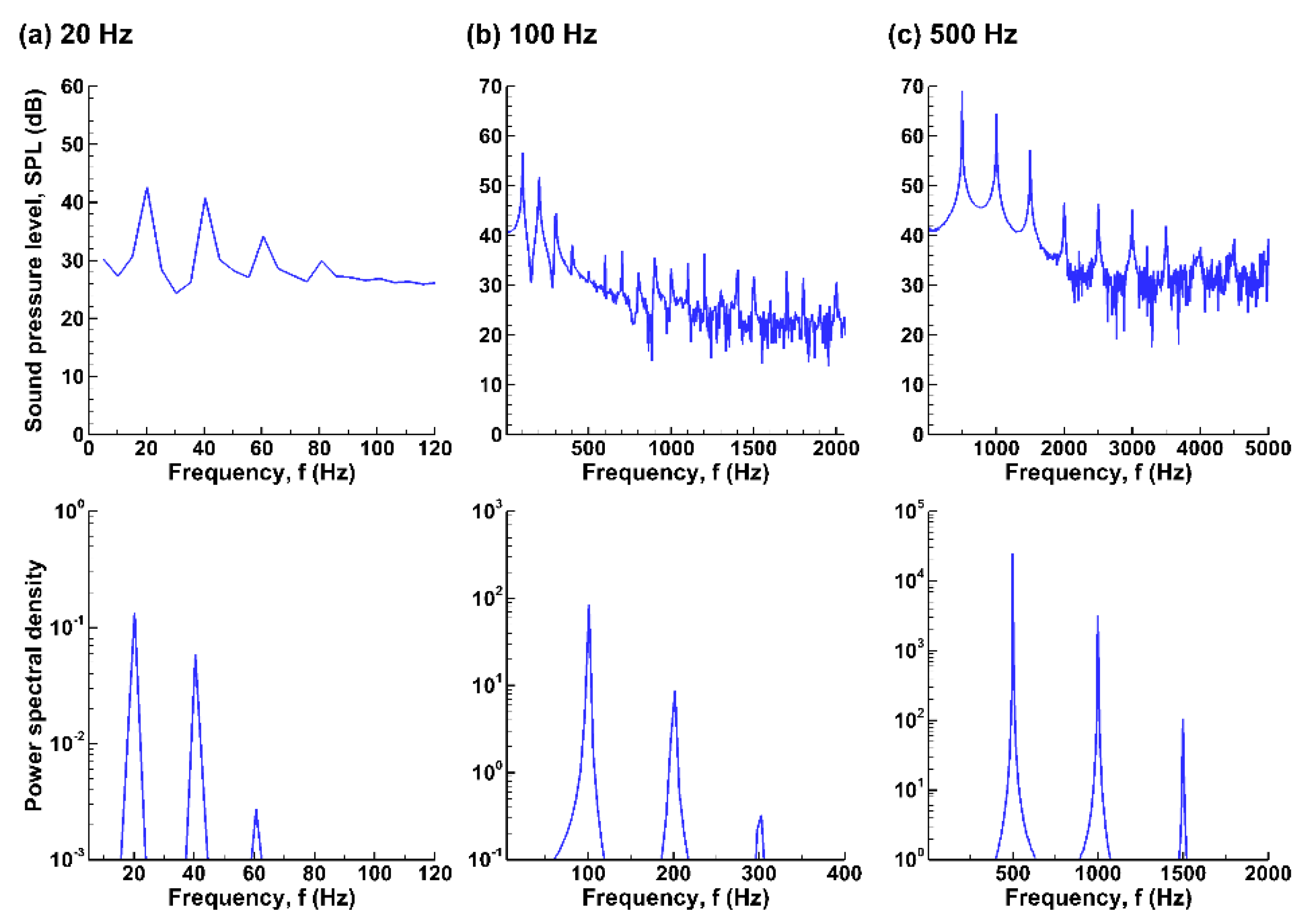
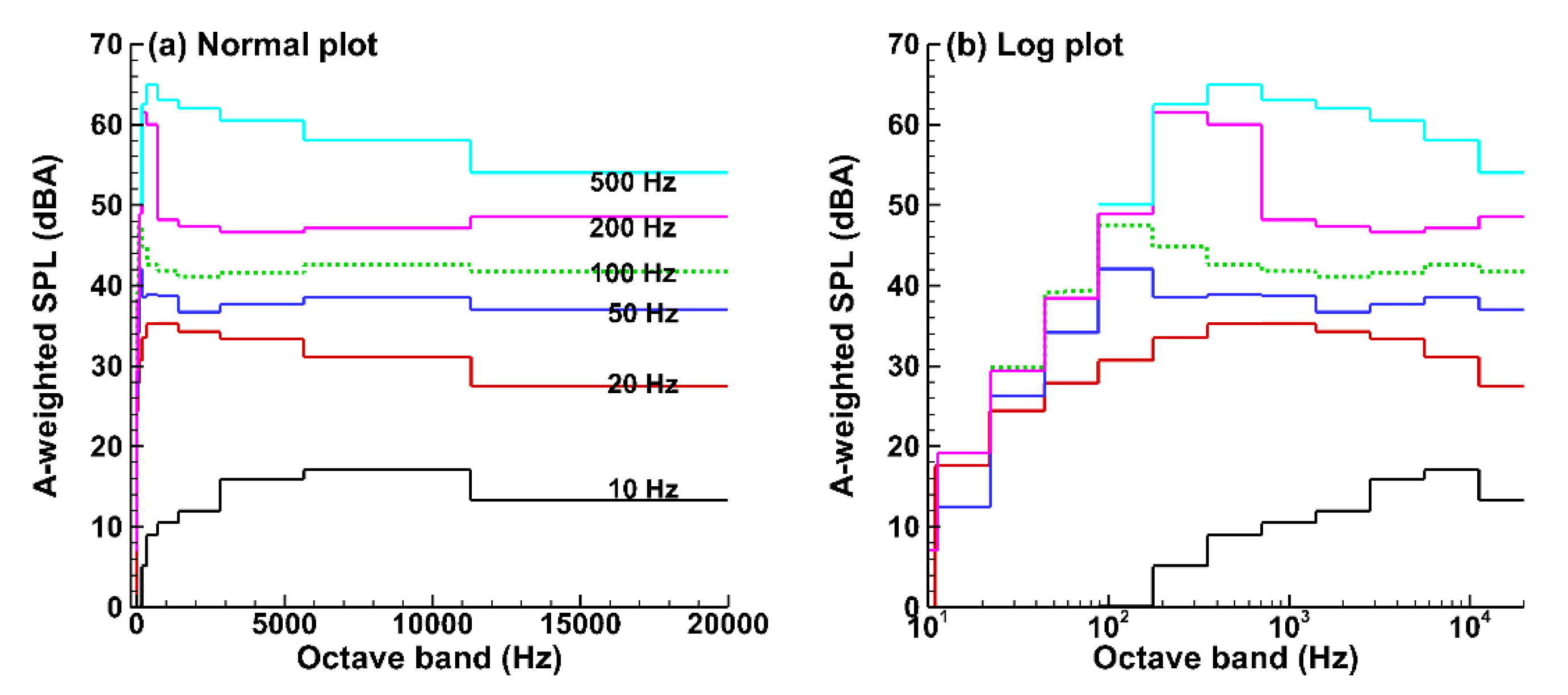
3.3. Sound Pressure and Acoustic Analyses
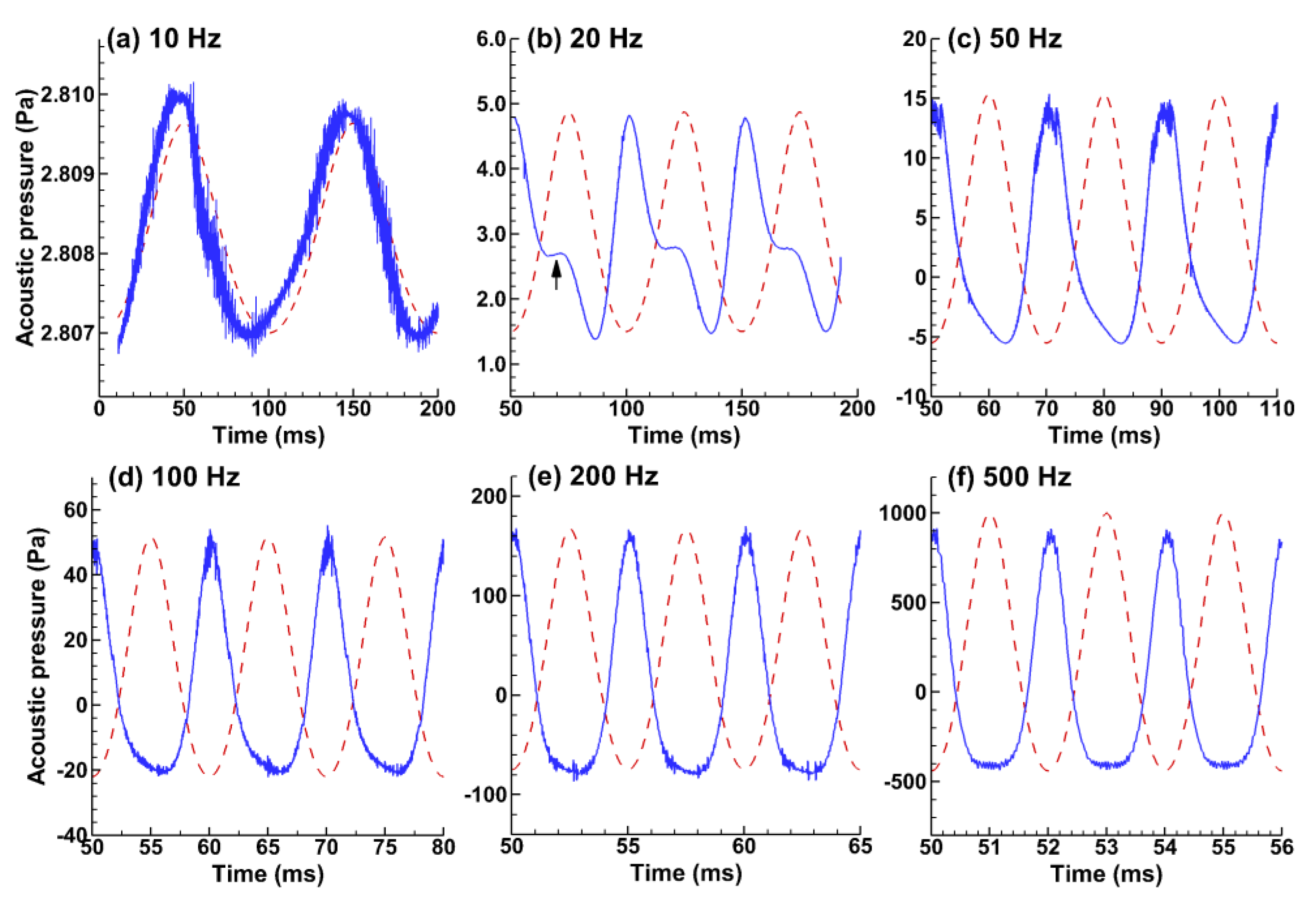
3.3.1. Pressure Signals at the Receiver (Mouth Opening)
3.3.2. Hilbert and Wavelet Transform Analyses
3.3.3. Fast Fourier Transform (FFT) Analyses
4. Discussion
4.1. Flow Responses to Glottal Vibrations
4.2. Is the Glottal Vibration Self-Sustained?
4.3. Effect of Glottal Vibrations on Acoustics
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colton, R.H. Physiological mechanisms of vocal frequency control: The role of tension. J. Voice 1988, 2, 208–220. [Google Scholar] [CrossRef]
- Triep, M.; Brücker, C. Three-dimensional nature of the glottal jet. J. Acoust. Soc. Am. 2010, 127, 1537–1547. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Muruguppan, S.; Gutmark, E.; Scherer, R. Vortical flow field during phonation in an excised canine larynx model. Ann. Otol. Rhinol. Laryngol. 2007, 116, 217–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihaescu, M.; Khosla, S.M.; Murugappan, S.; Gutmark, E.J. Unsteady laryngeal airflow simulations of the intra-glottal vortical structures. J. Acoust. Soc. Am. 2010, 127, 435–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattheus, W.; Brücker, C. Characteristics of the pulsating jet flow through a dynamic glottal model with a lens-like constriction. Biomed. Eng. Lett. 2018, 8, 309–320. [Google Scholar] [CrossRef]
- Brouns, M.; Verbanck, S.; Lacor, C. Influence of glottic aperture on the tracheal flow. J. Biomech. 2007, 40, 165–172. [Google Scholar] [CrossRef]
- Xi, J.; April Si, X.; Dong, H.; Zhong, H. Effects of glottis motion on airflow and energy expenditure in a human upper airway model. Eur. J. Mech. B Fluids 2018, 72, 23–37. [Google Scholar] [CrossRef]
- Patel, R.R.; Dubrovskiy, D.; Döllinger, M. Measurement of glottal cycle characteristics between children and adults: Physiological variations. J. Voice Off. J. Voice Found. 2014, 28, 476–486. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.L.; Titze, I.R. Vocal fold contact patterns based on normal modes of vibration. J. Biomech. 2018, 73, 177–184. [Google Scholar] [CrossRef]
- Stevens, K.N.; Hanson, H.M. Classification of glottal vibration from acoustic measurements. In Vocal Fold Physiology: Voice Quality Control; Fujimura, O., Hirano, M., Eds.; Singular: San Diego, CA, USA, 2017; Volume 9, pp. 147–170. [Google Scholar]
- Echternach, M.; Döllinger, M.; Sundberg, J.; Traser, L.; Richter, B. Vocal fold vibrations at high soprano fundamental frequencies. J. Acoust. Soc. Am. 2013, 133, El82–El87. [Google Scholar] [CrossRef]
- Sundberg, J. Vocal fold vibration patterns and modes of phonation. Folia. Phoniatr. Logop. 1995, 47, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Kakitsuba, N.; Sadaoka, T.; Kanai, R.; Fujiwara, Y.; Takahashi, H. Peculiar snoring in patients with multiple system atrophy: Its sound source, acoustic characteristics, and diagnostic significance. Ann. Otol. Rhinol. Laryngol. 1997, 106, 380–384. [Google Scholar] [PubMed]
- Isozaki, E.; Hayashi, M.; Hayashida, T.; Tanabe, H.; Hirai, S. Vocal cord abductor paralysis in multiple system atrophy--paradoxical movement of vocal cords during sleep. Rinsho Shinkeigaku Clin. Neurol. 1996, 36, 529–533. [Google Scholar]
- Bawa, R.; Ramadan, H.H.; Wetmore, S.J. Bilateral vocal cord paralysis with Shy-Drager syndrome. Otolaryngol. Head Neck Surg. 1993, 109, 911–914. [Google Scholar] [CrossRef] [PubMed]
- McBrien, F.; Spraggs, P.D.; Harcourt, J.P.; Croft, C.B. Abductor vocal fold palsy in the Shy-Drager syndrome presenting with snoring and sleep apnoea. J. Laryngol. Otol. 1996, 110, 681–682. [Google Scholar] [CrossRef]
- Kavey, N.B.; Whyte, J.; Blitzer, A.; Gidro-Frank, S. Sleep-related laryngeal obstruction presenting as snoring or sleep apnea. Laryngoscope 1989, 99, 851–854. [Google Scholar] [CrossRef]
- Ricci-Maccarini, A.; Bergamini, G.; Fustos, R. Proposal of a form for the collection of videolaryngostroboscopy basic findings. Eur. Arch. Otorhinolaryngol. 2018, 275, 1927–1933. [Google Scholar] [CrossRef] [Green Version]
- Chan, R.W. Ovine Vocal Fold Tissue Fatigue Response to Accumulated, Large-Amplitude Vibration Exposure at Phonatory Frequencies. J. Speech Lang. Hear Res. 2019, 62, 4291–4299. [Google Scholar] [CrossRef]
- Regner, M.F.; Tao, C.; Ying, D.; Olszewski, A.; Zhang, Y.; Jiang, J.J. The effect of vocal fold adduction on the acoustic quality of phonation: Ex vivo investigations. J. Voice Off. J. Voice Found. 2012, 26, 698–705. [Google Scholar] [CrossRef] [Green Version]
- Motie-Shirazi, M.; Zañartu, M.; Peterson, S.D.; Erath, B.D. Vocal fold dynamics in a synthetic self-oscillating model: Intraglottal aerodynamic pressure and energy. J. Acoust. Soc. Am. 2021, 150, 1332. [Google Scholar] [CrossRef]
- Si, X.; Xi, J.S.; Talaat, M.; Donepudi, R.; Su, W.-C.; Xi, J. Evaluation of impulse oscillometry in respiratory airway casts with varying obstruction phenotypes, locations, and complexities. J. Respir. 2022, 2, 44–58. [Google Scholar] [CrossRef]
- Hollien, H. Vocal fold dynamics for frequency change. J. Voice Off. J. Voice Found. 2014, 28, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, J.; Han, P.; Xi, J.; Dong, H. Effect of uvula length on airflow and pressure oscillation in a human pharynx model. In Proceedings of the ASME 2019 International Mechanical Engineering Congress and Exposition, Salt Lake City, UT, USA, 11–14 November 2019; p. 11697. [Google Scholar]
- Lou, Z.; Wang, J.; Daniero, J.J.; Dong, H.; Xi, J. Airflow characterization combined with 3D reconstruction of rabbit vocal cord based on in-vivo phonatory experiments. In Proceedings of the ASME 2020 Fluids Engineering Division Summer Meeting collocated with the ASME 2020 Heat Transfer Summer Conference and the ASME 2020 18th International Conference on Nanochannels, Microchannels, and Minichannels, Virtual, 13–15 July 2020; p. 23369. [Google Scholar]
- Farbos de Luzan, C.; Chen, J.; Mihaescu, M.; Khosla, S.M.; Gutmark, E. Computational study of false vocal folds effects on unsteady airflows through static models of the human larynx. J. Biomech. 2015, 48, 1248–1257. [Google Scholar] [CrossRef] [Green Version]
- Lodermeyer, A.; Bagheri, E.; Kniesburges, S.; Näger, C.; Probst, J.; Döllinger, M.; Becker, S. The mechanisms of harmonic sound generation during phonation: A multi-modal measurement-based approach. J. Acoust. Soc. Am. 2021, 150, 3485. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, W.; Frankel, S.H.; Mongeau, L. Computational aeroacoustics of phonation, part II: Effects of flow parameters and ventricular folds. J. Acoust. Soc. Am. 2002, 112, 2147–2154. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Longest, P.W. Evaluation of a drift flux model for simulating submicrometer aerosol dynamics in human upper tracheobronchial airways. Ann. Biomed. Eng. 2008, 36, 1714–1734. [Google Scholar] [CrossRef]
- Scheinherr, A.; Bailly, L.; Boiron, O.; Lagier, A.; Legou, T.; Pichelin, M.; Caillibotte, G.; Giovanni, A. Realistic glottal motion and airflow rate during human breathing. Med. Eng. Phys. 2015, 37, 829–839. [Google Scholar] [CrossRef]
- Scheinherr, A.; Bailly, L.; Boiron, O.; Legou, T.; Giovanni, A.; Caillibotte, G.; Pichelin, M. Glottal motion and its impact on the respiratory flow. Comput. Methods Biomech. Biomed. Engin. 2012, 15, 69–71. [Google Scholar] [CrossRef]
- Jackson, A.; Gulesian, P.; Mead, J. Glottal aperture during panting with voluntary limitation of tidal volume. J. Appl. Physiol. 1975, 39, 834–836. [Google Scholar] [CrossRef]
- Brancatisano, T.; Collett, P.W.; Engel, L.A. Respiratory movements of the vocal cords. J. Appl. Physiol. 1983, 54, 1269–1276. [Google Scholar] [CrossRef]
- England, S.J.; Bartlett, D., Jr.; Daubenspeck, J.A. Influence of human vocal cord movements on airflow and resistance during eupnea. J. Appl. Physiol. 1982, 52, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, Z. A computational study of vocal fold dehydration during phonation. IEEE Trans. Biomed. Eng. 2017, 64, 2938–2948. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B.S.; Sussman, R.G.; Lippmann, M. Ultrafine particle deposition in a human tracheobronchial cast. Aerosol Sci. Technol. 1990, 12, 1082–1093. [Google Scholar] [CrossRef]
- Nicoud, F.; Ducros, F. Subgrid-Scale Stress Modelling Based on the Square of the Velocity Gradient Tensor. Flow Turbul. Combust. 1999, 62, 183–200. [Google Scholar] [CrossRef]
- Xi, J.; Si, X.; Kim, J.; Su, G.; Dong, H. Modeling the pharyngeal anatomical effects on breathing resistance and aerodynamically generated sound. Med. Biol. Eng. Comput. 2014, 52, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Brentner, K.S.; Farassat, F. Modeling aerodynamically generated sound of helicopter rotors. Prog. Aerosp. Sci. 2003, 39, 83–120. [Google Scholar] [CrossRef] [Green Version]
- Lilley, G.M. The Radiated Noise from Isotropic Turbulence Revisited; NASA Contract Report 93–75; NASA Langley Research Center: Hampton, VA, USA, 1993. [Google Scholar]
- Salomons, E.M.; Janssen, S.A. Practical ranges of loudness levels of various types of environmental noise, including traffic noise, aircraft noise, and industrial noise. Int. J. Environ. Res. Public Health 2011, 8, 1847–1864. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.M.; Kim, S.T.; Mariani, S.; Cho, S.E.; Winkelman, J.W.; Park, K.H.; Kang, S.G. Difference in spectral power density of sleep EEG between patients with simple snoring and those with obstructive sleep apnoea. Sci. Rep. 2020, 10, 6135. [Google Scholar] [CrossRef] [Green Version]
- Longest, P.W.; Xi, J. Effectiveness of direct Lagrangian tracking models for simulating nanoparticle deposition in the upper airways. Aerosol Sci. Technol. 2007, 41, 380–397. [Google Scholar] [CrossRef]
- Xi, J.; Talaat, M.; Si, X.A. Two-way coupling and Kolmogorov scales on inhaler spray plume evolutions from Ventolin, ProAir, and Qvar. Math. Biosci. Eng. 2022, 19, 10915–10940. [Google Scholar] [CrossRef]
- Xi, J.; Wang, J.; Si, X.A.; Dong, H. Direct numerical simulations and acoustic analyses of flapping-uvula-induced flow evolutions within normal and constricted pharynx. Theor. Comput. Fluid Dyn. 2022. in review. [Google Scholar]
- Xi, J.; Wang, Z.; Talaat, K.; Glide-Hurst, C.; Dong, H. Numerical study of dynamic glottis and tidal breathing on respiratory sounds in a human upper airway model. Sleep Breath 2017, 22, 463–479. [Google Scholar] [CrossRef] [PubMed]
- Subbu, A.; Ray, A. Space Partitioning via Hilbert Transform for Symbolic Time Series Analysis. Appl. Phys. Lett. 2008, 92, 084107. [Google Scholar] [CrossRef]
- Jubran, B.A.; Hamdan, M.N.; Shabanneh, N.H.; Szepessy, S. Wavelet and chaos analysis of irregularities of vortex shedding. Mech. Res. Commun. 1998, 25, 583–591. [Google Scholar] [CrossRef]
- Grossmann, A.; Morlet, J. Decomposition of hardy functions into square integrable wavelets of constant shape. SIAM J. Math. Anal. 1984, 15, 723–736. [Google Scholar] [CrossRef] [Green Version]
- Honda, K. Physiological processes of speech production. In Handbook of Speech Processing; Benesty, J., Sondhi, M.M., Huang, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 7–26. [Google Scholar]
- Khosla, S.; Murugappan, S.; Gutmark, E. What can vortices tell us about vocal fold vibration and voice production. Curr. Opin. Otolaryngol. Head Neck Surg. 2008, 16, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.J.; Zhang, Y.; McGilligan, C. Chaos in voice, from modeling to measurement. J. Voice Off. J. Voice Found. 2006, 20, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Murugappan, S.; Paniello, R.; Ying, J.; Gutmark, E. Role of vortices in voice production: Normal versus asymmetric tension. Laryngoscope 2009, 119, 216–221. [Google Scholar] [CrossRef]
- Farbos de Luzan, C.; Oren, L.; Gutmark, E.; Khosla, S. Effects of vocal tract inertance on the glottal flow. J. Acoust. Soc. Am. 2016, 140, 3331. [Google Scholar] [CrossRef]
- Orlikoff, R.F.; Golla, M.E.; Deliyski, D.D. Analysis of longitudinal phase differences in vocal-fold vibration using synchronous high-speed videoendoscopy and electroglottography. J. Voice Off. J. Voice Found. 2012, 26, e813–e820. [Google Scholar] [CrossRef] [Green Version]
- Drioli, C.; Aichinger, P. Modelling sagittal and vertical phase differences in a lumped and distributed elements vocal fold model. Biomed. Signal Process. Control. 2021, 64, 102309. [Google Scholar] [CrossRef]
- Zhang, Z. Vibration in a self-oscillating vocal fold model with left-right asymmetry in body-layer stiffness. J. Acoust. Soc. Am. 2010, 128, EL279–EL285. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Chan, R.W. Viscoelastic properties of human aryepiglottic fold and ventricular fold tissues at phonatory frequencies. Laryngoscope 2018, 128, E296–E301. [Google Scholar] [CrossRef]
- Wang, J.; Xi, J.; Han, P.; Wongwiset, N.; Pontius, J.; Dong, H. Computational analysis of a flapping uvula on aerodynamics and pharyngeal wall collapsibility in sleep apnea. J. Biomech. 2019, 94, 88–98. [Google Scholar] [CrossRef]
- Xi, J.; Wang, J.; Si, X.A.; Zheng, S.; Donepudi, R.; Dong, H. Extracting signature responses from respiratory flows: Low-dimensional analyses on Direct Numerical Simulation-predicted wakes of a flapping uvula. Int. J. Numer. Meth. Biomed. Eng. 2020, 36, e3406. [Google Scholar] [CrossRef]
- Xue, Q.; Mittal, R.; Zheng, X.; Bielamowicz, S. A computational study of the effect of vocal-fold asymmetry on phonation. J. Acoust. Soc. Am. 2010, 128, 818–827. [Google Scholar] [CrossRef] [Green Version]
- de Vries, M.P.; Schutte, H.K.; Veldman, A.E.; Verkerke, G.J. Glottal flow through a two-mass model: Comparison of Navier-Stokes solutions with simplified models. J. Acoust. Soc. Am. 2002, 111, 1847–1853. [Google Scholar] [CrossRef] [PubMed]
- Eysholdt, U.; Rosanowski, F.; Hoppe, U. Vocal fold vibration irregularities caused by different types of laryngeal asymmetry. Eur. Arch. Otorhinolaryngol. 2003, 260, 412–417. [Google Scholar] [CrossRef]
- Ibrahim, W.H.; Gheriani, H.A.; Almohamed, A.A.; Raza, T. Paradoxical vocal cord motion disorder: Past, present and future. Postgrad. Med. J. 2007, 83, 164–172. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi, H.; Döllinger, M.; Kaltenbacher, M.; Kniesburges, S. Aerodynamic impact of the ventricular folds in computational larynx models. J. Acoust. Soc. Am. 2019, 145, 2376. [Google Scholar] [CrossRef]
- Birk, V.; Sutor, A.; Döllinger, M.; Bohr, C.; Kniesburges, S. Acoustic impact of ventricular folds on phonation studied in ex vivo human larynx models. Acta Acust. United Acust. 2016, 102, 244–256. [Google Scholar] [CrossRef]
- Schoder, S.; Maurerlehner, P.; Wurzinger, A.; Hauser, A.; Falk, S.; Kniesburges, S.; Döllinger, M.; Kaltenbacher, M. Aeroacoustic sound source characterization of the human voice production-perturbed convective wave equation. Appl. Sci. 2021, 11, 2614. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, J.; Talaat, M.; Si, X.; Dong, H. Flow Dynamics and Acoustics from Glottal Vibrations at Different Frequencies. Acoustics 2022, 4, 915-933. https://doi.org/10.3390/acoustics4040056
Xi J, Talaat M, Si X, Dong H. Flow Dynamics and Acoustics from Glottal Vibrations at Different Frequencies. Acoustics. 2022; 4(4):915-933. https://doi.org/10.3390/acoustics4040056
Chicago/Turabian StyleXi, Jinxiang, Mohamed Talaat, Xiuhua Si, and Haibo Dong. 2022. "Flow Dynamics and Acoustics from Glottal Vibrations at Different Frequencies" Acoustics 4, no. 4: 915-933. https://doi.org/10.3390/acoustics4040056





