A Protocol for Evaluating Digital Technology for Monitoring Sleep and Circadian Rhythms in Older People and People Living with Dementia in the Community
Abstract
:1. Introduction
1.1. The Need for Technology to Monitor Sleep and Circadian Rhythms Longitudinally
1.2. Gold-Standard Assessments of Sleep and Circadian Rhythms: Advantages and Disadvantages
1.3. Technology for Monitoring Sleep and Circadian Rhythms at Home: Current and Novel Approaches
1.4. Evaluating Technology: The Issues
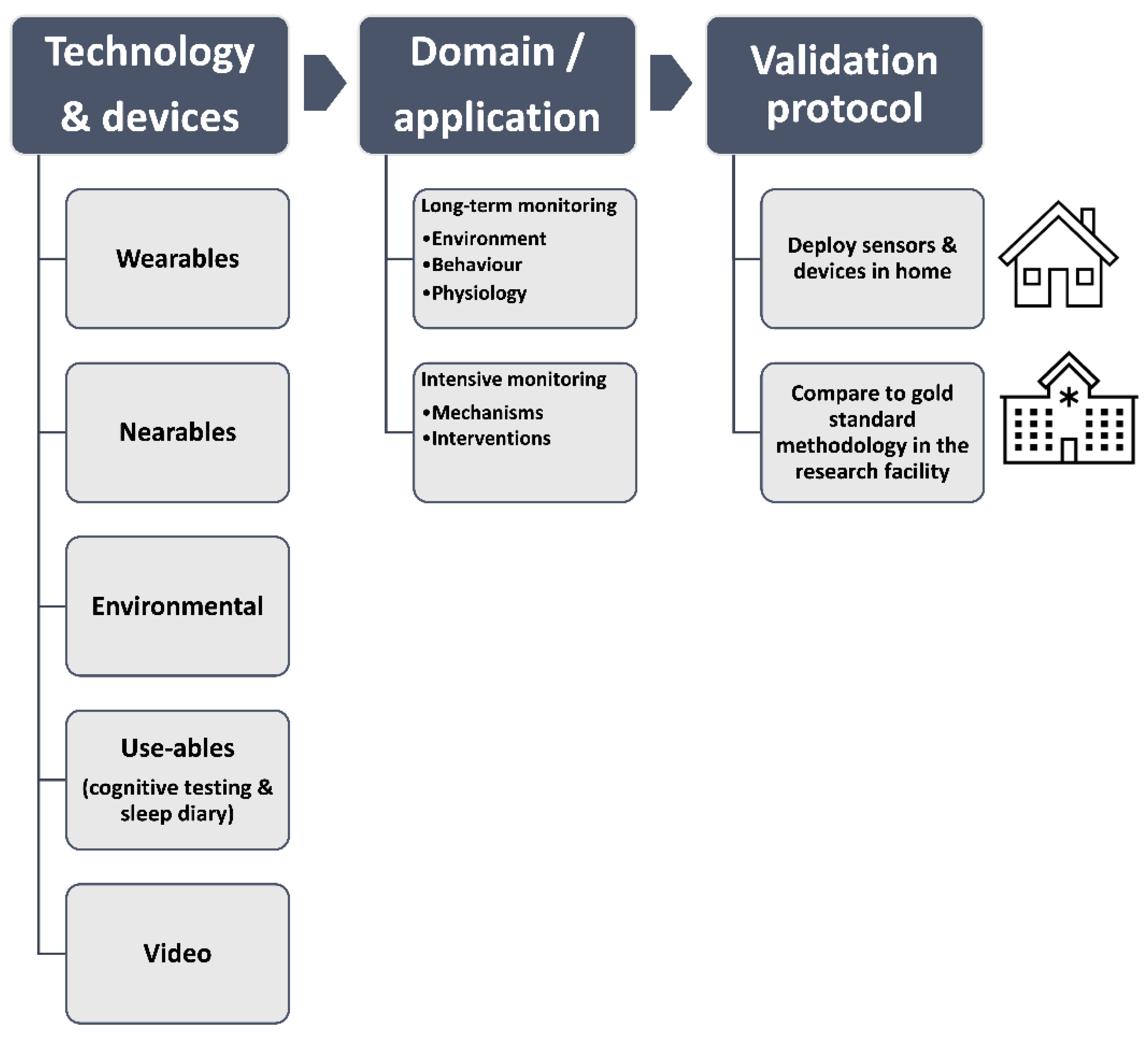
1.5. Our Approach to Technology Evaluation
1.5.1. Selection of Participants
1.5.2. Selection of Technology to Evaluate
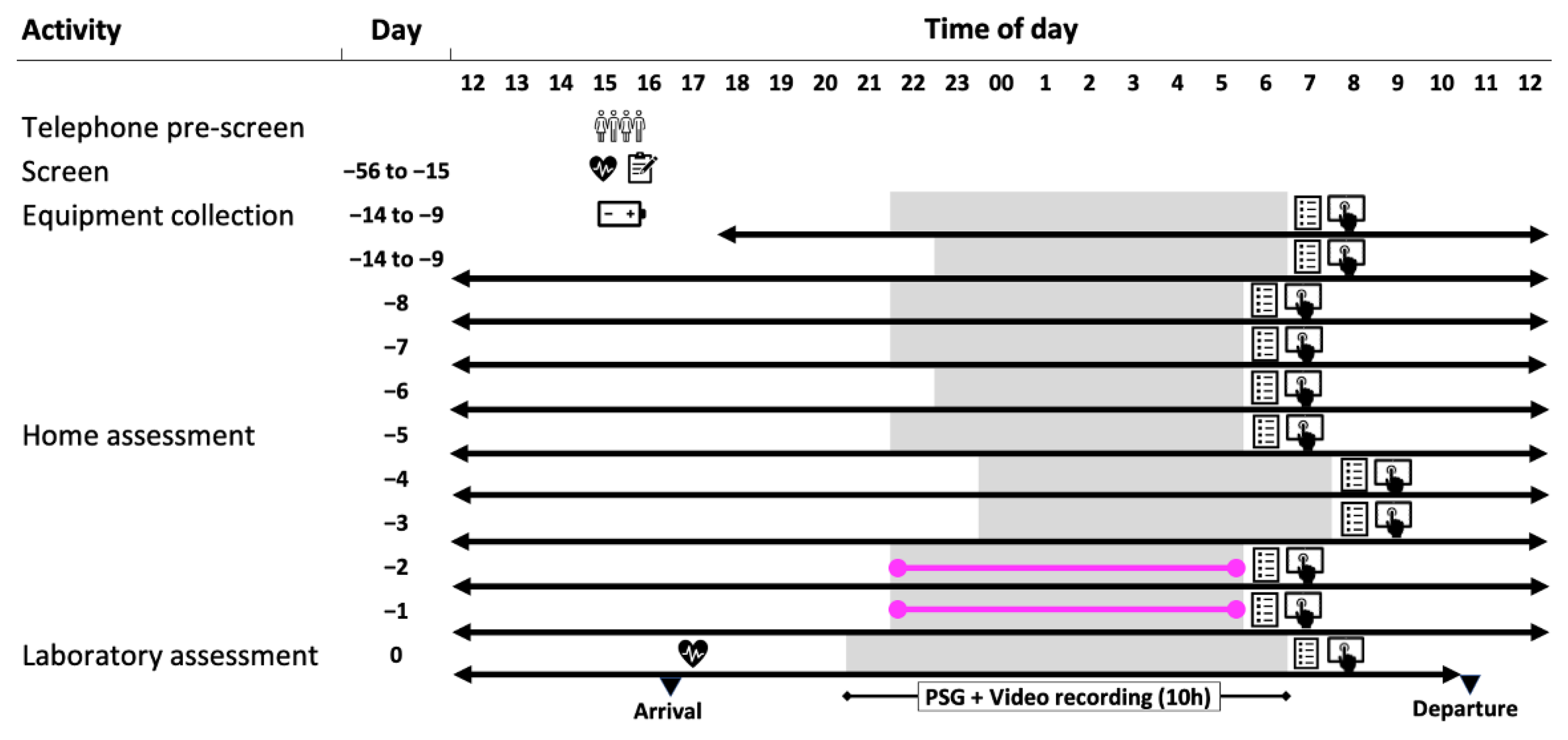
1.5.3. Study Protocol
2. Results
2.1. Recruitment and Participant Characterisation
2.2. Data Completeness
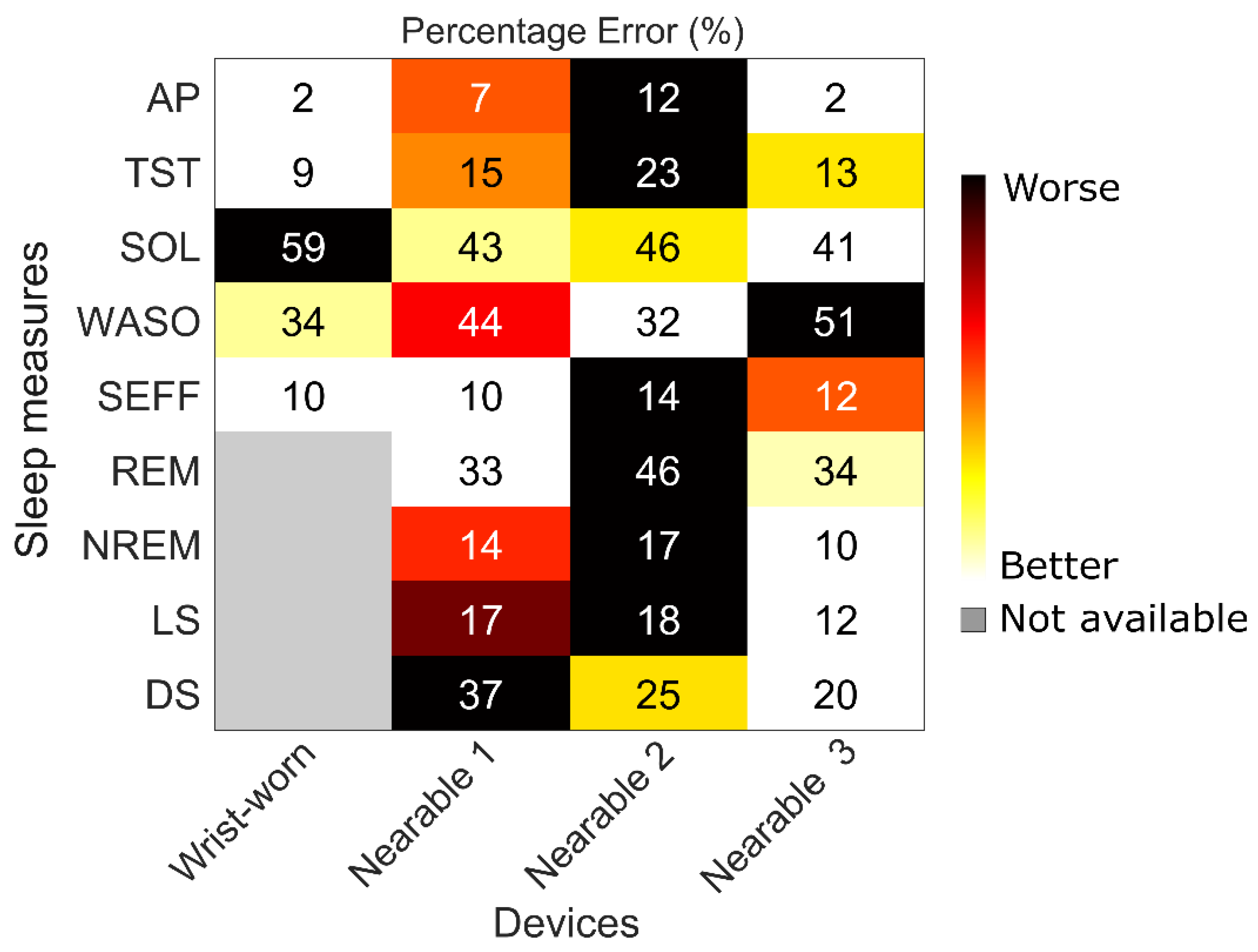
2.3. Device Acceptability
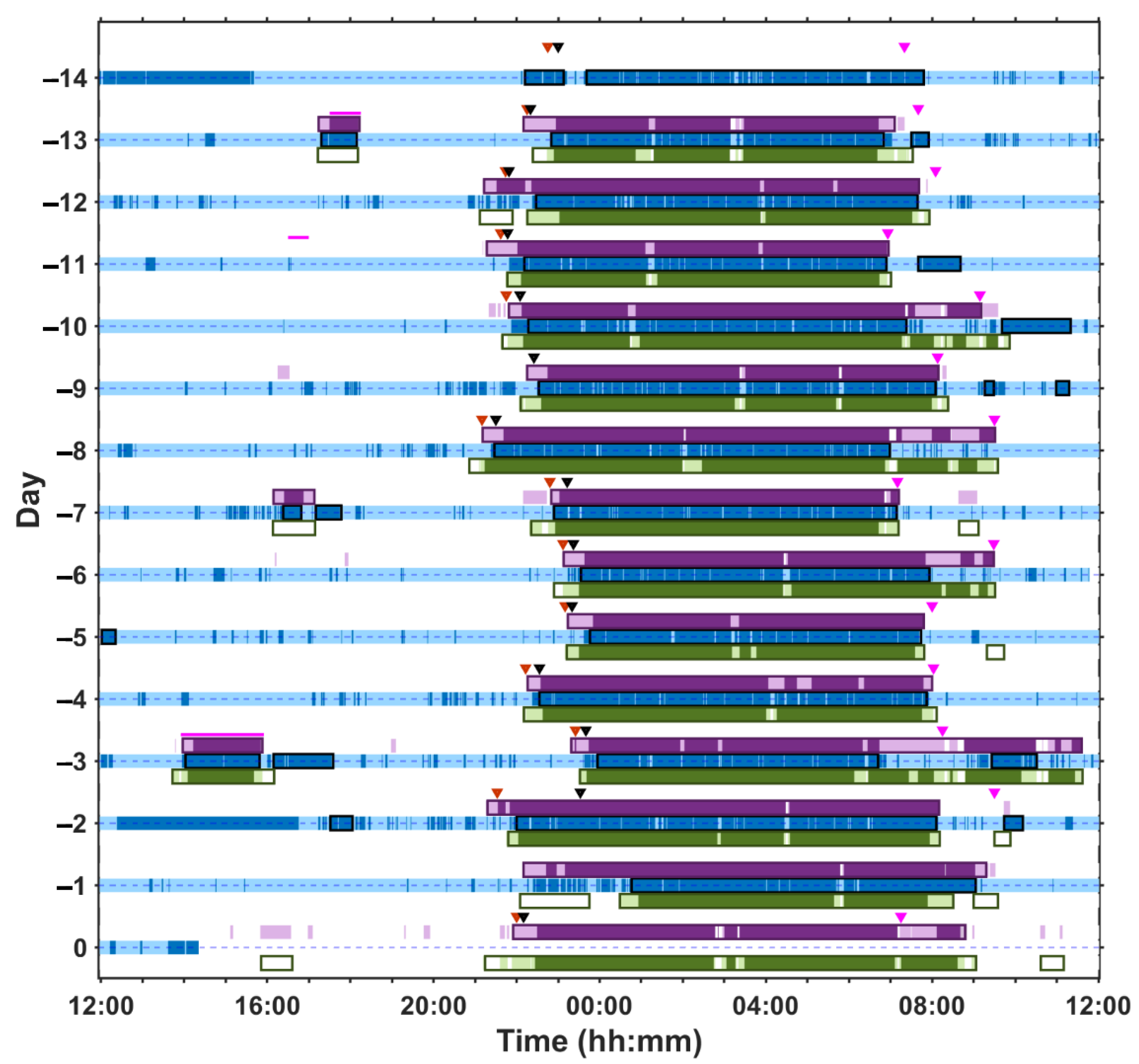
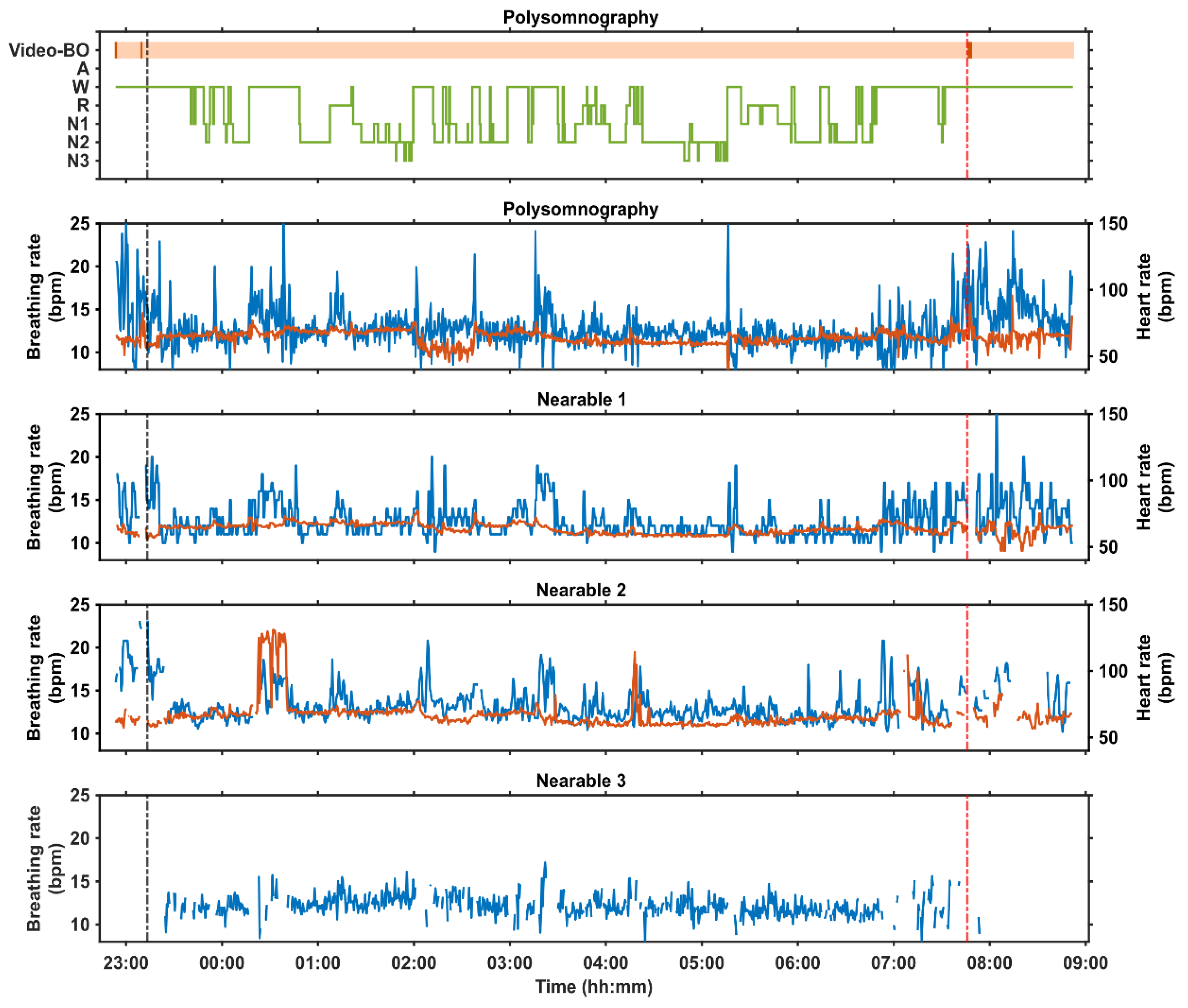
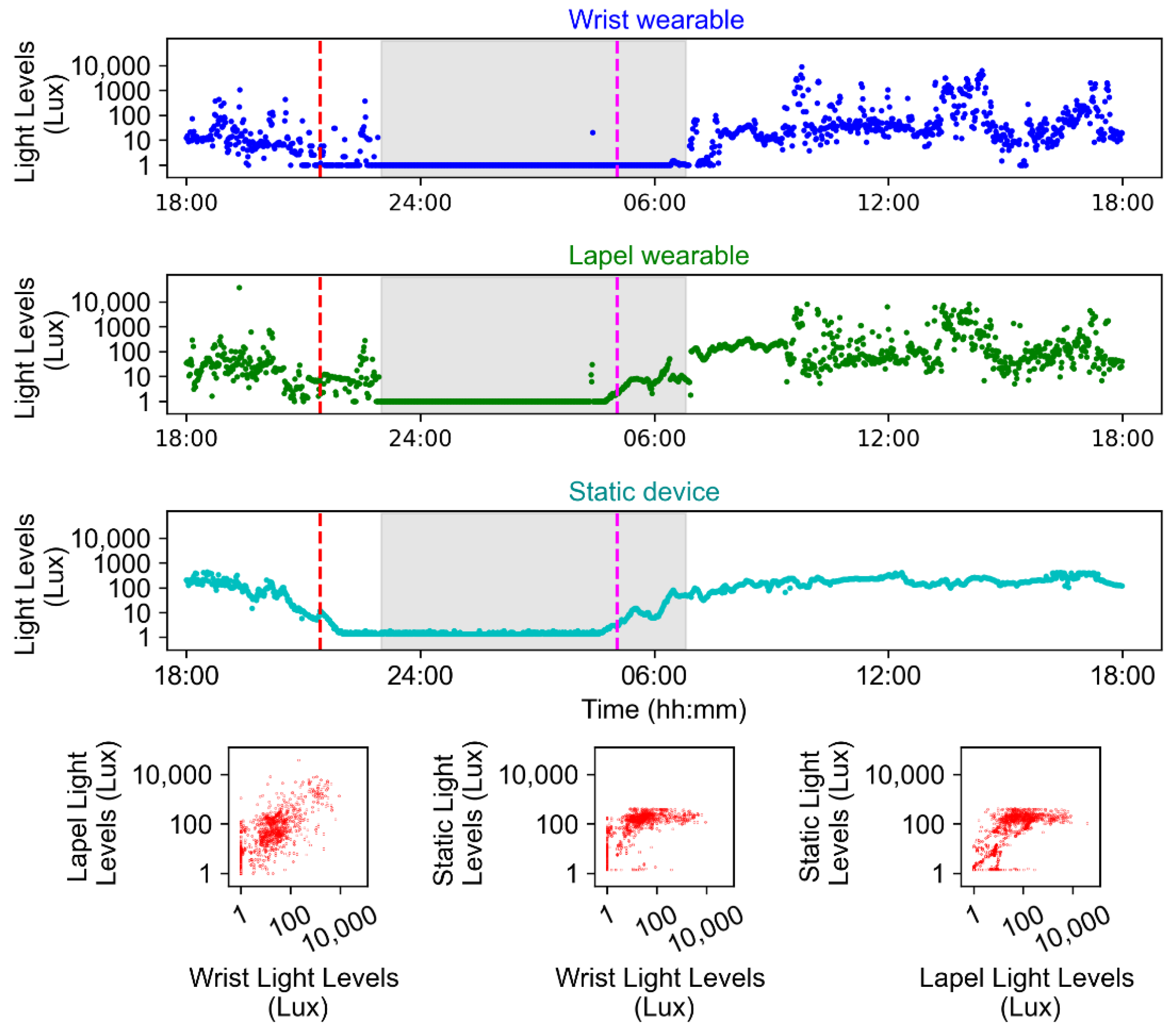
2.4. Examples of At-Home and In-Laboratory Recordings
3. Discussion
4. Materials and Methods
4.1. Study Design
4.1.1. Study Conduct
4.1.2. Participants
4.1.3. Longitudinal Monitoring At-Home
4.1.4. Overnight Laboratory Session
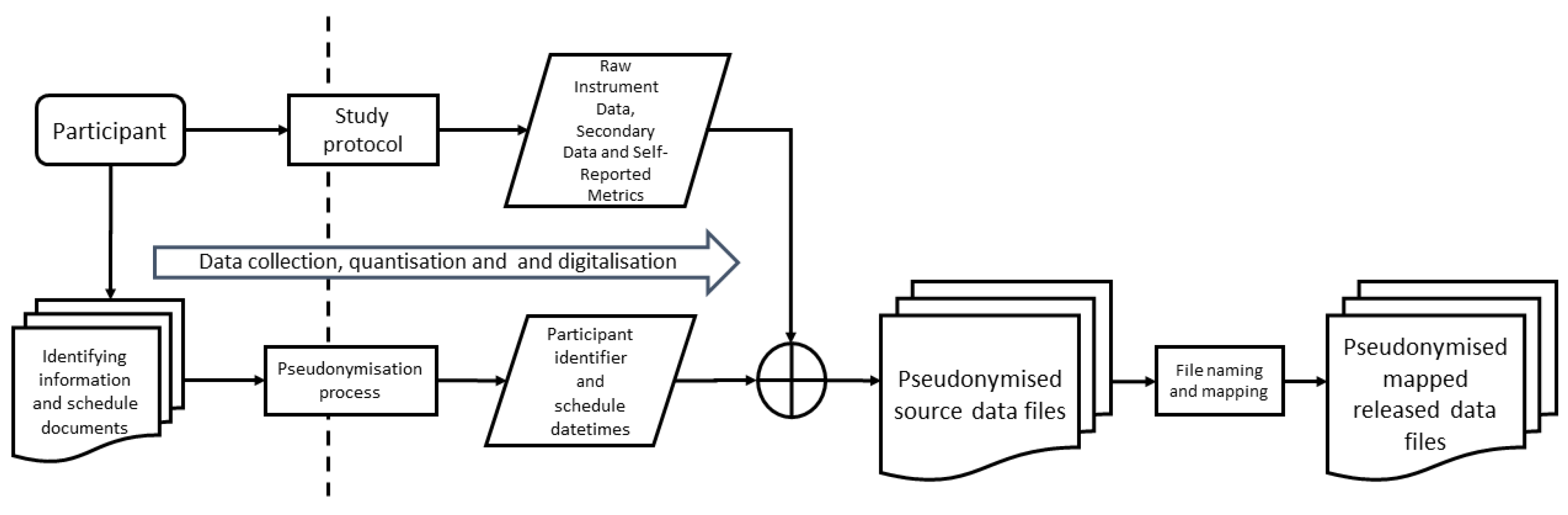
4.1.5. Device and Data Management
Device Allocation
Device Set-Up and Synchronisation
Data Acquisition
Data Mapping and File Name Convention
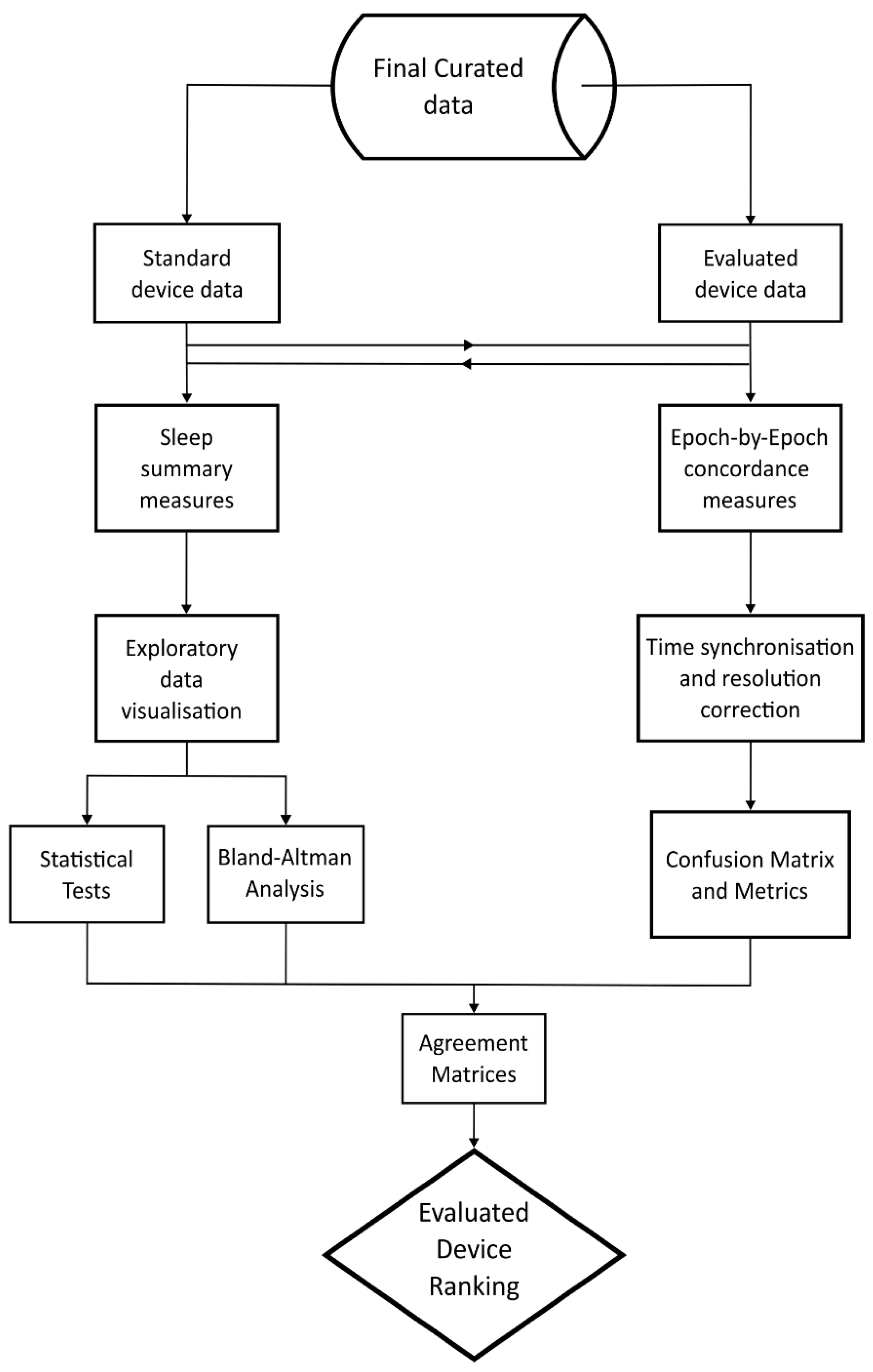
4.1.6. Data Processing and Analysis
4.1.7. Sleep Summary Measures
4.1.8. Epoch-By-Epoch (EBE) Concordance
4.1.9. Environmental Measures
4.1.10. Quality Assurance and Mitigating Issues Troubleshooting
4.1.11. Expertise Needed to Implement the Protocol
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mander, B.A.; Dave, A.; Lui, K.K.; Sprecher, K.E.; Berisha, D.; Chappel-Farley, M.G.; Chen, I.Y.; Riedner, B.A.; Heston, M.; Suridjan, I.; et al. Inflammation, tau pathology, and synaptic integrity associated with sleep spindles and memory prior to beta-amyloid positivity. Sleep 2022, 45, zsac135. [Google Scholar] [CrossRef] [PubMed]
- Meyer, N.; Harvey, A.G.; Lockley, S.W.; Dijk, D.J. Circadian rhythms and disorders of the timing of sleep. Lancet 2022, 400, 1061–1078. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.M.; Sletten, T.L.; Magee, M.; Gordon, C.; Lovato, N.; Bartlett, D.J.; Kennaway, D.J.; Lack, L.C.; Grunstein, R.R.; Lockley, S.W.; et al. Prevalence of Circadian Misalignment and Its Association with Depressive Symptoms in Delayed Sleep Phase Disorder. Sleep 2017, 40, zsw002. [Google Scholar] [CrossRef]
- Nedergaard, M.; Goldman, S.A. Glymphatic failure as a final common pathway to dementia. Science 2020, 370, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Winsky-Sommerer, R.; de Oliveira, P.; Loomis, S.; Wafford, K.; Dijk, D.J.; Gilmour, G. Disturbances of sleep quality, timing and structure and their relationship with other neuropsychiatric symptoms in Alzheimer’s disease and schizophrenia: Insights from studies in patient populations and animal models. Neurosci. Biobehav. Rev. 2019, 97, 112–137. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Holtzman, D.M. Bidirectional relationship between sleep and Alzheimer’s disease: Role of amyloid, tau, and other factors. Neuropsychopharmacology 2020, 45, 104–120. [Google Scholar] [CrossRef] [PubMed]
- Andre, C.; Champetier, P.; Rehel, S.; Kuhn, E.; Touron, E.; Ourry, V.; Landeau, B.; Le Du, G.; Mezenge, F.; Segobin, S.; et al. Rapid Eye Movement Sleep, Neurodegeneration, and Amyloid Deposition in Aging. Ann. Neurol. 2023, 93, 979–990. [Google Scholar] [CrossRef]
- Sani, T.P.; Bond, R.L.; Marshall, C.R.; Hardy, C.J.D.; Russell, L.L.; Moore, K.M.; Slattery, C.F.; Paterson, R.W.; Woollacott, I.O.C.; Wendi, I.P.; et al. Sleep symptoms in syndromes of frontotemporal dementia and Alzheimer’s disease: A proof-of-principle behavioural study. Eneurologicalsci 2019, 17, 100212. [Google Scholar] [CrossRef]
- Seda, G.; Matwiyoff, G.; Parrish, J.S. Effects of Obstructive Sleep Apnea and CPAP on Cognitive Function. Curr. Neurol. Neurosci. Rep. 2021, 21, 32. [Google Scholar] [CrossRef]
- Aini, N.; Chu, H.; Banda, K.J.; Chen, R.; Lee, T.Y.; Pien, L.C.; Liu, D.; Lai, Y.J.; Kang, X.L.; Chou, K.R. Prevalence of sleep-related breathing disorders and associated risk factors among people with dementia: A meta-analysis. Sleep. Med. 2023, 103, 51–61. [Google Scholar] [CrossRef]
- Lin, Y.Q.; Chen, S.D. RBD: A red flag for cognitive impairment in Parkinson’s disease? Sleep. Med. 2018, 44, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, Y.; Johnson, F.U.; Lei, L.; Choi, S.E.; Antonucci, T.C.; Robinson-Lane, S.G. Characteristics and Predictors of Sleep Among Spousal Care Dyads Living with Chronic Conditions. J. Gerontol. B Psychol. Sci. Soc. Sci. 2023, 78, S38–S47. [Google Scholar] [CrossRef] [PubMed]
- Van Hout, E.; Contreras, M.; Mioshi, E.; Kishita, N. Examining the Impact of Different Components of Sleep Quality on Anxiety Among Family Carers of People with Dementia. J. Geriatr. Psychiatry Neurol. 2023, 36, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Cha, E. Effect of Perceived Stress on Health-Related Quality of Life among Primary Caregiving Spouses of Patients with Severe Dementia: The Mediating Role of Depression and Sleep Quality. Int. J. Env. Res. Public. Health 2022, 19, 7962. [Google Scholar] [CrossRef]
- Benca, R.; Herring, W.J.; Khandker, R.; Qureshi, Z.P. Burden of Insomnia and Sleep Disturbances and the Impact of Sleep Treatments in Patients with Probable or Possible Alzheimer’s Disease: A Structured Literature Review. J. Alzheimers Dis. 2022, 86, 83–109. [Google Scholar] [CrossRef]
- Gibson, R.H.; Gander, P.H.; Jones, L.M. Understanding the sleep problems of people with dementia and their family caregivers. Dementia 2014, 13, 350–365. [Google Scholar] [CrossRef]
- Lee, D.R.; Thomas, A.J. Sleep in dementia and caregiving--assessment and treatment implications: A review. Int. Psychogeriatr. 2011, 23, 190–201. [Google Scholar] [CrossRef]
- Gibson, A.A.; Gale, J.; Stamatakis, E.; Lindley, R.I.; Fontana, L.; Cistulli, P.A.; Nassar, N. Impact of lifestyle risk factors on admission to nursing home care: A cohort study of 127 108 people aged 60 years and over. J. Epidemiol. Community Health 2023, 77, 744–751. [Google Scholar] [CrossRef]
- Wong, A.T.Y.; Reeves, G.K.; Floud, S. Total sleep duration and daytime napping in relation to dementia detection risk: Results from the Million Women Study. Alzheimer’s Dement. 2023, 19, 4978–4986. [Google Scholar] [CrossRef]
- Balouch, S.; Dijk, D.A.D.; Rusted, J.; Skene, S.S.; Tabet, N.; Dijk, D.J. Night-to-night variation in sleep associates with day-to-day variation in vigilance, cognition, memory, and behavioral problems in Alzheimer’s disease. Alzheimer’s Dement. 2022, 14, e12303. [Google Scholar] [CrossRef]
- Berry, R.; Brooks, R.; Gamaldo, C.E.; Harding, S.; Lloyd, R.; Marcus, C.; Vaughn, B. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications; The Americal Academy of Sleep Medicine: Darien, IL, USA, 2016. [Google Scholar]
- Dijk, D.J.; Duffy, J.F. Novel Approaches for Assessing Circadian Rhythmicity in Humans: A Review. J. Biol. Rhythm. 2020, 35, 421–438. [Google Scholar] [CrossRef]
- Skeldon, A.C.; Rodriguez Garcia, T.; Cleator, S.F.; della Monica, C.; Ravindran, K.K.G.; Revell, V.L.; Dijk, D.J. Method to determine whether sleep phenotypes are driven by endogenous circadian rhythms or environmental light by combining longitudinal data and personalised mathematical models. PLoS Comput. Biol. 2023, 19, e1011743. [Google Scholar] [CrossRef] [PubMed]
- Blume, C.; Santhi, N.; Schabus, M. ‘nparACT’ package for R: A free software tool for the non-parametric analysis of actigraphy data. MethodsX 2016, 3, 430–435. [Google Scholar] [CrossRef]
- Sletten, T.L.; Vincenzi, S.; Redman, J.R.; Lockley, S.W.; Rajaratnam, S.M. Timing of sleep and its relationship with the endogenous melatonin rhythm. Front. Neurol. 2010, 1, 137. [Google Scholar] [CrossRef] [PubMed]
- Burgess, H.J.; Wyatt, J.K.; Park, M.; Fogg, L.F. Home Circadian Phase Assessments with Measures of Compliance Yield Accurate Dim Light Melatonin Onsets. Sleep 2015, 38, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Lockley, S.W.; Skene, D.J.; Arendt, J.; Tabandeh, H.; Bird, A.C.; Defrance, R. Relationship between melatonin rhythms and visual loss in the blind. J. Clin. Endocrinol. Metab. 1997, 82, 3763–3770. [Google Scholar] [CrossRef] [PubMed]
- Lockley, S.W.; Skene, D.J.; James, K.; Thapan, K.; Wright, J.; Arendt, J. Melatonin administration can entrain the free-running circadian system of blind subjects. J. Endocrinol. 2000, 164, R1–R6. [Google Scholar] [CrossRef]
- Stone, J.E.; McGlashan, E.M.; Quin, N.; Skinner, K.; Stephenson, J.J.; Cain, S.W.; Phillips, A.J.K. The Role of Light Sensitivity and Intrinsic Circadian Period in Predicting Individual Circadian Timing. J. Biol. Rhythm. 2020, 35, 628–640. [Google Scholar] [CrossRef]
- Menczel Schrire, Z.; Gordon, C.J.; Palmer, J.R.; Murray, J.; Hickie, I.; Rogers, N.L.; Lewis, S.J.; Terpening, Z.; Pye, J.E.; Naismith, S.L.; et al. Actigraphic and melatonin alignment in older adults with varying dementia risk. Chronobiol. Int. 2023, 40, 91–102. [Google Scholar] [CrossRef]
- Duffy, J.F.; Zitting, K.M.; Chinoy, E.D. Aging and Circadian Rhythms. Sleep. Med. Clin. 2015, 10, 423–434. [Google Scholar] [CrossRef]
- Charman, W.N. Age, lens transmittance, and the possible effects of light on melatonin suppression. Ophthalmic Physiol. Opt. 2003, 23, 181–187. [Google Scholar] [CrossRef]
- Ancoli-Israel, S.; Klauber, M.R.; Jones, D.W.; Kripke, D.F.; Martin, J.; Mason, W.; Pat-Horenczyk, R.; Fell, R. Variations in circadian rhythms of activity, sleep, and light exposure related to dementia in nursing-home patients. Sleep 1997, 20, 18–23. [Google Scholar]
- de Zambotti, M.; Menghini, L.; Grandner, M.A.; Redline, S.; Zhang, Y.; Wallace, M.L.; Buxton, O.M. Rigorous performance evaluation (previously, “validation”) for informed use of new technologies for sleep health measurement. Sleep. Health 2022, 8, 263–269. [Google Scholar] [CrossRef]
- Green, S.F.; Frame, T.; Banerjee, L.V.; Gimson, A.; Blackman, J.; Morrison, H.; Lloyd, K.; Rudd, S.; Frederick Fotherby, W.G.; Bartsch, U.; et al. A systematic review of the validity of non-invasive sleep-measuring devices in mid-to-late life adults: Future utility for Alzheimer’s disease research. Sleep Med. Rev. 2022, 65, 101665. [Google Scholar] [CrossRef]
- Depner, C.M.; Cheng, P.C.; Devine, J.K.; Khosla, S.; de Zambotti, M.; Robillard, R.; Vakulin, A.; Drummond, S.P.A. Wearable technologies for developing sleep and circadian biomarkers: A summary of workshop discussions. Sleep 2020, 43, zsz254. [Google Scholar] [CrossRef]
- Chinoy, E.D.; Cuellar, J.A.; Huwa, K.E.; Jameson, J.T.; Watson, C.H.; Bessman, S.C.; Hirsch, D.A.; Cooper, A.D.; Drummond, S.P.A.; Markwald, R.R. Performance of seven consumer sleep-tracking devices compared with polysomnography. Sleep 2021, 44, zsaa291. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Li, Y.; Rueschman, M.N.; Winkelman, J.W.; Ellenbogen, J.M.; Solet, J.M.; Dulin, H.; Berkman, L.F.; Buxton, O.M. Measuring sleep: Accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep 2013, 36, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.T.; McCrae, C.S.; Cheung, J.; Martin, J.L.; Harrod, C.G.; Heald, J.L.; Carden, K.A. Use of Actigraphy for the Evaluation of Sleep Disorders and Circadian Rhythm Sleep-Wake Disorders: An American Academy of Sleep Medicine Systematic Review, Meta-Analysis, and GRADE Assessment. J. Clin. Sleep. Med. 2018, 14, 1209–1230. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, G.K.K.; della Monica, C.; Atzori, G.; Lambert, D.; Hassanin, H.; Revell, V.; Dijk, D.-J. Three Contactless Sleep Technologies Compared to Actigraphy and Polysomnography in a Heterogenous Group of Older Men and Women in a Model of Mild Sleep Disturbance: A Sleep Laboratory Study. JMIR Mhealth Uhealth 2023, 11, e46338. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, K.K.G.; della Monica, C.; Atzori, G.; Lambert, D.; Hassanin, H.; Revell, V.; Dijk, D.J. Contactless and Longitudinal Monitoring of Nocturnal Sleep and Daytime Naps in Older Men and Women: A Digital Health Technology Evaluation Study. Sleep 2023, 46, zsad194. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, K.K.G.; della Monica, C.; Atzori, G.; Lambert, D.; Revell, V.; Dijk, D.J. Evaluating the Empatica E4 Derived Heart Rate and Heart Rate Variability Measures in Older Men and Women. In Proceedings of the 2022 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Glasgow, UK, 11–15 July 2022; pp. 3370–3373. [Google Scholar] [CrossRef]
- Hammour, G.; Atzori, G.; della Monica, C.; Ravindran, K.K.G.; Revell, V.; Dijk, D.J.; Mandic, D.P. Hearables: Automatic Sleep Scoring from Single-Channel Ear-EEG in Older Adults. In Proceedings of the 2023 45th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Sydney, Australia, 24–27 July 2023; pp. 1–4. [Google Scholar] [CrossRef]
- Berry, R.B.; Wagner, M.H. Sleep Medicine Pearls; Elsevier Health Sciences: New York, NY, USA, 2015. [Google Scholar]
- NHS Digital. Mean Body Mass Index (BMI) of Adults in England 2021, by Gender and Age (in Kilograms Per Meters Squared) [Graph]. Available online: https://www.statista.com/statistics/375886/adult-s-body-mass-index-by-gender-and-age-in-england/ (accessed on 4 October 2022).
- Arnal, P.J.; Thorey, V.; Debellemaniere, E.; Ballard, M.E.; Bou Hernandez, A.; Guillot, A.; Jourde, H.; Harris, M.; Guillard, M.; Van Beers, P.; et al. The Dreem Headband compared to polysomnography for electroencephalographic signal acquisition and sleep staging. Sleep 2020, 43, zsaa097. [Google Scholar] [CrossRef]
- Toften, S.; Pallesen, S.; Hrozanova, M.; Moen, F.; Gronli, J. Validation of sleep stage classification using non-contact radar technology and machine learning (Somnofy(R)). Sleep Med. 2020, 75, 54–61. [Google Scholar] [CrossRef]
- Chinoy, E.D.; Cuellar, J.A.; Jameson, J.T.; Markwald, R.R. Performance of Four Commercial Wearable Sleep-Tracking Devices Tested Under Unrestricted Conditions at Home in Healthy Young Adults. Nat. Sci. Sleep 2022, 14, 493–516. [Google Scholar] [CrossRef]
- Kholghi, M.; Szollosi, I.; Hollamby, M.; Bradford, D.; Zhang, Q. A validation study of a ballistocardiograph sleep tracker against polysomnography. J. Clin. Sleep Med. 2022, 18, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Edouard, P.; Campo, D.; Bartet, P.; Yang, R.Y.; Bruyneel, M.; Roisman, G.; Escourrou, P. Validation of the Withings Sleep Analyzer, an under-the-mattress device for the detection of moderate-severe sleep apnea syndrome. J. Clin. Sleep Med. 2021, 17, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.A.; Toedebusch, C.D.; Redrick, T.; Freund, D.; McLeland, J.S.; Morris, J.C.; Holtzman, D.M.; Lucey, B.P. Comparison of single-channel EEG, actigraphy, and sleep diary in cognitively normal and mildly impaired older adults. Sleep Adv. 2020, 1, zpaa006. [Google Scholar] [CrossRef] [PubMed]
- Soreq, E.; Kolanko, M.A.; della Monica, C.; Ravindran, K.K.G.; Revell, V.L.; de Villèle, P.; Barnaghi, P.; Dijk, D.-J.; Sharp, D.J.; CRT group. Monitoring abnormal nocturnal behaviour in the homes of patients living with dementia. Alzheimer’s Dement. 2022, 18, e067936. [Google Scholar] [CrossRef]
- Molloy, D.W.; Standish, T.I. A guide to the standardized Mini-Mental State Examination. Int. Psychogeriatr. 1997, 9 (Suppl. S1), 87–94; discussion 143–150. [Google Scholar] [CrossRef] [PubMed]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Lawton, M.P.; Brody, E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef]
- Avery, K.; Donovan, J.; Peters, T.J.; Shaw, C.; Gotoh, M.; Abrams, P. ICIQ: A brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol. Urodyn. 2004, 23, 322–330. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Logsdon, R.G.; Gibbons, L.E.; McCurry, S.M.; Teri, L. Assessing quality of life in older adults with cognitive impairment. Psychosom. Med. 2002, 64, 510–519. [Google Scholar] [CrossRef]
- Nelson, H.; Willison, J. National Adult Reading Test (NART) Manual; NFER-Nelson: Windsor, UK, 1982. [Google Scholar]
- Netzer, N.C.; Stoohs, R.A.; Netzer, C.M.; Clark, K.; Strohl, K.P. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann. Intern. Med. 1999, 131, 485–491. [Google Scholar] [CrossRef]
- Horne, J.A.; Ostberg, O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol. 1976, 4, 97–110. [Google Scholar]
- Carney, C.E.; Buysse, D.J.; Ancoli-Israel, S.; Edinger, J.D.; Krystal, A.D.; Lichstein, K.L.; Morin, C.M. The consensus sleep diary: Standardizing prospective sleep self-monitoring. Sleep 2012, 35, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Bannon, A.; Rapeaux, A.; Constandinou, T.G. Tiresias: A low-cost networked UWB radar system for in-home monitoring of dementia patients. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Virtual, Mexico, 1–5 November 2021; pp. 7068–7072. [Google Scholar] [CrossRef]
- Malek-Ahmadi, M.; Su, Y.; Ghisays, V.; Luo, J.; Devadas, V.; Chen, Y.; Lee, W.; Protas, H.; Chen, K.; Zetterberg, H.; et al. Plasma NfL is associated with the APOE epsilon4 allele, brain imaging measurements of neurodegeneration, and lower recall memory scores in cognitively unimpaired late-middle-aged and older adults. Alzheimers Res. Ther. 2023, 15, 74. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K.; Galasko, D.; Perneczky, R.; Quevenco, F.C.; van der Flier, W.M.; Akinwonmi, A.; Carboni, M.; Jethwa, A.; Suridjan, I.; Zetterberg, H. The potential clinical value of plasma biomarkers in Alzheimer’s disease. Alzheimer’s Dement. 2023, 19, 5805–5816. [Google Scholar] [CrossRef] [PubMed]
- Mattsson-Carlgren, N.; Janelidze, S.; Palmqvist, S.; Cullen, N.; Svenningsson, A.L.; Strandberg, O.; Mengel, D.; Walsh, D.M.; Stomrud, E.; Dage, J.L.; et al. Longitudinal plasma p-tau217 is increased in early stages of Alzheimer’s disease. Brain 2020, 143, 3234–3241. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Chen, B.; Dai, Y.; Li, Y. A meta-analysis of the first-night effect in healthy individuals for the full age spectrum. Sleep Med. 2022, 89, 159–165. [Google Scholar] [CrossRef]
- Martin, J.L.; Hakim, A.D. Wrist actigraphy. Chest 2011, 139, 1514–1527. [Google Scholar] [CrossRef] [PubMed]
- Menghini, L.; Cellini, N.; Goldstone, A.; Baker, F.C.; de Zambotti, M. A standardized framework for testing the performance of sleep-tracking technology: Step-by-step guidelines and open-source code. Sleep 2021, 44, zsaa170. [Google Scholar] [CrossRef] [PubMed]
- Haghayegh, S.; Kang, H.A.; Khoshnevis, S.; Smolensky, M.H.; Diller, K.R. A comprehensive guideline for Bland-Altman and intra class correlation calculations to properly compare two methods of measurement and interpret findings. Physiol. Meas. 2020, 41, 055012. [Google Scholar] [CrossRef] [PubMed]
- Chicco, D.; Warrens, M.J.; Jurman, G. The Matthews Correlation Coefficient (MCC) is More Informative Than Cohen’s Kappa and Brier Score in Binary Classification Assessment. IEEE Access 2021, 9, 78368–78381. [Google Scholar] [CrossRef]
- Lucas, R.J.; Peirson, S.N.; Berson, D.M.; Brown, T.M.; Cooper, H.M.; Czeisler, C.A.; Figueiro, M.G.; Gamlin, P.D.; Lockley, S.W.; O’Hagan, J.B.; et al. Measuring and using light in the melanopsin age. Trends Neurosci. 2014, 37, 1–9. [Google Scholar] [CrossRef]
- Brown, T.M.; Brainard, G.C.; Cajochen, C.; Czeisler, C.A.; Hanifin, J.P.; Lockley, S.W.; Lucas, R.J.; Munch, M.; O’Hagan, J.B.; Peirson, S.N.; et al. Recommendations for daytime, evening, and nighttime indoor light exposure to best support physiology, sleep, and wakefulness in healthy adults. PLoS Biol. 2022, 20, e3001571. [Google Scholar] [CrossRef]


| Location | At Home/in Lab | Study—Cognitively Intact | Study—PLWD & Study Partner |
|---|---|---|---|
| Wearables | |||
| Wrist | At home & in Lab | Actiwatch Spectrum (Philips Respironics, Bend, OR, USA): activity; sleep estimates; white, red, green, and blue light. Withings Steel watch (Withings, Issy-les-Moulineaux, France): activity, sleep estimates, heart rate. AX3 (Axivity, Newcastle upon Tyne, UK): activity, white light. Apple watch (Apple, Cupertino, CA, USA): activity, sleep estimates, heart rate. | Withings ScanWatch (Withings, Issy-les-Moulineaux, France): activity, sleep estimates, heart rate. AX3 or AX6 (Axivity, Newcastle upon Tyne, UK): activity, white light. |
| In Lab only | E4 (Empatica, Cambridge, MA, USA): activity, wrist temperature, heart rate. | ||
| Head | At home & in Lab | Dreem Headband 2 (Dreem, Paris, France): EEG sleep, position, activity. | Dreem Headband 2 (Dreem, Paris, France): EEG sleep, position, activity. |
| In Lab only | Marvels ear-EEG sensor prototype developed by Imperial College (Imperial College, London, UK) | Marvels ear-EEG sensor prototype developed by Imperial College (Imperial College, London, UK) Somnomedics Home Sleep Test (SOMNOmedics GmbHTM, Randersacker, Germany): EEG sleep. | |
| Nearables | |||
| Under mattress | At home & in Lab | Withings Sleep Mattress Analyser (Withings, Issy-les-Moulineaux, France): sleep, breathing rate, heart rate, activity, bed occupancy. EMFIT QS (EMFIT Limited, Vaajakoski, Finland): sleep, breathing rate, heart rate, activity, bed occupancy. | Withings Sleep Mattress Analyser (Withings, Issy-les-Moulineaux, France): sleep, breathing rate, heart rate, activity, bed occupancy. |
| In Lab only | N.A. | N.A. | |
| Nightstand | At home & in Lab | N.A. | Somnofy (VitalThings AS, Tønsberg, Norway): sleep, breathing rate, bed occupancy. |
| In Lab only | Somnofy (VitalThings AS, Tønsberg, Norway): sleep, breathing rate, bed occupancy. Tiresias networked radar system prototype (Imperial College, London, UK) [64]: sleep, breathing rate, bed occupancy. | Tiresias networked radar system prototype (Imperial College, London, UK) [64]: sleep, breathing rate, bed occupancy. | |
| Environmental | |||
| One worn at level of collarbone when awake & one hung in living space | At home & in Lab | HOBO (Tempcon, Ford, UK): white light, temperature. | HOBO (Tempcon, Ford, UK): white light, temperature. |
| In Lab only | N.A. | N.A. | |
| Usables | |||
| N.A. | At home & in Lab | Electronic tablet with Cognitron and sleep diary. | Electronic tablet with Cognitron and sleep diary. |
| In Lab only | N.A. | N.A. | |
| Video | |||
| Sleep Lab | At home & in Lab | N.A. | N.A. |
| In Lab only | Somnomedics video camera (SOMNOmedics GmbHTM, Randersacker, Germany). | Somnomedics video camera (SOMNOmedics GmbHTM, Randersacker, Germany). | |
| Potential Issue | Mitigation |
|---|---|
| Device synchronization: Devices may not be time synchronized if they were set up/downloaded/analysed on different systems. This could be due to the fact that some devices will use timestamps on local machines whereas others use UTC. This has previously been identified as being critical for epoch-by-epoch analysis [36]. | Possible solutions could be using a physiological signal, e.g., eye blinks or moving the wrist, as a synchronizing signal for cross correlation. |
| Missing data: This could occur due to equipment malfunction, user error, data signal loss, data storage insufficiency, or user error (e.g., wearing the device incorrectly, not using the device when required, forgetting to update apps, forgetting to enter data, unplugging or obstructing nearable devices). | Potential mitigations include: (a) ensure participants are thoroughly trained in the use of all equipment and provide instructions to take home, (b) where possible, remotely monitor data acquisition and follow up if needed, (c) test all equipment before use. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
della Monica, C.; Ravindran, K.K.G.; Atzori, G.; Lambert, D.J.; Rodriguez, T.; Mahvash-Mohammadi, S.; Bartsch, U.; Skeldon, A.C.; Wells, K.; Hampshire, A.; et al. A Protocol for Evaluating Digital Technology for Monitoring Sleep and Circadian Rhythms in Older People and People Living with Dementia in the Community. Clocks & Sleep 2024, 6, 129-155. https://doi.org/10.3390/clockssleep6010010
della Monica C, Ravindran KKG, Atzori G, Lambert DJ, Rodriguez T, Mahvash-Mohammadi S, Bartsch U, Skeldon AC, Wells K, Hampshire A, et al. A Protocol for Evaluating Digital Technology for Monitoring Sleep and Circadian Rhythms in Older People and People Living with Dementia in the Community. Clocks & Sleep. 2024; 6(1):129-155. https://doi.org/10.3390/clockssleep6010010
Chicago/Turabian Styledella Monica, Ciro, Kiran K. G. Ravindran, Giuseppe Atzori, Damion J. Lambert, Thalia Rodriguez, Sara Mahvash-Mohammadi, Ullrich Bartsch, Anne C. Skeldon, Kevin Wells, Adam Hampshire, and et al. 2024. "A Protocol for Evaluating Digital Technology for Monitoring Sleep and Circadian Rhythms in Older People and People Living with Dementia in the Community" Clocks & Sleep 6, no. 1: 129-155. https://doi.org/10.3390/clockssleep6010010





