Temporal Considerations in Brain Metastases Radiation Therapy: The Intersection of Chronobiology and Patient Profiles
Abstract
:1. Introduction
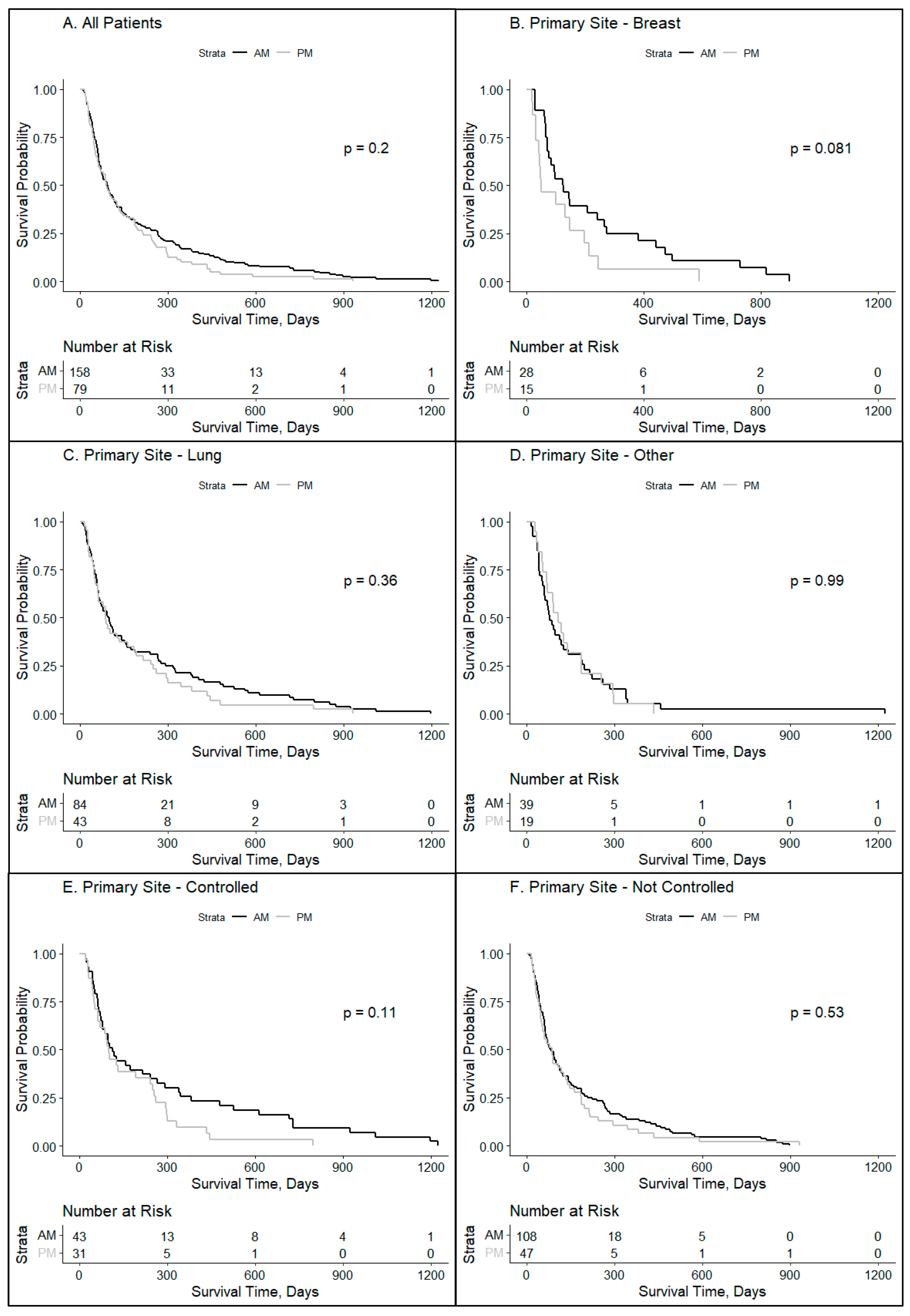
2. Results
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Takahashi, J.S.; Hong, H.K.; Ko, C.H.; McDearmon, E.L. The genetics of mammalian circadian order and disorder: Implications for physiology and disease. Nat. Rev. Genet. 2008, 9, 764–775. [Google Scholar] [CrossRef]
- Diamantopoulou, Z.; Castro-Giner, F.; Schwab, F.D.; Foerster, C.; Saini, M.; Budinjas, S.; Strittmatter, K.; Krol, I.; Seifert, B.; Heinzelmann-Schwarz, V.; et al. The metastatic spread of breast cancer accelerates during sleep. Nature 2022, 607, 156–162. [Google Scholar] [CrossRef]
- Wang, C.; Barnoud, C.; Cenerenti, M.; Sun, M.; Caffa, I.; Kizil, B.; Bill, R.; Liu, Y.; Pick, R.; Garnier, L.; et al. Dendritic cells direct circadian anti-tumour immune responses. Nature 2023, 614, 136–143. [Google Scholar] [CrossRef]
- Kim, D.W.; Byun, J.M.; Lee, J.O.; Kim, J.K.; Koh, Y. Chemotherapy delivery time affects treatment outcomes of female patients with diffuse large B cell lymphoma. JCI Insight 2023, 8, 164767. [Google Scholar] [CrossRef]
- Haus, E. Chronobiology of the mammalian response to ionizing radiation. Potential applications in oncology. Chronobiol. Int. 2002, 19, 77–100. [Google Scholar] [CrossRef]
- Bermúdez-Guzmán, L.; Blanco-Saborío, A.; Ramírez-Zamora, J.; Lovo, E. The Time for Chronotherapy in Radiation Oncology. Front. Oncol. 2021, 11, 687672. [Google Scholar] [CrossRef] [PubMed]
- Nelson, N.; Lombardo, J.; Matlack, L.; Smith, A.; Hines, K.; Shi, W.; Simone, N.L. Chronoradiobiology of Breast Cancer: The Time Is Now to Link Circadian Rhythm and Radiation Biology. Int. J. Mol. Sci. 2022, 23, 1331. [Google Scholar] [CrossRef]
- Chan, S.; Rowbottom, L.; McDonald, R.; Zhang, L.; Bjarnason, G.A.; Tsao, M.; Chow, E. Could time of whole brain radiotherapy delivery impact overall survival in patients with multiple brain metastases? Ann. Palliat. Med. 2016, 5, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Badiyan, S.N.; Ferraro, D.J.; Yaddanapudi, S.; Drzymala, R.E.; Lee, A.Y.; Silver, S.A.; Dyk, P.; DeWees, T.; Simpson, J.R.; Rich, K.M.; et al. Impact of time of day on outcomes after stereotactic radiosurgery for non-small cell lung cancer brain metastases. Cancer 2013, 119, 3563–3569. [Google Scholar] [CrossRef] [PubMed]
- Rahn, D.A.; Ray, D.K.; Schlesinger, D.J.; Steiner, L.; Sheehan, J.P.; O’Quigley, J.M.; Rich, T. Gamma knife radiosurgery for brain metastasis of nonsmall cell lung cancer: Is there a difference in outcome between morning and afternoon treatment? Cancer 2011, 117, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Kabolizadeh, P.; Wegner, R.; Bernard, M.; Heron, D.; Mintz, A.; Burton, S. The effect of treatment time on outcome in non-small cell lung cancer brain metastases treated with stereotactic radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, S301. [Google Scholar] [CrossRef]
- Straif, K.; Baan, R.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Bouvard, V.; Altieri, A.; Benbrahim-Tallaa, L.; Cogliano, V. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol. 2007, 8, 1065–1066. [Google Scholar] [CrossRef]
- He, C.; Anand, S.T.; Ebell, M.H.; Vena, J.E.; Robb, S.W. Circadian disrupting exposures and breast cancer risk: A meta-analysis. Int. Arch. Occup. Environ. Health 2015, 88, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Saenz, A.; Sánchez de Miguel, A.; Espinosa, A.; Valentin, A.; Aragonés, N.; Llorca, J. Evaluating the Association between Artificial Light-at-Night Exposure and Breast and Prostate Cancer Risk in Spain (MCC-Spain Study). Environ. Health Perspect. 2018, 126, 047011. [Google Scholar] [CrossRef] [PubMed]
- deHaro, D.; Kines, K.J.; Sokolowski, M.; Dauchy, R.T.; Streva, V.A.; Hill, S.M.; Hanifin, J.P.; Brainard, G.C.; Blask, D.E.; Belancio, V.P. Regulation of L1 expression and retrotransposition by melatonin and its receptor: Implications for cancer risk associated with light exposure at night. Nucleic Acids Res. 2014, 42, 7694–7707. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; He, C.; Zhao, W.; Hu, N.; Long, D. Circadian clock and cell cycle: Cancer and chronotherapy. Acta Histochem. 2021, 123, 151816. [Google Scholar] [CrossRef] [PubMed]
- Reszka, E.; Przybek, M.; Muurlink, O.; Pepłonska, B. Circadian gene variants and breast cancer. Cancer Lett. 2017, 390, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, L.D.; Bussard, K.M. The Clock Is Ticking: Countdown to Metastases. PLoS Genet. 2016, 12, e1006299. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.; Chang-Claude, J.; Critchley, A.M.; Kyriacou, C.; Lavers, S.; Rattay, T.; Webb, A.; Azria, D.; Brookes, A.; Burr, T.; et al. Genetic Variants Predict Optimal Timing of Radiotherapy to Reduce Side-effects in Breast Cancer Patients. Clin. Oncol. (R. Coll. Radiol.) 2019, 31, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lv, H.; Ji, M.; Wang, Z.; Wu, W. Low circadian clock genes expression in cancers: A meta-analysis of its association with clinicopathological features and prognosis. PLoS ONE 2020, 15, e0233508. [Google Scholar] [CrossRef]
- Chan, S.; Zhang, L.; Rowbottom, L.; McDonald, R.; Bjarnason, G.A.; Tsao, M.; Barnes, E.; Danjoux, C.; Popovic, M.; Lam, H.; et al. Effects of circadian rhythms and treatment times on the response of radiotherapy for painful bone metastases. Ann. Palliat. Med. 2017, 6, 14–25. [Google Scholar] [CrossRef]
- Steele, T.A.; St Louis, E.K.; Videnovic, A.; Auger, R.R. Circadian Rhythm Sleep-Wake Disorders: A Contemporary Review of Neurobiology, Treatment, and Dysregulation in Neurodegenerative Disease. Neurotherapeutics 2021, 18, 53–74. [Google Scholar] [CrossRef]
- Hamilton, T. Influence of environmental light and melatonin upon mammary tumour inductino. Br. J. Surg. 1969, 56, 764–766. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.T.; Choo, K.B.; Hou, M.F.; Yeh, K.T.; Kuo, S.J.; Chang, J.G. Deregualted expression of the PER1, PER2, and PER3 genes in breast cancers. Carcinogenesis 2005, 26, 1241–1246. [Google Scholar] [CrossRef]
- Innominato, P.F.; Roche, V.P.; Palesh, O.G.; Ulusakarya, A.; Spiegel, D.; Levi, F.A. The circadian timing system in clinical oncology. Ann. Med. 2014, 46, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Kubatka, P.; Zubor, P.; Busselberg, D.; Kwon, T.K.; Adamek, M.; Petrovic, D.; Opatrilova, R.; Gazdikova, K.; Caprnda, M.; Rodrigo, L.; et al. Melatonin and breast cancer: Evidences from preclinical and human studies. Crit. Rev. Oncol. Hematol. 2018, 122, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Nakamura, Y.; Korkmaz, A.; Manchester, L.C.; Tan, D.X.; Sugino, N.; Reiter, R.J. Melatonin and the ovary: Physiological and pathophysiological impications. Fertil. Sterlity 2008, 92, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Marzouk, M.A.; Adhikari, S.; Wright, T.D.; Miller, B.P.; Matossian, M.D.; Elliott, S.; Wright, M.; Alzoubi, M.; Collins-Burow, B.M.; et al. Pharmacological, Mechanistic, and Pharmacokinetic Assessment of Novel Melatonin-Tamoxifen Drug Conjugates as Breast Cancer Drugs. Mol. Pharmacol. 2019, 96, 272–296. [Google Scholar] [CrossRef] [PubMed]
- Ball, L.J.; Palesh, O.; Kriegsfeld, L.J. The Pathophysiologic Role of Disrupted Circadian and Neuroendocrine Rhythms in Breast Carcinogenesis. Endocr. Rev. 2016, 37, 450–466. [Google Scholar] [CrossRef] [PubMed]
- Linder, S.; Hoogstraat, M.; Stelloo, S.; Eickhoff, N.; Schuurman, K.; de Barros, H.; Alkemade, M.; Bekers, E.M.; Severson, T.M.; Sanders, J.; et al. Drug-Induced Epigenomic Plasticity Reprograms Circadian Rhythm Regulation to Drive Prostate Cancer toward Androgen Independence. Cancer Discov. 2022, 12, 2074–2097. [Google Scholar] [CrossRef]
- Zhu, Y.; Brown, H.N.; Zhang, Y.; Stevens, R.G.; Zheng, T. Period3 structural variation: A circadian biomarker associated with breast cancer in young women. Cancer Epidemiol. Biomark. Prev. 2005, 14, 268–270. [Google Scholar] [CrossRef]
- Duffy, J.F.; Cain, S.W.; Chang, A.M.; Phillips, A.J.; Munch, M.Y.; Gronfier, C.; Wyatt, J.K.; Dijk, D.J.; Wright, K.P., Jr.; Czeisler, C.A. Sex difference in the near-24-hour insrinsic period of the human circadian timing system. Proc. Natl. Acad. Sci. USA 2011, 108, 15602–15608. [Google Scholar] [CrossRef]
- Maury, E.; Ramsey, K.M.; Bass, J. Circadian rhytms and metabolic syndrome: From experimental genetics to human disease. Circ. Res. 2010, 106, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J.F.; Zitting, K.M.; Chinoy, E.D. Aging and Circadian rhythms. Sleep. Med. Clin. 2015, 10, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.L.; McWhirter, L.; Diniz Behn, C.; Bubar, K.M.; Kaar, J.L.; Pyle, L.; Rahat, H.; Garcia-Reyes, Y.; Carreau, A.M.; Wright, K.P.; et al. Morning circadian misalignment is associated with insulin resistance in girls with obesity and polycystic ovarian syndrome. J. Clin. Endocrinol. Metab. 2019, 104, 3525–3534. [Google Scholar] [CrossRef]
- Dasari, S.S.; Archer, M.; Mohamed, N.E.; Tewari, A.K.; Figueiro, M.G.; Kypinaou, N. Circadian Rhythm Disruption as a contributor to racial disparities in prostate cancer. Cancers 2002, 14, 5116. [Google Scholar] [CrossRef]
- Smith, M.R.; Burgess, H.J.; Fogg, L.F.; Eastman, C.I. Racial differences in the human endogenous circadian period. PLoS ONE 2009, 4, e6014. [Google Scholar] [CrossRef] [PubMed]
- Ota, S.M.; Kong, X.; Hut, R.; Suchecki, D.; Meerlo, P. The impact of stress and stress hormones on endogenous clocks and circadian rhythms. Front. Neuroendocr. 2023, 63, 100931. [Google Scholar] [CrossRef]
- Walker, W.H.; Borniger, J.C. Molecular Mechanisms of Cancer-Induced Sleep Disruption. Int. J. Mol. Sci. 2019, 20, 2780. [Google Scholar] [CrossRef]
- Gaspar, L.; Scott, C.; Rotman, M.; Asbell, S.; Phillips, T.; Wasserman, T.; McKenna, W.G.; Byhardt, R. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int. J. Radiat. Oncol. Biol. Phys. 1997, 37, 745–751. [Google Scholar] [CrossRef]


| Patients | ≥80% AM Treatment % (n = 158) | ≥80% PM Treatment % (n = 79) | All Patients |
|---|---|---|---|
| Deceased | 133 (84.18%) | 71 (89.87%) | 204 (86.08%) |
| Alive | 25 (15.82%) | 8 (10.13%) | 33 (13.92%) |
| Total | 158 | 79 | 237 |
| Sex | ≥80% AM | ≥80% PM | All ≥ 80% |
| Female | 100 (63.29%) | 47 (59.49%) | 147 (62.03%) |
| Male | 58 (36.71%) | 32 (40.51%) | 90 (37.97%) |
| Age at Dx | ≥80% AM | ≥80% PM | All ≥ 80% |
| <65 Years | 76 (48.10%) | 41 (51.90%) | 117 (49.37%) |
| ≥65 Years | 82 (51.90%) | 38 (48.10%) | 120 (50.63%) |
| Primary Site | ≥80% AM | ≥80% PM | All ≥ 80% |
| Breast | 28 (17.72%) | 15 (18.99%) | 43 (18.14%) |
| Lung | 84 (53.16%) | 43 (54.43%) | 127 (53.59%) |
| Other | 39 (24.68%) | 19 (24.05%) | 58 (24.47%) |
| Unknown | 7 (4.43%) | 2 (2.53%) | 9 (3.80%) |
| Primary Controlled | ≥80% AM | ≥80% PM | All ≥ 80% |
| Yes | 43 (27.22%) | 31 (39.24%) | 74 (31.22%) |
| No | 108 (68.35%) | 47 (59.49%) | 155 (65.40%) |
| N/A | 7 (4.43%) | 1 (1.27%) | 8 (3.38%) |
| KPS Index | ≥80% AM | ≥80% PM | All ≥ 80% |
| ≥70 | 97 (61.39%) | 47 (59.49%) | 144 (60.76%) |
| <70 | 33 (20.89%) | 16 (20.25%) | 49 (20.68%) |
| N/A | 28 (17.72%) | 16 (20.25%) | 44 (18.57%) |
| RPA Group | ≥80% AM | ≥80% PM | All ≥ 80% |
| Class 1 | 17 (10.76%) | 16 (20.25%) | 33 (13.92%) |
| Class 2 | 80 (50.63%) | 31 (39.24%) | 111 (46.84%) |
| Class 3 | 32 (20.25%) | 17 (21.52%) | 49 (20.68%) |
| N/A | 29 (18.35%) | 15 (18.99%) | 44 (18.57%) |
| Race/Ethnicity | ≥80% AM | ≥80% PM | All ≥ 80% |
| Asian | 4 (2.53%) | 1 (1.27%) | 5 (2.11%) |
| Black | 24 (15.19%) | 15 (18.99%) | 39 (16.46%) |
| Hispanic | 4 (2.53%) | 1 (1.27%) | 5 (2.11%) |
| White | 111 (70.25%) | 53 (67.09%) | 164 (69.25%) |
| N/A | 15 (9.49%) | 9 (11.39%) | 24 (10.13%) |
| BMI | ≥80% AM | ≥80% PM | All ≥ 80% |
| <25 | 60 (37.97%) | 31 (39.24%) | 91 (38.40%) |
| 25–30 | 48 (30.38%) | 21 (26.58%) | 69 (29.11%) |
| >30 | 38 (24.05%) | 22 (27.85%) | 60 (25.32%) |
| N/A | 12 (7.59%) | 5 (6.33%) | 17 (7.17%) |
| Patient Zip Code Median Income | ≥80% AM | ≥80% PM | All ≥ 80% |
| <$57,550 K | 52 (32.91%) | 27 (34.18%) | 79 (33.33%) |
| >$57,500 K | 106 (67.09%) | 52 (65.82%) | 158 (66.67%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nelson, N.G.; Burke, S.E.; Cappelli, L.; Matlack, L.E.; Smith, A.P.; Francois, N.; Lombardo, J.F.; Shah, Y.B.; Wen, K.-Y.; Shafi, A.A.; et al. Temporal Considerations in Brain Metastases Radiation Therapy: The Intersection of Chronobiology and Patient Profiles. Clocks & Sleep 2024, 6, 200-210. https://doi.org/10.3390/clockssleep6010014
Nelson NG, Burke SE, Cappelli L, Matlack LE, Smith AP, Francois N, Lombardo JF, Shah YB, Wen K-Y, Shafi AA, et al. Temporal Considerations in Brain Metastases Radiation Therapy: The Intersection of Chronobiology and Patient Profiles. Clocks & Sleep. 2024; 6(1):200-210. https://doi.org/10.3390/clockssleep6010014
Chicago/Turabian StyleNelson, Nicolas G., Sara E. Burke, Louis Cappelli, Lauren E. Matlack, Alexandria P. Smith, Noelle Francois, Joseph F. Lombardo, Yash B. Shah, Kuang-Yi Wen, Ayesha A. Shafi, and et al. 2024. "Temporal Considerations in Brain Metastases Radiation Therapy: The Intersection of Chronobiology and Patient Profiles" Clocks & Sleep 6, no. 1: 200-210. https://doi.org/10.3390/clockssleep6010014






