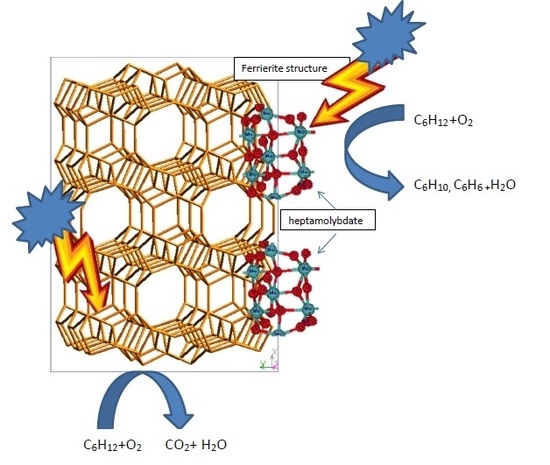
UV Light Driven Selective Oxidation of Cyclohexane in Gaseous Phase Using Mo-Functionalized Zeolites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Preparation and Characterization
2.2. Photocatalytic Activity Tests
- Feed section;
- Reaction section; and
- Gas composition analysis section.
- CH% conversion = 100 × (moles of inlet CH – moles of outlet CH)/(moles of inlet CH)
- BE% selectivity = 100 × (moles of outlet BE)/(moles of inlet CH – moles of outlet CH)
- CO2% selectivity = 100 × (moles of outlet CO2)/6 × (moles of inlet CH – moles of outlet CH)
3. Results and Discussion
3.1. Physical-Chemical Characterization Results
3.2. Photocatalytic Activity Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Giannotti, C.; Richter, C. Photocatalysed oxidation of cyclohexane by W10O324− irradiation with natural sunlight. Int. J. Photoenergy 1999, 1, 69–73. [Google Scholar] [CrossRef]
- Paz, Y. Preferential photodegradation—Why and how? Comptes Rendus Chim. 2006, 9, 774–787. [Google Scholar] [CrossRef]
- Corma, A.; Garcia, H. Zeolite-Based photocatalysts. Chem. Commun. 2004, 1443–1459. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Pang, W.; Yu, J.; Huo, Q.; Chen, J. Chemistry of Zeolites and Related Porous Materials: Synthesis and Structure; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Aramendía, M.; Colmenares, J.; López-Fernández, S.; Marinas, A.; Marinas, J.; Urbano, F. Screening of different zeolite-based catalysts for gas-phase selective photooxidation of propan-2-ol. Catal. Today 2007, 129, 102–109. [Google Scholar] [CrossRef]
- Calza, P.; Pazé, C.; Pelizzetti, E.; Zecchina, A. Shape-Selective photocatalytic transformation of phenols in an aqueous medium. Chem. Commun. 2001, 2130–2131. [Google Scholar] [CrossRef]
- Sagatelian, Y.; Sharabi, D.; Paz, Y. Enhanced photodegradation of diisopropyl methyl phosphonate by the “adsorb & shuttle” approach. J. Photochem. Photobiol. A Chem. 2005, 174, 253–260. [Google Scholar]
- Guo, Y.; Zu, B.; Dou, X. Zeolite-Based photocatalysts: A promising strategy for efficient photocatalysis. J. Thermodyn. Catal. 2013, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, S. Zeolite photochemistry: Impact of zeolites on photochemistry and feedback from photochemistry to zeolite science. J. Photochem. Photobiol. C Photochem. Rev. 2003, 4, 19–49. [Google Scholar] [CrossRef]
- Juan, Z.; Dishun, Z.; Liyan, Y.; Yongbo, L. Photocatalytic oxidation dibenzothiophene using TS-1. Chem. Eng. J. 2010, 156, 528–531. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Z.; Wang, Y.; Li, Y.; Qu, L.; E, L.; Ya, J.; Huang, P. Photocatalysis of TiO2 nanoparticles supported on natural zeolite. Mater. Technol. 2012, 27, 267–271. [Google Scholar] [CrossRef]
- White, J.C.; Dutta, P.K. Assembly of nanoparticles in zeolite Y for the photocatalytic generation of hydrogen from water. J. Phys. Chem. C 2011, 115, 2938–2947. [Google Scholar] [CrossRef]
- Bossmann, S.H.; Turro, C.; Schnabel, C.; Pokhrel, M.R.; Payawan, L.M.; Baumeister, B.; Wörner, M. Ru(bpy)32+/TiO2-codoped zeolites: Synthesis, characterization, and the role of TiO2 in electron transfer photocatalysis. J. Phys. Chem. B 2001, 105, 5374–5382. [Google Scholar] [CrossRef]
- Yamashita, H.; Ichihashi, Y.; Anpo, M.; Hashimoto, M.; Louis, C.; Che, M. Photocatalytic decomposition of NO at 275 K on titanium oxides included within Y-zeolite cavities: The structure and role of the active sites. J. Phys. Chem. 1996, 100, 16041–16044. [Google Scholar] [CrossRef]
- Rasmussen, S.B.; Portela, R.; Suárez, S.; Coronado, J.M.; Rojas-Cervantes, M.L.; Avila, P.; Sánchez, B. Hybrid TiO2—SiMgOx composite for combined chemisorption and photocatalytic elimination of gaseous H2S. Ind. Eng. Chem. Res. 2010, 49, 6685–6690. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Thompson, R.L.; Baltrus, J.; Matranga, C. Visible light photoreduction of CO2 using CdSe/Pt/TiO2 heterostructured catalysts. J. Phys. Chem. Lett. 2009, 1, 48–53. [Google Scholar] [CrossRef]
- Ikeue, K.; Yamashita, H.; Anpo, M. Photocatalytic reduction of CO2 with H2O on titanium oxides prepared within the FSM-16 mesoporous zeolite. Chem. Lett. 1999, 28, 1135–1136. [Google Scholar] [CrossRef]
- Durgakumari, V.; Subrahmanyam, M.; Rao, K.S.; Ratnamala, A.; Noorjahan, M.; Tanaka, K. An easy and efficient use of TiO2 supported HZSM-5 and TiO2+ HZSM-5 zeolite combinate in the photodegradation of aqueous phenol and p-chlorophenol. Appl. Catal. A Gen. 2002, 234, 155–165. [Google Scholar] [CrossRef]
- Sarno, G.; Vaiano, V.; Sannino, D.; Ciambelli, P. Photocatalytic applications with TiO2-zeolites composites anchored on ceramic tiles. Chem. Eng. 2015, 43. [Google Scholar] [CrossRef]
- Ramamurthy, V. Controlling photochemical reactions via confinement: Zeolites. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 145–166. [Google Scholar] [CrossRef]
- Shimizu, K.-i.; Akahane, H.; Kodama, T.; Kitayama, Y. Selective photo-oxidation of benzene over transition metal-exchanged BEA zeolite. Appl. Catal. A Gen. 2004, 269, 75–80. [Google Scholar] [CrossRef]
- Blatter, F.; Sun, H.; Vasenkov, S.; Frei, H. Photocatalyzed oxidation in zeolite cages. Catal. Today 1998, 41, 297–309. [Google Scholar] [CrossRef]
- Ciambelli, P.; Sannino, D.; Palo, E.; Gargano, G.; Balboni, E.; Martucci, A.; Dalconi, M.; Alberti, A. Relevance of Co, Ag-ferrierite catalysts acidity and cation siting to CH4-NOx-SCR activity. Nuovo Cim. 2008, 123, 1583–1595. [Google Scholar]
- Çulfaz, A.; Yilmaz, A.K. Synthesis and characterization of ferrierite. Cryst. Res. Technol. 1985, 20, 11–19. [Google Scholar] [CrossRef]
- Sannino, D.; Vaiano, V.; Ciambelli, P.; Murcia, J.; Hidalgo, M.; Navío, J.A. Gas-Phase photocatalytic partial oxidation of cyclohexane to cyclohexanol and cyclohexanone on Au/TiO2 photocatalysts. J. Adv. Oxid. Technol. 2013, 16, 71–82. [Google Scholar] [CrossRef] [Green Version]
- Vaiano, V.; Sannino, D.; Ciambelli, P. Sustainable gas phase selective photocatalytic oxidation of cyclohexane on MoOx/TiO2/SiO2 catalysts. Chem. Eng. 2014, 39, 565–570. [Google Scholar]
- Ciambelli, P.; Sannino, D.; Palma, V.; Vaiano, V.; Mazzei, R.S.; Eloy, P.; Gaigneaux, E.M. Photocatalytic cyclohexane oxidehydrogenation on sulphated MoOx/γ-Al2O3 catalysts. Catal. Today 2009, 141, 367–373. [Google Scholar] [CrossRef]
- Ciambelli, P.; Sannino, D.; Palma, V.; Vaiano, V. The effect of sulphate doping on nanosized and catalysts in cyclohexane photooxidative dehydrogenation. Int. J. Photoenergy 2008, 2008, 258631. [Google Scholar]
- Ciambelli, P.; Sannino, D.; Palo, E.; Ruggiero, A. Improved stability of Co-ferrierite catalyst by Mn in dry–wet cycles of lean CH4-SCR of NOx. Top. Catal. 2007, 42, 177–181. [Google Scholar] [CrossRef]
- Ciambelli, P.; Sannino, D.; Palma, V.; Vaiano, V. Photocatalysed selective oxidation of cyclohexane to benzene on MoOx/TiO2. Catal. Today 2005, 99, 143–149. [Google Scholar]
- Einaga, H.; Futamura, S.; Ibusuki, T. Heterogeneous photocatalytic oxidation of benzene, toluene, cyclohexene and cyclohexane in humidified air: Comparison of decomposition behavior on photoirradiated TiO2 catalyst. Appl. Catal. B Environ. 2002, 38, 215–225. [Google Scholar] [CrossRef]
- ALOthman, Z.A. A review: Fundamental aspects of silicate mesoporous materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef] [Green Version]
- Ciambelli, P.; Corbo, P.; Gaudino, M.C.; Migliardini, F.; Sannino, D. Investigation of Co-based DeNOx catalysts for the treatment of natural gas lean-burn engine exhaust. Top. Catal. 2001, 16-17, 413–417. [Google Scholar] [CrossRef]
- Pirone, R.; Ciambelli, P.; Moretti, G.; Russo, G. Nitric oxide decomposition over Cu-exchanged ZSM-5 with high SiAl ratio. Appl. Catal. B: Environ. 1996, 8, 197–207. [Google Scholar] [CrossRef]
- Xu, Y.; Shu, Y.; Liu, S.; Huang, J.; Guo, X. Interaction between ammonium heptamolybdate and NH4 ZSM-5 zeolite: The location of Mo species and the acidity of Mo/HZSM-5. Catal. Lett. 1995, 35, 233–243. [Google Scholar] [CrossRef]
- Mejri, I.; Ayari, F.; Mhamdi, M.; Delahay, G.; Ksibi, Z.; Ghorbel, A. SCR of NO by NH3 catalyzed by Mo-and V-exchanged zeolite: Effect of Mo precursor salt. Microporous Mesoporous Mater. 2016, 220, 239–246. [Google Scholar] [CrossRef]
- Afanasiev, P.; Geantet, C.; Breysse, M.; Coudurier, G.; Vedrine, J.C. Influence of preparation method on the acidity of MoO3 (WO3)/ZrO2 catalysts. J. Chem. Soc. Faraday Trans. 1994, 90, 193–202. [Google Scholar] [CrossRef]
- Matralis, H.; Theret, S.; Bastians, P.; Ruwet, M.; Grange, P. Selective catalytic reduction of nitric oxide with ammonia using MoO3/ TiO2: Catalyst structure and activity. Appl. Catal. B: Environ. 1995, 5, 271–281. [Google Scholar] [CrossRef]
- Lietti, L.; Alemany, J.; Forzatti, P.; Busca, G.; Ramis, G.; Giamello, E.; Bregani, F. Reactivity of V2O5- WO3/TiO2 catalysts in the selective catalytic reduction of nitric oxide by ammonia. Catal. Today 1996, 29, 143–148. [Google Scholar] [CrossRef]
- Ng, K.Y.S.; Gulari, E. Molybdena on titania: I. Preparation and characterization by raman and fourier transform infrared spectroscopy. J. Catal. 1985, 92, 340–354. [Google Scholar] [CrossRef] [Green Version]
- Quincy, R.B.; Houalla, M.; Proctor, A.; Hercules, D.M. Surface structure and thiophene hydrodesulfurization activity of molybdenum/titania catalysts. J. Phys. Chem. 1989, 93, 5882–5888. [Google Scholar] [CrossRef]
- Tagiev, D.B.; Minachev, K.M. Catalytic properties of zeolites towards oxidation reactions. Russ. Chem. Rev. 1981, 50, 1009. [Google Scholar] [CrossRef]
- Aliev, A.; Shabanova, Z.; Nadzhaf-Kuliev, U.; Medzhidova, S. Oxidative dehydrogenation of cyclohexane over modified zeolite catalysts. Pet. Chem. 2016, 56, 639–645. [Google Scholar] [CrossRef]
- Sun, H.; Blatter, F.; Frei, H. Cyclohexanone from cyclohexane and O2 in a zeolite under visible light with complete selectivity. J. Am. Chem. Soc. 1996, 118, 6873–6879. [Google Scholar] [CrossRef]
- Palma, V.; Sannino, D.; Vaiano, V.; Ciambelli, P. Fluidized-Bed reactor for the intensification of gas-phase photocatalytic oxidative dehydrogenation of cyclohexane. Ind. Eng. Chem. Res. 2010, 49, 10279–10286. [Google Scholar] [CrossRef]
- Ciambelli, P.; Sannino, D.; Palma, V.; Vaiano, V.; Bickley, R.I. Reaction mechanism of cyclohexane selective photo-oxidation to benzene on molybdena/titania catalysts. Appl. Catal. A Gen. 2008, 349, 140–147. [Google Scholar] [CrossRef]
- Ciambelli, P.; Sannino, D.; Palma, V.; Vaiano, V.; Eloy, P.; Dury, F.; Gaigneaux, E.M. Tuning the selectivity of MoOx supported catalysts for cyclohexane photo oxidehydrogenation. Catal. Today 2007, 128, 251–257. [Google Scholar] [CrossRef]
- Maldotti, A.; Amadelli, R.; Vitali, I.; Borgatti, L.; Molinari, A. CH2Cl2-assisted functionalization of cycloalkenes by photoexcited (nBu4N)4W10O32 heterogenized on SiO2. J. Mol. Catal. A Chem. 2003, 204, 703–711. [Google Scholar] [CrossRef]










| Bulk Density, g/cm3 | 0.40 |
| Pores Diameter, Å | 4.0 |
| SiO2, Dry wt % | 84.9 |
| Al2O3, Dry wt % | 8.6 |
| Na2O, Dry wt % | 1.5 |
| K2O, Dry wt % | 5.6 |
| K2O/Al2O3 | 0.7 |
| Na2O/Al2O3 | 0.28 |
| SiO2/Al2O3 | 16.8 |
| Catalyst | Nominal MoO3 Content (wt %) |
|---|---|
| AFer | - |
| 5MoAFer | 5.0 |
| 20MoAFer | 20.0 |
| Catalyst | Microporous Volume (cm3/g) | Measured MoO3 Content (wt %) | Equivalent Band Gap Energy (eV) |
|---|---|---|---|
| AFer | 0.130 | - | - |
| 5MoAFer | 0.045 | 4.7 | 3.2 |
| 20MoAFer | 0.024 | 18.5 | 3.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaiano, V.; Sannino, D. UV Light Driven Selective Oxidation of Cyclohexane in Gaseous Phase Using Mo-Functionalized Zeolites. Surfaces 2019, 2, 546-559. https://doi.org/10.3390/surfaces2040040
Vaiano V, Sannino D. UV Light Driven Selective Oxidation of Cyclohexane in Gaseous Phase Using Mo-Functionalized Zeolites. Surfaces. 2019; 2(4):546-559. https://doi.org/10.3390/surfaces2040040
Chicago/Turabian StyleVaiano, Vincenzo, and Diana Sannino. 2019. "UV Light Driven Selective Oxidation of Cyclohexane in Gaseous Phase Using Mo-Functionalized Zeolites" Surfaces 2, no. 4: 546-559. https://doi.org/10.3390/surfaces2040040