Catalysis Mediated by 2D Black Phosphorus Either Pristine or Decorated with Transition Metals Species
Abstract
:1. Introduction
2. Synthesis and Exfoliation of Black Phosphorus
Ambient Stability of Exfoliated Black Phosphorus
3. Chemisorption Studies on Exfoliated Black Phosphorus
4. Catalytic Applications of Pristine Black Phosphorus
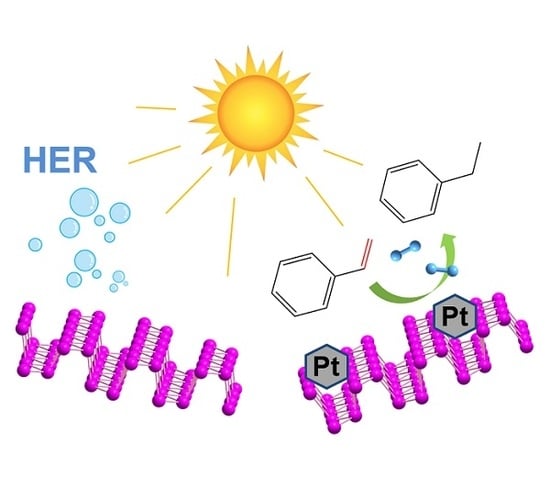
4.1. HER and OER Processes
4.2. Electroreduction of N2 to NH4+
4.3. Alkylation of Soft Nucleophiles with Esters
5. Metal Nanoparticles Decorated Black Phosphorus
5.1. Cobalt
5.2. Nickel
5.3. Palladium
5.4. Platinum
5.5. Silver
5.6. Gold
6. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Grazianetti, C.; Martella, C.; Molle, A. The Xenes Generations: A Taxonomy of Epitaxial Single-Element 2D Materials. Phys. Status Solidi RRL 2019, 14, 1900439. [Google Scholar] [CrossRef] [Green Version]
- Manzeli, S.; Ovchinnikov, D.; Pasquier, D.; Yazyev, O.V.; Kis, A. 2D transition metal dichalcogenides. Nat. Rev. Mater. 2017, 2, 17033. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Xie, L.M. Two-dimensional transition metal dichalcogenide alloys: Preparation, characterization and applications. Nanoscale 2015, 7, 18392–18401. [Google Scholar] [CrossRef]
- Bridgman, P.W. Two new modifications of phosphorus. J. Am. Chem. Soc. 1914, 36, 1344–1363. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Yu, Y.; Ye, G.J.; Ge, Q.; Ou, X.; Wu, H.; Feng, D.; Chen, X.H.; Zhang, Y. Black phosphorus field-effect transistors. Nat. Nanotechnol. 2014, 9, 372–377. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Neal, A.T.; Zhu, Z.; Luo, Z.; Xu, X.; Tománek, D.; Ye, P.D. Phosphorene: An Unexplored 2D Semiconductor with a High Hole Mobility. ACS Nano 2014, 8, 4033–4041. [Google Scholar] [CrossRef] [Green Version]
- Batmunkh, M.; Bat-Erdene, M.; Shapter, J.G. Black Phosphorus: Synthesis and Application for Solar Cells. Adv. Energy Mater. 2017, 8, 1701832. [Google Scholar] [CrossRef]
- Liu, H.; Hu, K.; Yan, D.; Chen, R.; Zou, Y.; Liu, H.; Wang, S. Recent Advances on Black Phosphorus for Energy Storage, Catalysis, and Sensor Applications. Adv. Mater. 2018, 30, 1800295. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lai, C.; Zeng, G.; Huang, D.; Qin, L.; Zhang, M.; Cheng, M.; Liu, X.; Yi, H.; Zhou, C.; et al. Black Phosphorus, a Rising Star 2D Nanomaterial in the Post-Graphene Era: Synthesis, Properties, Modifications, and Photocatalysis Applications. Small 2019, 15, e1804565. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.; Wang, M.; Zhu, X.; Rodin, A.; Su, H.; Neto, A.H.C. Phosphorene: From theory to applications. Nat. Rev. Mater. 2016, 1, 16061. [Google Scholar] [CrossRef]
- Jiang, J.-W.; Park, H. Negative poisson’s ratio in single-layer black phosphorus. Nat. Commun. 2014, 5, 4727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, H.; Lin, X.; Xu, Z.; Chu, D. Recent developments in black phosphorus transistors. J. Mater. Chem. C 2015, 3, 8760–8775. [Google Scholar] [CrossRef]
- Hyun, C.; Kim, J.H.; Lee, J.-Y.; Lee, G.-H.; Kim, K.S. Atomic scale study of black phosphorus degradation. RSC Adv. 2020, 10, 350–355. [Google Scholar] [CrossRef] [Green Version]
- Tan, W.C.; Wang, L.; Feng, X.; Chen, L.; Huang, L.; Huang, X.; Ang, K.-W. Recent Advances in Black Phosphorus-Based Electronic Devices. Adv. Electron. Mater. 2018, 5, 1800666. [Google Scholar] [CrossRef]
- Li, P.; Zhang, D.; Liu, J.; Chang, H.; Sun, Y.; Yin, N. Air-Stable Black Phosphorus Devices for Ion Sensing. ACS Appl. Mater. Interfaces 2015, 7, 24396–24402. [Google Scholar] [CrossRef]
- Donarelli, M.; Ottaviano, L.; Giancaterini, L.; Fioravanti, G.; Perrozzi, F.; Cantalini, C. Exfoliated black phosphorus gas sensing properties at room temperature. 2D Mater. 2016, 3, 025002. [Google Scholar] [CrossRef]
- Miao, J.; Cai, L.; Zhang, S.; Nah, J.; Yeom, J.; Wang, C. Air-Stable Umidity Sensor Using Few-Layer Black Phosphorus. ACS Appl. Mater. Interfaces 2017, 9, 10019–10026. [Google Scholar] [CrossRef]
- Huang, M.; Wang, M.; Chen, C.; Ma, Z.; Li, X.; Han, J.; Wu, Y. Broadband Black-Phosphorus Photodetectors with High Responsivity. Adv. Mater. 2016, 28, 3481–3485. [Google Scholar] [CrossRef]
- Viti, L.; Hu, J.; Coquillat, D.; Knap, W.; Tredicucci, A.; Politano, A.; Vitiello, M.S. Black Phosphorus Terahertz Photodetectors. Adv. Mater. 2015, 27, 5567–5572. [Google Scholar] [CrossRef] [Green Version]
- Viti, L.; Politano, A.; Zhang, K.; Vitiello, M.S. Thermoelectric terahertz photodetectors based on selenium-doped black phosphorus flakes. Nanoscale 2019, 11, 1995–2002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latiff, N.M.; Teo, W.Z.; Sofer, Z.; Fisher, A.C.; Pumera, M. The Cytotoxicity of Layered Black Phosphorus. Chem. A Eur. J. 2015, 21, 13991–13995. [Google Scholar] [CrossRef] [PubMed]
- Qu, G.; Xia, T.; Zhou, W.; Zhang, X.; Zhang, H.; Hu, L.; Shi, J.; Yu, X.; Jiang, G. Property–Activity Relationship of Black Phosphorus at the Nano–Bio Interface: From Molecules to Organisms. Chem. Rev. 2020, 120, 2288–2346. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, X.; Shao, W.; Chen, S.; Xie, J.; Zhang, X.; Wang, J.; Xie, Y. Ultrathin Black Phosphorus Nanosheets for Efficient Singlet Oxygen Generation. J. Am. Chem. Soc. 2015, 137, 11376–11382. [Google Scholar] [CrossRef] [PubMed]
- Raucci, M.G.; Fasolino, I.; Caporali, M.; Serrano-Ruiz, M.; Soriente, A.; Peruzzini, M.; Ambrosio, L. Exfoliated Black Phosphorus Promotes in Vitro Bone Regeneration and Suppresses Osteosarcoma Progression through Cancer-Related Inflammation Inhibition. ACS Appl. Mater. Interfaces 2019, 11, 9333–9342. [Google Scholar] [CrossRef]
- Ienco, A.; Manca, G.; Peruzzini, M.; Mealli, C. Modelling strategies for the covalent functionalization of 2D phosphorene. Dalton Trans. 2018, 47, 17243–17256. [Google Scholar] [CrossRef] [Green Version]
- Brown, A.; Rundqvist, S. Refinement of the crystal structure of black phosphorus. Acta Crystallogr. 1965, 19, 684–685. [Google Scholar] [CrossRef]
- Lange, S.; Schmidt, P.; Nilges, T. Au3SnP7@Black Phosphorus: An Easy Access to Black Phosphorus. Inorg. Chem. 2007, 46, 4028. [Google Scholar] [CrossRef]
- Köpf, M.; Eckstein, N.; Pfister, D.; Grotz, C.; Krüger, I.; Greiwe, M.; Hansen, T.; Kohlmann, H.; Nilges, T. Access and in situ growth of phosphorene-precursor black phosphorus. J. Cryst. Growth 2014, 405, 6–10. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liu, D.; Huang, H.; Yang, N.; Yu, B.; Wen, M.; Wang, X.; Chu, P.K.; Yu, X. In-Plane Black Phosphorus/Dicobalt Phosphide Heterostructure for Efficient Electrocatalysis. Angew. Chem. Int. Ed. 2018, 57, 2600–2604. [Google Scholar] [CrossRef]
- Serrano-Ruiz, M.; Caporali, M.; Ienco, A.; Piazza, V.; Heun, S.; Peruzzini, M. The Role of Water in the Preparation and Stabilization of High-Quality Phosphorene Flakes. Adv. Mater. Interfaces 2015, 3, 1500441. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhou, Q.; Shi, L.; Chen, Q.; Wang, J. Recent advances in oxidation and degradation mechanisms of ultrathin 2D materials under ambient conditions and their passivation strategies. J. Mater. Chem. A 2019, 7, 4291–4312. [Google Scholar] [CrossRef]
- Pei, J.; Gai, X.; Yang, J.; Wang, X.; Yu, Z.; Choi, D.-Y.; Luther-Davies, B.; Lu, Y. Producing air-stable monolayers of phosphorene and their defect engineering. Nat. Commun. 2016, 7, 10450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolognesi, M.; Moschetto, S.; Trapani, M.; Prescimone, F.; Ferroni, C.; Manca, G.; Ienco, A.; Borsacchi, S.; Caporali, M.; Muccini, M.; et al. Noncovalent Functionalization of 2D Black Phosphorus with Fluorescent Boronic Derivatives of Pyrene for Probing and Modulating the Interaction with Molecular Oxygen. ACS Appl. Mater. Interfaces 2019, 11, 22637–22647. [Google Scholar] [CrossRef] [PubMed]
- Gusmão, R.; Sofer, Z.; Pumera, M. Functional Protection of Exfoliated Black Phosphorus by Noncovalent Modification with Anthraquinone. ACS Nano 2018, 12, 5666–5673. [Google Scholar] [CrossRef]
- Caporali, M.; Serrano-Ruiz, M.; Telesio, F.; Heun, S.; Nicotra, G.; Spinella, C.; Peruzzini, M. Decoration of exfoliated black phosphorus with nickel nanoparticles and its application in catalysis. Chem. Commun. 2017, 53, 10946–10949. [Google Scholar] [CrossRef] [Green Version]
- Abellán, G.; Wild, S.; Lloret, V.; Scheuschner, N.; Gillen, R.; Mundloch, U.; Maultzsch, J.; Varela, M.; Hauke, F.; Hirsch, A. Fundamental Insights into the Degradation and Stabilization of Thin Layer Black Phosphorus. J. Am. Chem. Soc. 2017, 139, 10432–10440. [Google Scholar] [CrossRef] [Green Version]
- Matthews, P.D.; Hirunpinyopas, W.; Lewis, D.J.; Brent, J.; McNaughter, P.D.; Zeng, N.; Thomas, A.; O’Brien, P.; Derby, B.; Bissett, M.A.; et al. Black phosphorus with near-superhydrophobic properties and long-term stability in aqueous media. Chem. Commun. 2018, 54, 3831–3834. [Google Scholar] [CrossRef] [Green Version]
- Kou, L.; Frauenheim, T.; Chen, C. Phosphorene as a Superior Gas Sensor: Selective Adsorption and Distinct I–V Response. J. Phys. Chem. Lett. 2014, 5, 2675–2681. [Google Scholar] [CrossRef] [Green Version]
- Ou, P.; Song, P.; Liu, X.; Song, J. Superior Sensing Properties of Black Phosphorus as Gas Sensors: A Case Study on the Volatile Organic Compounds. Adv. Theory Simul. 2018, 2, 1800103. [Google Scholar] [CrossRef]
- Huang, Y.; Qiao, J.; He, K.; Bliznakov, S.; Sutter, E.; Chen, X.; Luo, D.; Meng, F.; Su, D.; Decker, J.; et al. Interaction of Black Phosphorus with Oxygen and Water. Chem. Mater. 2016, 28, 8330–8339. [Google Scholar] [CrossRef] [Green Version]
- Abbas, A.N.; Liu, B.; Chen, L.; Ma, Y.; Cong, S.; Aroonyadet, N.; Köpf, M.; Nilges, T.; Zhou, C. Black Phosphorus Gas Sensors. ACS Nano 2015, 9, 5618–5624. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.Y.; Yu, Z.Y.; Shen, H.-Y.; Sun, X.L.; Wan, N.; Yu, H. CO Adsorption on Metal-Decorated Phosphorene. ACS Omega 2018, 3, 3957–3965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, G.; Jung, S.; Jang, S.; Kim, J. Platinum-functionalized black phosphorus hydrogen sensors. Appl. Phys. Lett. 2017, 110, 242103. [Google Scholar] [CrossRef]
- Appl, M. Ammonia: Principles and Industrial Practice; Wiley-VCH: Hoboken, NJ, USA, 2007; ISBN 978-3-527-61388-5. [Google Scholar]
- Erisman, J.W.; Sutton, M.A.; Galloway, J.; Klimont, Z.; Winiwarter, W. How a century of ammonia synthesis changed the world. Nat. Geosci. 2008, 1, 636–639. [Google Scholar] [CrossRef]
- De Klerk, A. Fischer-Tropsch Refining; Wiley-VCH: Hoboken, NJ, USA, 2011; ISBN 9783527326051. [Google Scholar]
- Mahmoudi, H.; Mahmoudi, M.; Doustdar, O.; Jahangiri, H.; Tsolakis, A.; Gu, S.; LechWyszynski, M. A review of Fischer Tropsch synthesis process, mechanism, surface chemistry and catalyst formulation. Biofuels Eng. 2017, 2, 11–31. [Google Scholar] [CrossRef]
- Tanimu, A.; Alhooshani, K. Advanced Hydrodesulfurization Catalysts: A Review of Design and Synthesis. Energy Fuels 2019, 33, 2810–2838. [Google Scholar] [CrossRef]
- Coker, A.K. Petroleum Refining Design and Applications Handbook. Pet. Refin. Des. Appl. Handb. 2018, 1, 305–338. [Google Scholar]
- Liu, X.; Dai, L. Carbon-based metal-free catalysts. Nat. Rev. Mater. 2016, 1, 16064. [Google Scholar] [CrossRef]
- Abellán, G.; Lloret, V.; Mundloch, U.; Marcia, M.; Neiss, C.; Görling, A.; Varela, M.; Hauke, F.; Hirsch, A. Noncovalent Functionalization of Black Phosphorus. Angew. Chem. Int. Ed. 2016, 55, 14557–14562. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Zhang, Z.; Lei, Z.; Wang, X.; Tan, Y.; Cheng, N.; Sun, X. Encapsulating Pt Nanoparticles inside a Derived Two-Dimensional Metal–Organic Frameworks for the Enhancement of Catalytic Activity. ACS Appl. Mater. Interfaces 2020, 12, 10359–10368. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, L.; Zhao, P.; Lee, L.Y.S.; Wong, K.-Y. Recent Advances in Electrocatalytic Hydrogen Evolution Using Nanoparticles. Chem. Rev. 2019, 120, 851–918. [Google Scholar] [CrossRef] [PubMed]
- Mayorga-Martinez, C.C.; Latiff, N.M.; Eng, A.Y.S.; Sofer, Z.; Pumera, M. Black Phosphorus Nanoparticle Labels for Immunoassays via Hydrogen Evolution Reaction Mediation. Anal. Chem. 2016, 88, 10074–10079. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, T.; Sun, Z.; Chen, H.; Guan, J.; Chen, X.; Ji, H.; Du, P.; Yang, S. Black Phosphorus Revisited: A Missing Metal-Free Elemental Photocatalyst for Visible Light Hydrogen Evolution. Adv. Mater. 2017, 29, 1605776. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.Z.; Kwong, C.W.; Davey, K.; Qiao, S. 2D phosphorene as a water splitting photocatalyst: Fundamentals to applications. Energy Environ. Sci. 2016, 9, 709–728. [Google Scholar] [CrossRef]
- Muduli, S.K.; Varrla, E.; Xu, Y.; Kulkarni, S.A.; Katre, A.; Chakraborty, S.; Chen, S.; Sum, T.C.; Xu, R.; Mathews, N. Evolution of hydrogen by few-layered black phosphorus under visible illumination. J. Mater. Chem. A 2017, 5, 24874–24879. [Google Scholar] [CrossRef]
- You, H.; Jia, Y.; Wu, Z.; Wang, F.; Huang, H.; Wang, Y. Room-temperature pyro-catalytic hydrogen generation of 2D few-layer black phosphorene under cold-hot alternation. Nat. Commun. 2018, 9, 2889. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Q.; Xu, L.; Chen, N.; Zhang, H.; Dai, L.; Wang, S. Facile Synthesis of Black Phosphorus: An Efficient Electrocatalyst for the Oxygen Evolving Reaction. Angew. Chem. Int. Ed. 2016, 55, 13849–13853. [Google Scholar] [CrossRef]
- Ren, X.; Zhou, J.; Qi, X.; Liu, Y.; Huang, Z.; Li, Z.; Ge, Y.; Dhanabalan, S.C.; Ponraj, J.S.; Wang, S.; et al. Few-Layer Black Phosphorus Nanosheets as Electrocatalysts for Highly Effcient Oxygen Evolution Reaction. Adv. Energy Mater. 2017, 7, 1700396. [Google Scholar] [CrossRef]
- Shi, F.; Huang, K.; Wang, Y.; Zhang, W.; Li, L.; Wang, X.; Feng, S. Black Phosphorus-Modified Co3O4 through Tuning the Electronic Structure for Enhanced Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2019, 11, 17459–17466. [Google Scholar] [CrossRef]
- Fryzuk, M.D.; Johnson, S. The continuing story of dinitrogen activation. Co ord. Chem. Rev. 2000, 200, 379–409. [Google Scholar] [CrossRef]
- Légaré, M.-A.; Bélanger-Chabot, G.; Dewhurst, R.D.; Welz, E.; Krummenacher, I.; Engels, B.; Braunschweig, H. Nitrogen fixation and reduction at boron. Science 2018, 359, 896–900. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Chen, G.-F.; Ding, L.-X.; Wang, H. Advanced Non-metallic Catalysts for Electrochemical Nitrogen Reduction under Ambient Conditions. Chem. Eur. J. 2019, 25, 12464–12485. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, L.-X.; Chen, G.-F.; Yang, X.; Wang, H. Ammonia Synthesis Under Ambient Conditions: Selective Electroreduction of Dinitrogen to Ammonia on Black Phosphorus Nanosheets. Angew. Chem. Int. Ed. 2019, 58, 2612–2616. [Google Scholar] [CrossRef]
- Lloret, V.; Rivero-Crespo, M.; Vidal-Moya, J.A.; Wild, S.; Doménech-Carbó, A.; Heller, B.S.J.; Shin, S.; Steinrück, H.-P.; Maier, F.; Hauke, F.; et al. Few layer 2D pnictogens catalyze the alkylation of soft nucleophiles with esters. Nat. Commun. 2019, 10, 509. [Google Scholar] [CrossRef] [Green Version]
- Trost, B.M.; Van Vranken, D.L. Asymmetric Transition Metal-Catalyzed Allylic Alkylations. Chem. Rev. 1996, 96, 395–422. [Google Scholar] [CrossRef]
- Sueki, S.; Kuninobu, Y. Copper-Catalyzed N- and O-Alkylation of Amines and Phenols using Alkylborane Reagents. Org. Lett. 2013, 15, 1544–1547. [Google Scholar] [CrossRef]
- Shi, F.; Geng, Z.; Huang, K.; Liang, Q.; Zhang, Y.; Sun, Y.; Cao, J.; Feng, S. Cobalt Nanoparticles/Black Phosphorus Nanosheets: An Efficient Catalyst for Electrochemical Oxygen Evolution. Adv. Sci. 2018, 5, 1800575. [Google Scholar] [CrossRef]
- Oger, C.; Balas, L.; Durand, T.; Galano, J.-M. ChemInform Abstract: Are Alkyne Reductions Chemo-, Regio-, and Stereoselective Enough To Provide Pure (Z)-Olefins in Polyfunctionalized Bioactive Molecules? Chem. Rev. 2013, 44, 1313–1350. [Google Scholar] [CrossRef]
- Delgado, J.A.; Benkirane, O.; Claver, C.; Curulla-Ferré, D.; Godard, C. Advances in the preparation of highly selective nanocatalysts for the semi-hydrogenation of alkynes using colloidal approaches. Dalton Trans. 2017, 46, 12381–12403. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Li, C.; Zhou, J.; Zhang, S.; Rao, D.; He, S.; Wei, M.; Evans, D.G.; Duan, X. Metal Phosphides Derived from Hydrotalcite Precursors toward the Selective Hydrogenation of Phenylacetylene. ACS Catal. 2015, 5, 5756–5765. [Google Scholar] [CrossRef]
- Li, C.; Chen, Y.; Zhang, S.; Zhou, J.; Wang, F.; He, S.; Wei, M.; Evans, D.G.; Duan, X. Nickel-Gallium Intermetallic Nanocrystal Catalysts in the Semihydrogenation of Phenylacetylene. ChemCatChem 2014, 6, 824–831. [Google Scholar] [CrossRef]
- Erokhin, A.V.; Lokteva, E.; Yermakov, A.; Boukhvalov, D.; Maslakov, K.I.; Golubina, E.V.; Uimin, M. Phenylacetylene hydrogenation on Fe@C and Ni@C core–shell nanoparticles: About intrinsic activity of graphene-like carbon layer in H2 activation. Carbon 2014, 74, 291–301. [Google Scholar] [CrossRef]
- Vanni, M.; Serrano-Ruiz, M.; Telesio, F.; Heun, S.; Banchelli, M.; Matteini, P.; Mio, A.M.; Nicotra, G.; Spinella, C.; Caporali, S.; et al. Black Phosphorus/Palladium Nanohybrid: Unraveling the Nature of P–Pd Interaction and Application in Selective Hydrogenation. Chem. Mater. 2019, 31, 5075–5080. [Google Scholar] [CrossRef] [Green Version]
- Orlandi, M.; Brenna, D.; Harms, R.; Jost, S.; Benaglia, M. Recent Developments in the Reduction of Aromatic and Aliphatic Nitro Compounds to Amines. Org. Process. Res. Dev. 2016, 22, 430–445. [Google Scholar] [CrossRef]
- Kovacic, P.; Somanathan, R. Nitroaromatic compounds: Environmental toxicity, carcinogenicity, mutagenicity, therapy and mechanism. J. Appl. Toxicol. 2014, 34, 810–824. [Google Scholar] [CrossRef]
- Costantino, F.; Nocchetti, M.; Bastianini, M.; Lavacchi, A.; Caporali, M.; Liguori, F. Robust Zirconium Phosphate–Phosphonate Nanosheets Containing Palladium Nanoparticles as Efficient Catalyst for Alkynes and Nitroarenes Hydrogenation Reactions. ACS Appl. Nano Mater. 2018, 1, 1750–1757. [Google Scholar] [CrossRef]
- Wu, T.; Fan, J.; Li, Q.; Shi, P.; Xu, Q.; Min, Y. Palladium Nanoparticles Anchored on Anatase Titanium Dioxide-Black Phosphorus Hybrids with Heterointerfaces: Highly Electroactive and Durable Catalysts for Ethanol Electrooxidation. Adv. Energy Mater. 2017, 8, 1701799. [Google Scholar] [CrossRef]
- Wu, T.; Ma, Y.; Qu, Z.-B.; Fan, J.; Li, Q.; Shi, P.; Xu, Q.; Min, Y. Black Phosphorus–Graphene Heterostructure-Supported Pd Nanoparticles with Superior Activity and Stability for Ethanol Electro-oxidation. ACS Appl. Mater. Interfaces 2019, 11, 5136–5145. [Google Scholar] [CrossRef]
- Bai, L.; Wang, X.; Tang, S.; Kang, Y.; Wang, J.; Yu, Y.; Zhou, Z.-K.; Ma, C.; Zhang, X.; Jiang, J.; et al. Black Phosphorus/Platinum Heterostructure: A Highly Efficient Photocatalyst for Solar-Driven Chemical Reactions. Adv. Mater. 2018, 30, e1803641. [Google Scholar] [CrossRef]
- Wang, X.; Bai, L.; Lu, J.; Zhang, X.; Liu, D.; Yang, H.; Wang, J.; Chu, P.K.; Ramakrishna, S.; Yu, X.F. Rapid Activation of Platinum with Black Phosphorus for Efficient Hydrogen Evolution. Angew. Chem. Int. Ed. 2019, 58, 19060–19066. [Google Scholar] [CrossRef]
- Tian, B.; Tian, B.; Smith, B.; Scott, M.C.; Lei, Q.; Hua, R.; Tian, Y.; Liu, Y. Facile bottom-up synthesis of partially oxidized black phosphorus nanosheets as metal-free photocatalyst for hydrogen evolution. Proc. Natl. Acad. Sci. USA 2018, 115, 4345–4350. [Google Scholar] [CrossRef] [Green Version]
- Lei, W.; Zhang, T.; Liu, P.; Rodriguez, J.A.; Liu, G.; Liu, M.-H. Bandgap- and Local Field-Dependent Photoactivity of Ag/Black Phosphorus Nanohybrids. ACS Catal. 2016, 6, 8009–8020. [Google Scholar] [CrossRef]
- Hu, J.; Guo, Z.; McWilliams, P.E.; Darges, J.E.; Druffel, D.; Moran, A.M.; Warren, S. Band Gap Engineering in a 2D Material for Solar-to-Chemical Energy Conversion. Nano Lett. 2015, 16, 74–79. [Google Scholar] [CrossRef]
- Wu, Q.; Liang, M.; Zhang, S.; Liu, X.; Wang, F. Development of functional black phosphorus nanosheets with remarkable catalytic and antibacterial performance. Nanoscale 2018, 10, 10428–10435. [Google Scholar] [CrossRef]
- Huang, H.; Xiao, Q.; Wang, J.; Yu, X.; Wang, H.; Zhang, H.; Chu, P.K. Black phosphorus: A two-dimensional reductant for in situ nanofabrication. npj 2D Mater. Appl. 2017, 1, 20. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Liu, C.-Y. Au/graphene hydrogel: Synthesis, characterization and its use for catalytic reduction of 4-nitrophenol. J. Mater. Chem. 2012, 22, 8426. [Google Scholar] [CrossRef]
- Kuroda, K.; Ishida, T.; Haruta, M. Reduction of 4-nitrophenol to 4-aminophenol over Au nanoparticles deposited on PMMA. J. Mol. Catal. A Chem. 2009, 298, 7–11. [Google Scholar] [CrossRef]
- Wu, X.; Lu, C.; Zhou, Z.; Yuan, G.; Xiong, R.; Zhang, X. Green synthesis and formation mechanism of cellulose nanocrystal-supported gold nanoparticles with enhanced catalytic performance. Environ. Sci. Nano 2014, 1, 71. [Google Scholar] [CrossRef]
- Zhu, M.; Cai, X.; Fujitsuka, M.; Zhang, J.; Majima, T. Au/La2Ti2O7Nanostructures Sensitized with Black Phosphorus for Plasmon-Enhanced Photocatalytic Hydrogen Production in Visible and Near-Infrared Light. Angew. Chem. Int. Ed. 2017, 56, 2064–2068. [Google Scholar] [CrossRef]


































© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanni, M.; Caporali, M.; Serrano-Ruiz, M.; Peruzzini, M. Catalysis Mediated by 2D Black Phosphorus Either Pristine or Decorated with Transition Metals Species. Surfaces 2020, 3, 132-167. https://doi.org/10.3390/surfaces3020012
Vanni M, Caporali M, Serrano-Ruiz M, Peruzzini M. Catalysis Mediated by 2D Black Phosphorus Either Pristine or Decorated with Transition Metals Species. Surfaces. 2020; 3(2):132-167. https://doi.org/10.3390/surfaces3020012
Chicago/Turabian StyleVanni, Matteo, Maria Caporali, Manuel Serrano-Ruiz, and Maurizio Peruzzini. 2020. "Catalysis Mediated by 2D Black Phosphorus Either Pristine or Decorated with Transition Metals Species" Surfaces 3, no. 2: 132-167. https://doi.org/10.3390/surfaces3020012