Membrane-Assisted Condenser
Abstract
:1. Introduction
2. Description of the Technology
- -
- traditional condensers represent the easiest process even if corrosion phenomena due to the presence of acid pollutant in the waste gases stream are their main limitation;
- -
- adsorption of water by a liquid or solid desiccant is another valid alternative despite desiccant losses, cost and regeneration of adsorbent, and low quality of water are the main drawbacks;
- -
- high energy consumption due to the high pressure requirements is associated with the utilization of dense membrane for the recovery of water vapor from the gaseous streams.
- -
- no corrosion phenomena because membrane can be fabricated from almost any chemically resistant polymers with hydrophobic intrinsic properties;
- -
- energy consumption of a MC is lower with respect to that of either cryogenic separation or dense membrane, due to the high difference in boiling point between the gases and water in the first process and due to the high pressure requirements in the second process;
- -
- with respect to liquid or solid sorption, a MC is not effected by problems such as desiccant losses, cost and regeneration of adsorbent, and low quality of the produced water.
3. Methods
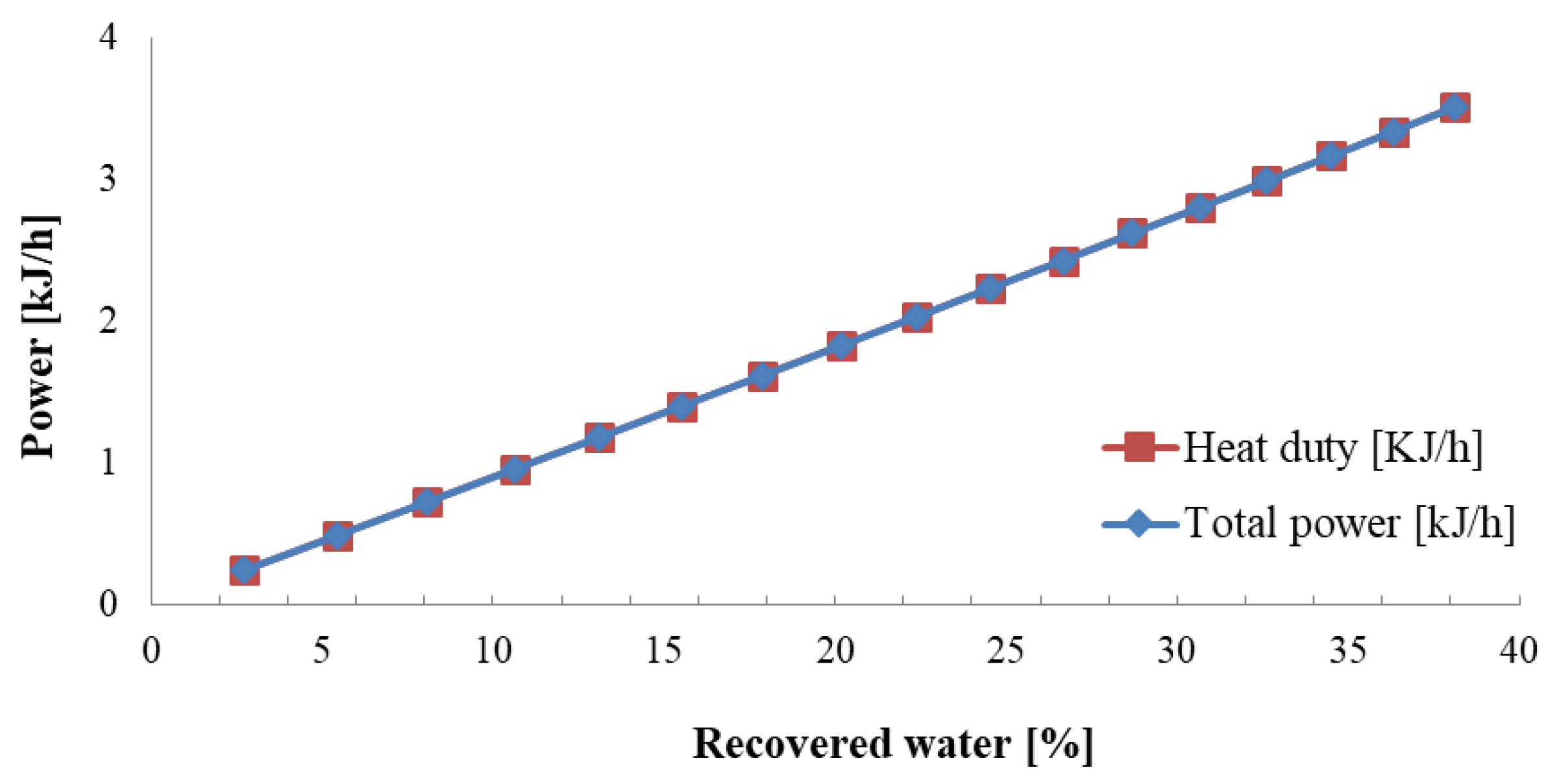
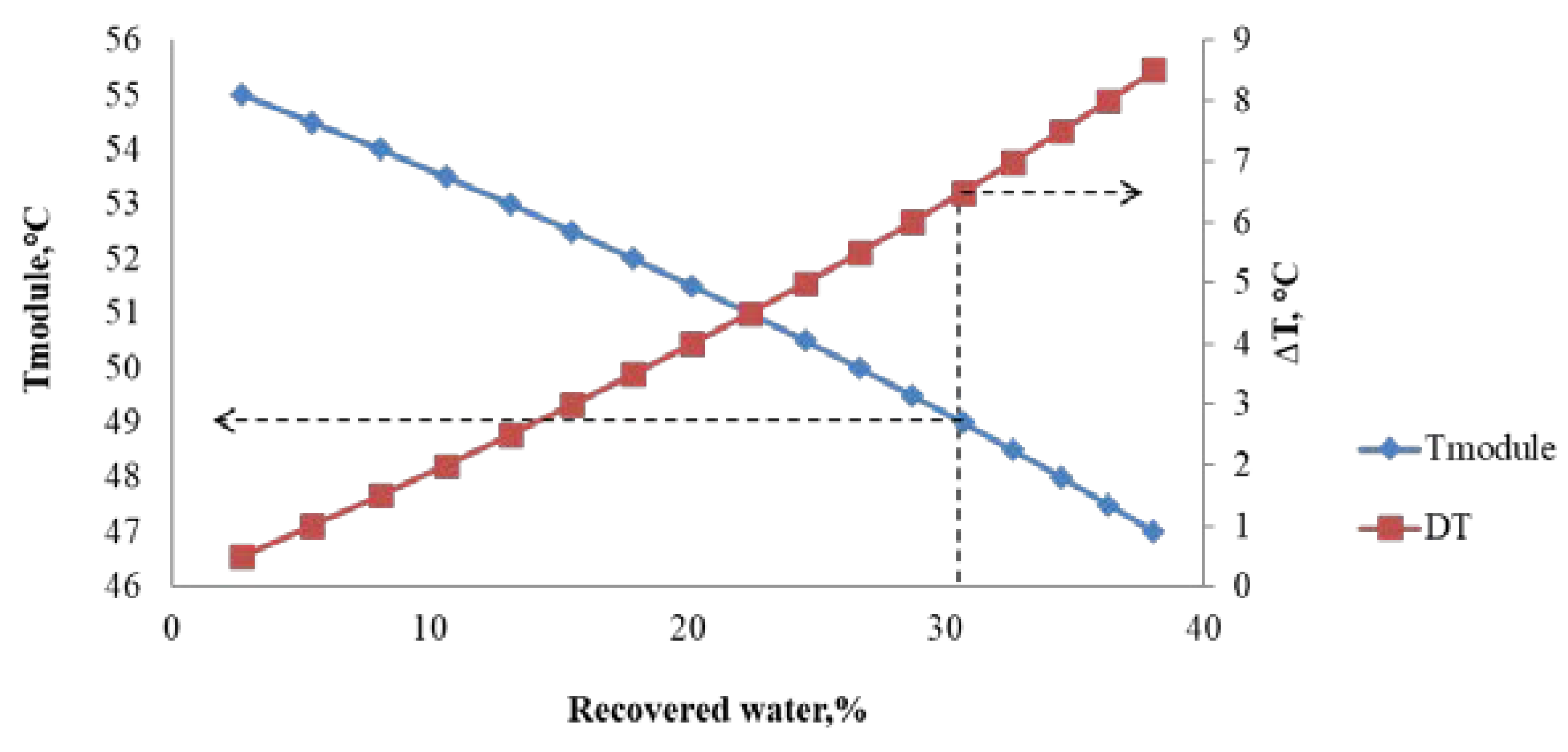
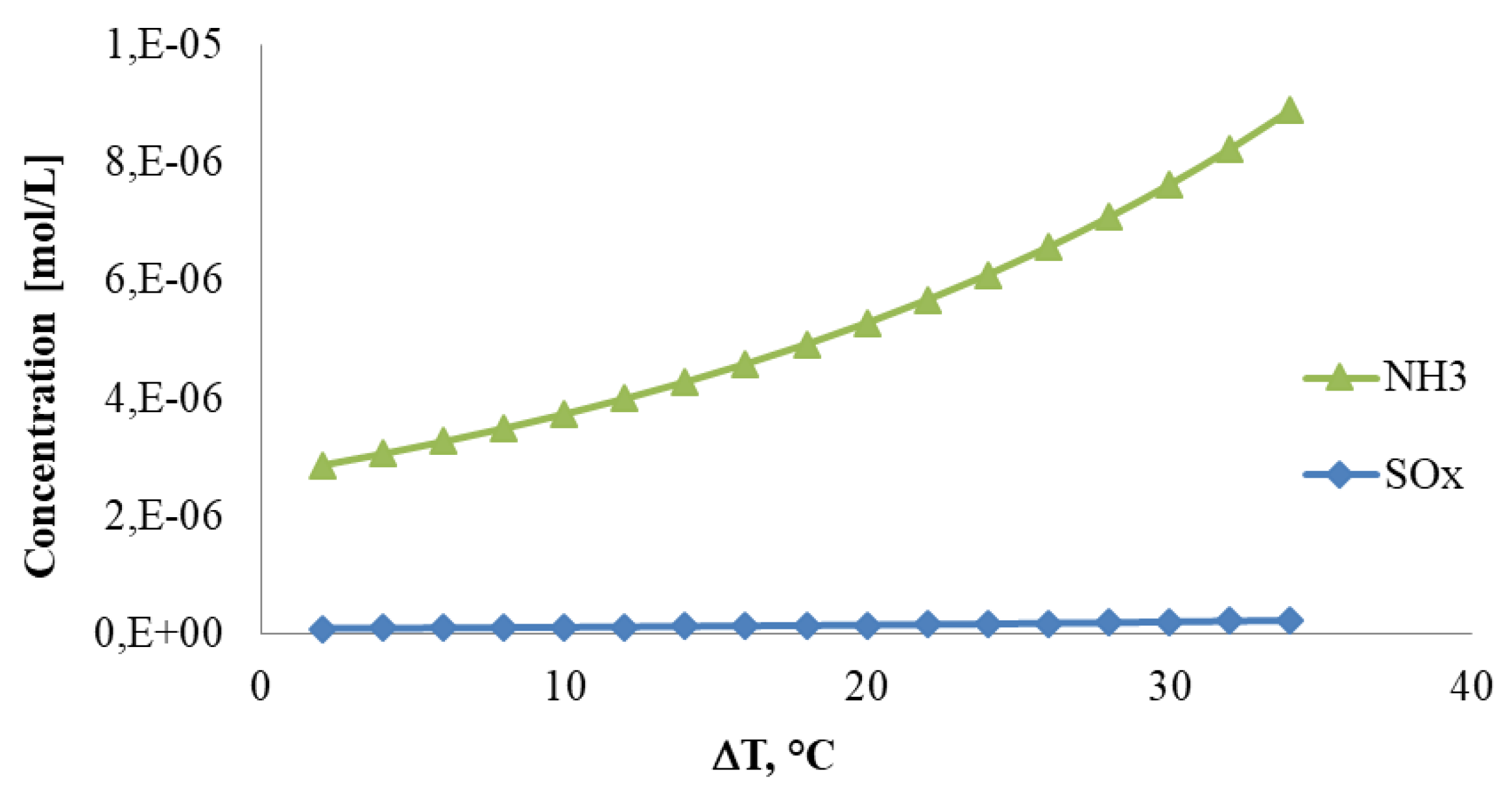
4. Results
5. Conclusions
- -
- it does not suffer corrosion phenomena of traditional condensers because membrane can be fabricated from almost any chemically resistant polymers with hydrophobic intrinsic properties;
- -
- it can control the concentration of contaminants in the recovered liquid water.
Author Contributions
Conflicts of Interest
References
- Drioli, E.; Stankiewicz, A.I.; Macedonio, F. Membrane Engineering in Process Intensification—An Overview. J. Membr. Sci. 2011, 380, 1–8. [Google Scholar] [CrossRef]
- Macedonio, F.; Drioli, E. Membrane Engineering for Green Process Engineering. Engineering 2017, 3, 290–298. [Google Scholar] [CrossRef]
- Drioli, E.; Criscuoli, A.; Macedonio, F. Membrane-Based Desalination: An Integrated Approach; IWA Publishig: London, UK, 2011. [Google Scholar]
- Macedonio, F.; Drioli, E. Chapter 6. Advanced membrane based desalination systems for water and minerals extraction from the sea. In Desalination Sustainability: A Technical, Socioeconomic, and Environmental Approach; Arafat, H., Ed.; Elsevier: New York, NY, USA, 2017; pp. 237–259. [Google Scholar]
- De Bartolo, L.; Curcio, E.; Drioli, E. Membrane Systems: For Bioartificial Organs and Regenerative Medicine; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2017. [Google Scholar]
- Macedonio, F.; Brunetti, A.; Barbieri, G.; Drioli, E. Membrane Condenser as a new technology for water recovery from humidified “waste” gaseous streams. Ind. Eng. Chem. Res. 2013, 52, 1160–1167. [Google Scholar] [CrossRef]
- Drioli, E.; Santoro, S.; Simone, S.; Barbieri, G.; Brunetti, A.; Macedonio, F.; Figoli, A. ECTFE membrane preparation for recovery of humidified gas streams using membrane condenser. React. Funct. Polym. 2014, 79, 1–7. [Google Scholar] [CrossRef]
- Brunetti, A.; Santoro, S.; Macedonio, F.; Figoli, A.; Drioli, E.; Barbieri, G. Waste gaseous streams: From environmental issue to source of water by using membrane condensers. CLEAN Soil Air Water 2014, 42, 1145–1153. [Google Scholar] [CrossRef]
- Macedonio, F.; Cersosimo, M.; Brunetti, A.; Barbieri, G.; Drioli, E. Water Recovery from Humidified Waste Gas Streams: Quality Control Using Membrane Condenser Technology. Chem. Eng. Process. Process Intensif. 2014, 86, 196–203. [Google Scholar] [CrossRef]
- Macedonio, F.; Brunetti, A.; Barbieri, G.; Drioli, E. Membrane condenser configurations for water recovery from waste gases. Sep. Purif. Technol. 2017, 181, 60–68. [Google Scholar] [CrossRef]
- Quist-Jensen, C.A.; Macedonio, F.; Horbez, D.; Drioli, E. Reclamation of sodium sulfate from industrial wastewater by using membrane distillation and membrane crystallization. Desalination 2017, 401, 112–119. [Google Scholar] [CrossRef]
- Di Profio, G.; Tucci, S.; Curcio, E.; Drioli, E. Selective glycine polymorph crystallization by using microporous membranes. Cryst. Growth Des. 2017, 7, 526–530. [Google Scholar] [CrossRef]
- Yanagishita, T.; Tomabechi, Y.; Nishio, K.; Masuda, H. Preparation of monodisperse SiO2 nanoparticles by membrane emulsification using ideally ordered anodic porous alumina. Langmuir 2004, 20, 554–555. [Google Scholar] [CrossRef] [PubMed]
- Banat, F.; Jumah, R.; Garaibeh, M. Exploitation of solar energy collected by solar stills for desalination by membrane distillation. Renew. Energy 2002, 25, 293–305. [Google Scholar] [CrossRef]
- Shim, W.G.; He, K.; Gray, S.; Moon, I.S. Solar energy assisted direct contact membrane distillation (DCMD) process for seawater desalination. Sep. Purif. Technol. 2015, 143, 94–104. [Google Scholar] [CrossRef]
- Michels, B.; Adamczyk, F.; Koch, J. Retrofit of a Flue Gas Heat Recovery System at the Mehrum Power Plant. An Example of Power Plant Lifetime Evaluation in Practice. In Proceedings of the POWER-GEN Europe Conference 2004, Barcelona, Spain, 25–27 May 2004; pp. 10–11. [Google Scholar]
- Folkedahl, B.; Weber, G.F.; Collings, M.E. Water Extraction from Coal-Fired Power Plant Flue Gas; Final Report, DOE Cooperative Agreement No. DE-FC26-03NT41907; National Energy Technology Laboratory: Pittsburgh, PA, USA, 2006.
- Scovazzo, P.; Hoehn, A.; Todd, P. Membrane Porosity and Hydrophilic Membrane-Based Dehumidification Performance. J. Membr. Sci. 2000, 167, 217–225. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drioli, E.; Macedonio, F. Membrane-Assisted Condenser. Clean Technol. 2019, 1, 2-8. https://doi.org/10.3390/cleantechnol1010002
Drioli E, Macedonio F. Membrane-Assisted Condenser. Clean Technologies. 2019; 1(1):2-8. https://doi.org/10.3390/cleantechnol1010002
Chicago/Turabian StyleDrioli, Enrico, and Francesca Macedonio. 2019. "Membrane-Assisted Condenser" Clean Technologies 1, no. 1: 2-8. https://doi.org/10.3390/cleantechnol1010002





