Exploring the Combined Effects of Different Nitrogen Sources and Chabazite Zeolite-Tuff on Nitrogen Dynamics in an Acidic Sandy-Loam Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil
2.2. N Sources and Natural Chabazite Tuff
2.3. Experimental Set-Up
2.4. Analytical Techniques
2.5. Calculations and Statistical Analysis
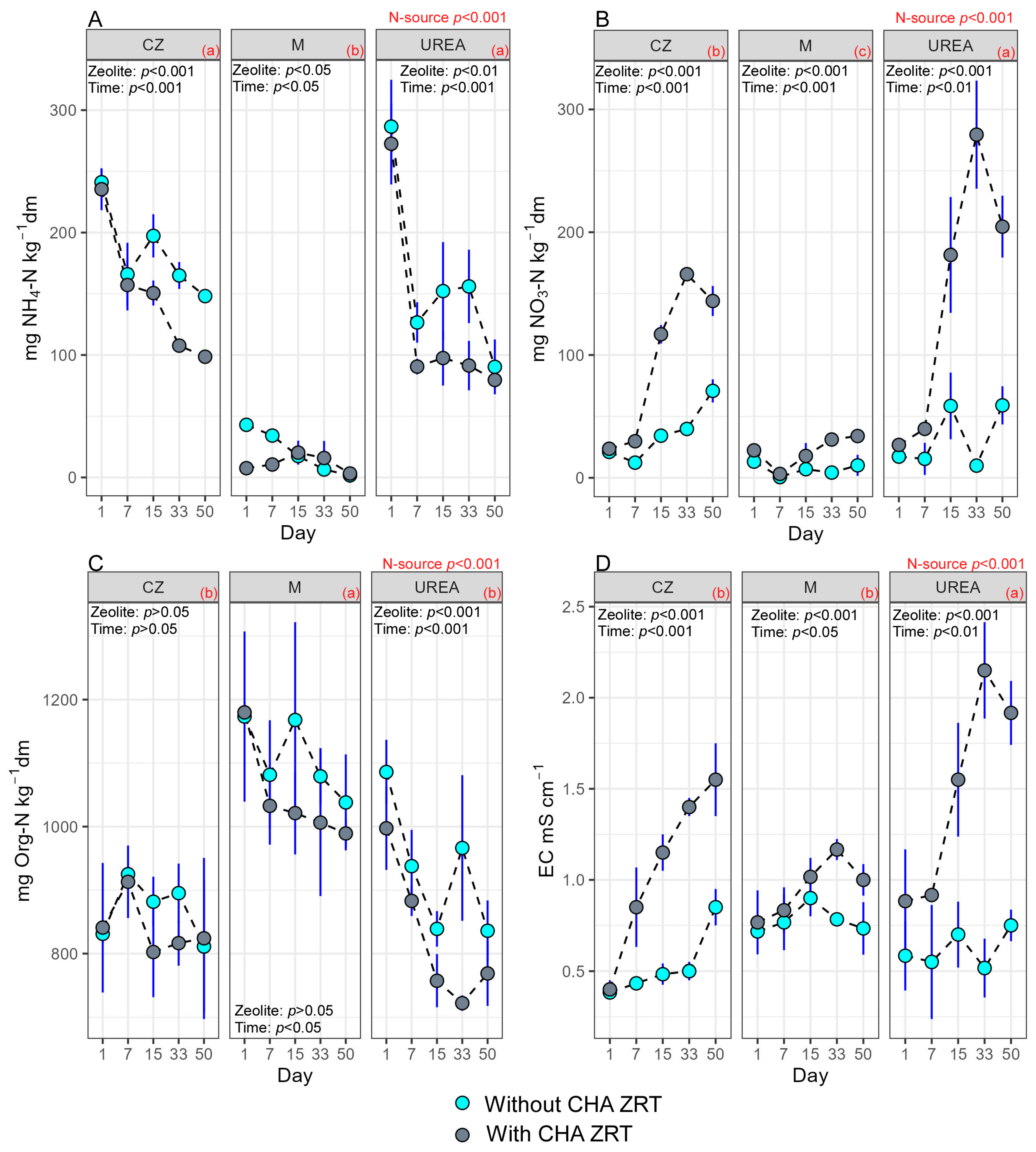
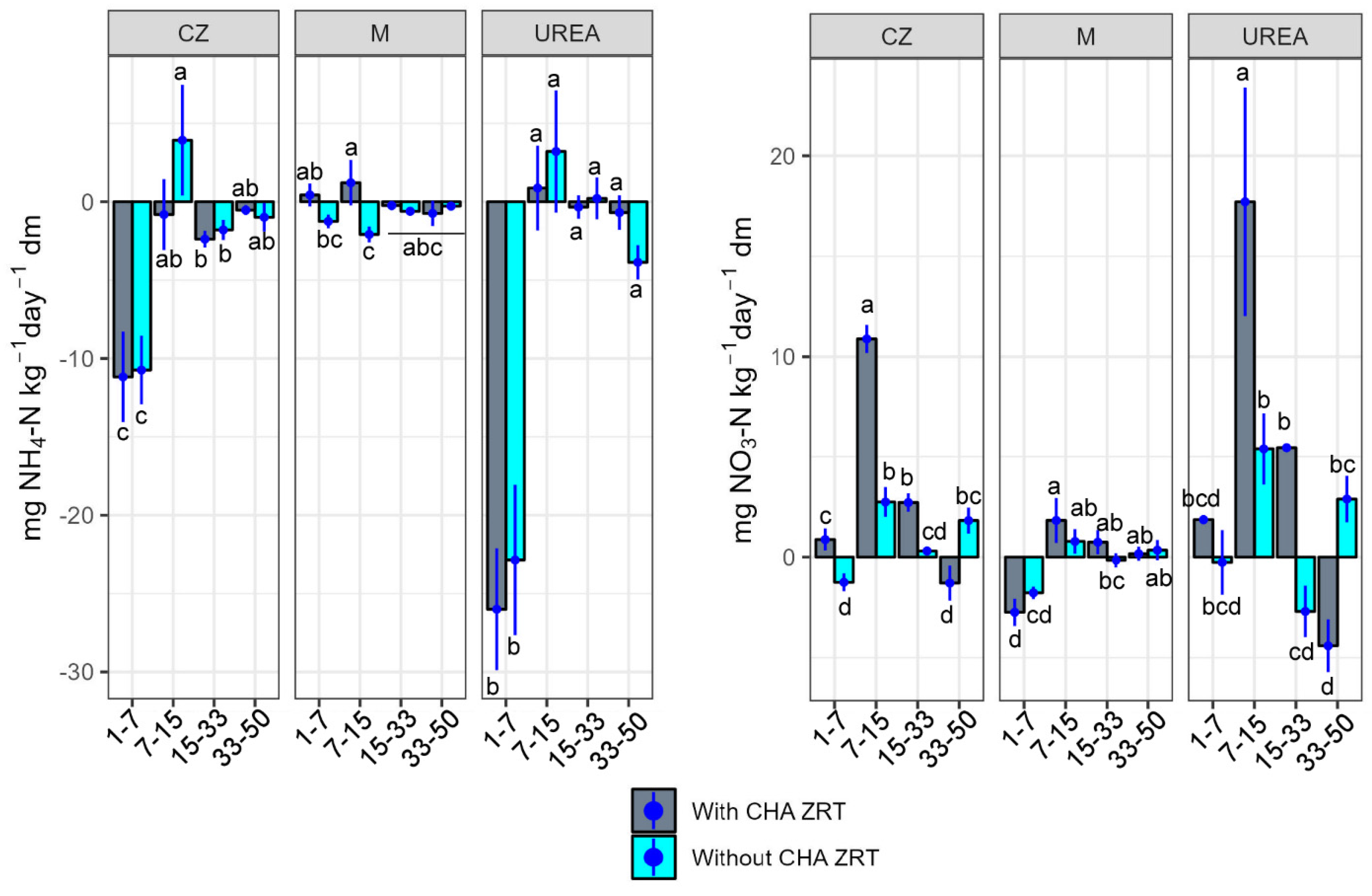
3. Results
3.1. Effects of the Different N Sources
3.2. Effects of Natural CHA Zeolite Tuff as Soil Improver
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Parameter | Units | 10% CHA-ZRT | N SOURCE: CZ | ||||
|---|---|---|---|---|---|---|---|
| Presence | Sampling Day | ||||||
| 1 | 7 | 15 | 33 | 50 | |||
| pH | No | 6.36a | 5.94b | 5.64c | 5.47c | 4.84de | |
| (0.12) | (0.05) | (0.09) | (0.20) | (0.09) | |||
| Yes | 6.45a | 5.62c | 5.13d | 4.77e | 4.64e | ||
| (0.10) | (0.12) | (0.06) | (0.04) | (0.02) | |||
| EC | mS cm−1 | No | 0.38d | 0.43d | 0.48d | 0.50d | 0.85c |
| (0.03) | (0.03) | (0.06) | (0.05) | (0.10) | |||
| Yes | 0.40d | 0.85c | 1.15bc | 1.40ab | 1.55a | ||
| (0.05) | (0.22) | (0.10) | (0.05) | (0.20) | |||
| NH4+-N | mg kg−1 | No | 241a | 166cd | 197bc | 165cd | 148de |
| (11) | (26) | (18) | (11) | (4) | |||
| Yes | 235ab | 157cd | 151d | 108ef | 98.6f | ||
| (17) | (21) | (10) | (4) | (2.8) | |||
| NO3−-N | mg kg−1 | No | 21.1fg | 12.3g | 34.3ef | 39.8e | 70.8d |
| (2.5) | (0.6) | (5.3) | (4.7) | (9.4) | |||
| Yes | 23.6efg | 29.8efg | 117c | 166a | 144b | ||
| (0.9) | (4.6) | (8) | (3) | (12) | |||
| Org-N | mg kg−1 | No | 831a | 925a | 882a | 895a | 811a |
| (13) | (13) | (39) | (47) | (104) | |||
| Yes | 841a | 913a | 803a | 817a | 824a | ||
| (102) | (57) | (71) | (35) | (127) | |||
| TN | mg kg−1 | No | 1093a | 1103a | 1113a | 1100a | 1030a |
| (6) | (15) | (21) | (40) | (113) | |||
| Yes | 1100a | 1100a | 1070a | 1090a | 1067a | ||
| (87) | (78) | (76) | (35) | (132) | |||
| Cl− | mg kg−1 | No | 16.7bc | 28.4abc | 27.8abc | 15.0c | 14.3c |
| (2.9) | (6.5) | (7.4) | (1.1) | (1.8) | |||
| Yes | 19.8abc | 33.8ab | 34.9a | 17.3abc | 16.5bc | ||
| (1.3) | (14.3) | (7.2) | (1.5) | (1.1) | |||
| SO42− | mg kg−1 | No | 9.40d | 15.3c | 19.5bc | 22.8bc | 19.5bc |
| (0.81) | (1.0) | (0.9) | (2.7) | (1.4) | |||
| Yes | 8.98d | 20.4b | 23.5ab | 25.5a | 23.9ab | ||
| (0.20) | (1.5) | (2.2) | (1.9) | (2.3) | |||
| Parameter | Units | 10% CHA-ZRT | N SOURCE: M | ||||
|---|---|---|---|---|---|---|---|
| Presence | Sampling Day | ||||||
| 1 | 7 | 15 | 33 | 50 | |||
| pH | No | 7.08abc | 7.36a | 7.12ab | 6.78c | 6.83bc | |
| (0.12) | (0.18) | (0.21) | (0.03) | (0.04) | |||
| Yes | 6.92bc | 7.33a | 7.01bc | 6.88bc | 6.80c | ||
| (0.05) | (0.06) | (0.09) | (0.04) | (0.08) | |||
| EC | mS cm−1 | No | 0.72b | 0.77b | 0.90ab | 0.78b | 0.73b |
| (0.03) | (0.15) | (0.10) | (0.03) | (0.14) | |||
| Yes | 0.77b | 0.83b | 1.02ab | 1.17a | 1.00ab | ||
| (0.18) | (0.13) | (0.10) | (0.06) | (0.09) | |||
| NH4+-N | mg kg−1 | No | 43.0a | 34.2ab | 17.5bcd | 6.49cd | 1.74d |
| (2.0) | (1.1) | (4.1) | (2.61) | (0.75) | |||
| Yes | 7.56cd | 10.6cd | 20.2bc | 15.9cd | 3.21d | ||
| (3.83) | (1.9) | (9.8) | (13.9) | (0.56) | |||
| NO3−-N | mg kg−1 | No | 13.1cde | 0.73e | 7.00de | 4.24de | 10.1cde |
| (2.4) | (0.18) | (4.79) | (1.59) | (8.6) | |||
| Yes | 22.4abc | 3.17de | 17.8bcd | 31.2ab | 34.0a | ||
| (3.7) | (1.79) | (10.5) | (5.5) | (4.2) | |||
| Org-N | mg kg−1 | No | 1173a | 1082a | 1168a | 1079a | 1038a |
| (134) | (86) | (154) | (45) | (76) | |||
| Yes | 1180a | 1033a | 1021a | 1006a | 989a | ||
| (16) | (61) | (65) | (116) | (23) | |||
| TN | mg kg−1 | No | 1230a | 1117a | 1193a | 1090a | 1050a |
| (135) | (85) | (150) | (44) | (75) | |||
| Yes | 1210a | 1047a | 1060a | 1053a | 1027a | ||
| (10) | (59) | (66) | (110) | (23) | |||
| Cl− | mg kg−1 | No | 65.3ab | 70.7ab | 74.7ab | 82.4a | 53.0b |
| (15.4) | (8.6) | (15.4) | (3.5) | (11.1) | |||
| Yes | 73.7ab | 67.3ab | 78.2ab | 69.8ab | 56.2ab | ||
| (12.2) | (6.6) | (2.0) | (3.3) | (6.7) | |||
| SO42− | mg kg−1 | No | 44.4d | 119c | 172abc | 40.2d | 135bc |
| (0.7) | (12) | (52) | (3.3) | (17) | |||
| Yes | 35.7d | 113c | 188ab | 224a | 136bc | ||
| (4.5) | (21) | (13) | (15) | (21) | |||
| Parameter | Units | 10% CHA-ZRT | N SOURCE: U | ||||
|---|---|---|---|---|---|---|---|
| Presence | Sampling Day | ||||||
| 1 | 7 | 15 | 33 | 50 | |||
| pH | No | 7.99a | 6.72bc | 6.01cde | 6.46cd | 5.59defg | |
| (0.06) | (0.61) | (0.32) | (0.11) | (0.44) | |||
| Yes | 7.60ab | 5.81cdef | 5.22efg | 4.95fg | 4.85g | ||
| (0.23) | (0.13) | (0.39) | (0.24) | (0.23) | |||
| EC | mS cm−1 | No | 0.58b | 0.55b | 0.70b | 0.52b | 0.75b |
| (0.19) | (0.31) | (0.18) | (0.16) | (0.09) | |||
| Yes | 0.88b | 0.92b | 1.55a | 2.15a | 1.92a | ||
| (0.28) | (0.03) | (0.31) | (0.26) | (0.18) | |||
| NH4+-N | mg kg−1 | No | 287a | 127cd | 152bc | 156cd | 90.3bc |
| (38) | (17) | (40) | (30) | (22.4) | |||
| Yes | 273a | 90.5cd | 97.5d | 91.4ef | 79.6c | ||
| (33) | (6.4) | (22.5) | (20.2) | (7.5) | |||
| NO3−-N | mg kg−1 | No | 17.2c | 15.4c | 58.5c | 9.89c | 59.0c |
| (2.8) | (13.0) | (27.1) | (4.09) | (15.5) | |||
| Yes | 26.8c | 39.8c | 182b | 279a | 205b | ||
| (1.2) | (2.3) | (47) | (44) | (25) | |||
| Org-N | mg kg−1 | No | 1086a | 938ab | 839bc | 966ab | 836bc |
| (51) | (57) | (28) | (115) | (48) | |||
| Yes | 997ab | 883bc | 758c | 722c | 769c | ||
| (66) | (24) | (42) | (12) | (51) | |||
| TN | mg kg−1 | No | 1390a | 1080c | 1050c | 1133bc | 987c |
| (75) | (61) | (40) | (127) | (42) | |||
| Yes | 1297ab | 1013c | 1037c | 1093c | 1053c | ||
| (95) | (31) | (21) | (29) | (68) | |||
| Cl− | mg kg−1 | No | 15.6b | 22.4ab | 21.7ab | 16.2b | 12.0b |
| (2.7) | (3.1) | (4.2) | (4.0) | (2.2) | |||
| Yes | 24.4ab | 22.9ab | 37.9a | 16.0b | 14.7b | ||
| (16.3) | (3.8) | (8.7) | (2.3) | (2.1) | |||
| SO42− | mg kg−1 | No | 8.14ef | 12.3de | 14.3bcd | 4.81f | 18.3bc |
| (0.29) | (1.1) | (1.3) | (0.74) | (2.6) | |||
| Yes | 9.49e | 14.1cd | 18.5ab | 22.6a | 18.1bc | ||
| (2.67) | (0.4) | (1.5) | (1.2) | (0.9) | |||
References
- Gobinath, R.; Ganapathy, G.P.; Gayathiri, E.; Salunkhe, A.A.; Pourghasemi, H.R. Ecoengineering practices for soil degradation protection of vulnerable hill slopes. In Computers in Earth and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2022; pp. 255–270. [Google Scholar] [CrossRef]
- Daniels, W.L. The Nature and Properties of Soils, 15th Edition Ray R. Weil and Nyle C. Brady. Pearson Press, Upper Saddle River NJ, 2017. 1086 p. $164.80. ISBN-10: 0-13-325448-8; ISBN-13: 978-0-13-325448-8. Also available as eText for $67.99. Soil Sci. Soc. Am. J. 2016, 80, 1428. [Google Scholar] [CrossRef]
- Maximillian, J.; Brusseau, M.L.; Glenn, E.P.; Matthias, A.D. Pollution and Environmental Perturbations in the Global System. In Environmental and Pollution Science; Academic Press: London, UK, 2019. [Google Scholar]
- Verma, S.; Sharma, P.K. Effect of long-term manuring and fertilizers on carbon pools, soil structure, and sustainability under different cropping systems in wet-temperate zone of northwest Himalayas. Biol. Fertil. Soils 2007, 44, 235–240. [Google Scholar] [CrossRef]
- Chen, R.; Senbayram, M.; Blagodatsky, S.; Myachina, O.; Dittert, K.; Lin, X.; Blagodatskaya, E.; Kuzyakov, Y. Soil C and N availability determine the priming effect: Microbial N mining and stoichiometric decomposition theories. Glob. Chang. Biol. 2014, 20, 2356–2367. [Google Scholar] [CrossRef]
- Dodds, W.K.; Smith, V.H. Nitrogen, phosphorus, and eutrophication in streams. Inl. Waters 2016, 6, 155–164. [Google Scholar] [CrossRef]
- Bijay-Singh; Craswell, E. Fertilizers and nitrate pollution of surface and ground water: An increasingly pervasive global problem. SN Appl. Sci. 2021, 3, 518. [Google Scholar] [CrossRef]
- Dawson, C.J.; Hilton, J. Fertiliser availability in a resource-limited world: Production and recycling of nitrogen and phosphorus. Food Policy 2011, 36, S14–S22. [Google Scholar] [CrossRef]
- Pankievicz, V.C.S.; Irving, T.B.; Maia, L.G.S.; Ané, J.-M. Are we there yet? The long walk towards the development of efficient symbiotic associations between nitrogen-fixing bacteria and non-leguminous crops. BMC Biol. 2019, 17, 99. [Google Scholar] [CrossRef]
- Boaretto, R.M.; Mattos, D.; Quaggio, J.A.; Cantarella, H.; Trivelin, P.C.O. Absorption of 15NH3 volatilized from urea by Citrus trees. Plant Soil 2013, 365, 283–290. [Google Scholar] [CrossRef]
- Soares, J.R.; Cantarella, H.; Menegale, M.L.d.C. Ammonia volatilization losses from surface-applied urea with urease and nitrification inhibitors. Soil Biol. Biochem. 2012, 52, 82–89. [Google Scholar] [CrossRef]
- Cantarella, H.; Mattos, D.; Quaggio, J.A.; Rigolin, A.T. Fruit yield of Valencia sweet orange fertilized with different N sources and the loss of applied N. Nutr. Cycl. Agroecosystems 2003, 67, 215–223. [Google Scholar] [CrossRef]
- Ferretti, G.; Galamini, G.; Medoro, V.; Faccini, B. Amount and speciation of N leached from a sandy soil fertilized with urea, liquid digestate, struvite and NH4 -enriched chabazite zeolite-tuff. Soil Use Manag. 2022, 39, 456–473. [Google Scholar] [CrossRef]
- Al-Zahrani, S.M. Controlled-release of fertilizers: Modelling and simulation. Int. J. Eng. Sci. 1999, 37, 1299–1307. [Google Scholar] [CrossRef]
- Keiblinger, K.M.; Kral, R.M. Sustainable intensification of agricultural production: A review of four soil amendments. Bodenkultur 2018, 69, 141–153. [Google Scholar] [CrossRef]
- Ming, D.W.; Allen, E.R. Use of natural zeolites in agronomy, horticulture, and environmental soil remediation. Rev. Mineral. Geochem. 2001, 45, 618–654. [Google Scholar] [CrossRef]
- Di Giuseppe, D.; Ferretti, G.; Faccini, B.; Blasi, E.; Passeri, N.; Bianchini, G.; Coltorti, M. Is it possible to cultivate corn in a sustainable way using a quarry waste? Period. Mineral. 2016, 85, 179–183. [Google Scholar] [CrossRef]
- Azarpour, E.; Motamed, M.K.; Moraditochaee, M.; Bozorgi, H.R. Effects of zeolite application and nitrogen fertilization on yield components of Cowpea (Vigna unguiculata L.). World Appl. Sci. J. 2011, 14, 687–692. [Google Scholar]
- Nakhli, S.A.A.; Delkash, M.; Bakhshayesh, B.E.; Kazemian, H. Application of Zeolites for Sustainable Agriculture: A Review on Water and Nutrient Retention. Water Air Soil Pollut. 2017, 228, 464. [Google Scholar] [CrossRef]
- Ferretti, G.; Galamini, G.; Medoro, V.; Coltorti, M.; Di Giuseppe, D.; Faccini, B. Impact of sequential treatments with natural and na-exchanged chabazite zeolite-rich tuff on pig-slurry chemical composition. Water 2020, 12, 310. [Google Scholar] [CrossRef]
- Delkash, M.; Bakhshayesh, B.E.; Kazemian, H. Using zeolitic adsorbents to cleanup special wastewater streams: A review. Microporous Mesoporous Mater. 2015, 214, 224–241. [Google Scholar] [CrossRef]
- Dwairi, I.M. Evaluation of jordanian zeolite tuff as a controlled slow-release fertilizer for NH4+. Environ. Geol. 1998, 34, 1–4. [Google Scholar] [CrossRef]
- Ferretti, G.; Di Giuseppe, D.; Faccini, B.; Coltorti, M. Mitigation of sodium risk in a sandy agricultural soil by the use of natural zeolites. Environ. Monit. Assess. 2018, 190, 646. [Google Scholar] [CrossRef]
- Innovative Biological Technologies for Lesser Developed Countries—Workshop Proceedings (Washington, DC: U.S. Congress, Office of Technology Assessment, OTA13P-F-29, July 1985). Available online: https://ota.fas.org/reports/8512.pdf (accessed on 10 January 2024).
- Passaglia, E. Zeoliti Naturali, Zeolititi e Loro Applicazioni; Arvan: Mira, Italy, 2008; ISBN 9788887801194. [Google Scholar]
- Coombs, D.S.; Alberti, A.; Armbruster, T.; Artioli, G.; Colella, C.; Galli, E.; Grice, J.D.; Liebau, F.; Mandarino, J.A.; Minato, H.; et al. Recommended nomenclature for zeolite minerals: Report of the subcommittee on zeolites of the International Mineralogical Association, Commission on new Minerals and Mineral names. Can. Mineral. 1997, 35, 1571–1606. [Google Scholar] [CrossRef]
- Moshoeshoe, M.; Nadiye-Tabbiruka, M.S.; Obuseng, V. A Review of the Chemistry, Structure, Properties and Applications of Zeolites. Am. J. Mater. Sci. 2017, 7, 196–221. [Google Scholar] [CrossRef]
- Galli, E.; Passaglia, E. Natural zeolites in environmental engineering. In Zeolites in Chemical Engineering; Holzapfel, H., Ed.; Verlag ProcessEng Engineering GmbH: Wien, Austria, 2011; pp. 392–416. [Google Scholar]
- Mumpton, F.A. La roca magica: Uses of natural zeolites in agriculture and industry. Proc. Natl. Acad. Sci. USA 1999, 96, 3463–3470. [Google Scholar] [CrossRef] [PubMed]
- Ouki, S.K.; Kavannagh, M. Performance of natural zeolites for the treatment of mixed metal-contaminated effluents. Waste Manag. Res. 1997, 15, 383–394. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Passaglia, E. Rietveld structure refinement of NH4-exchanged natural chabazite. Eur. J. Mineral. 2006, 18, 351–359. [Google Scholar] [CrossRef]
- Galamini, G.; Ferretti, G.; Medoro, V.; Tescaro, N.; Faccini, B.; Coltorti, M. Isotherms, Kinetics, and Thermodynamics of NH4+ Adsorption in Raw Liquid Manure by Using Natural Chabazite Zeolite-Rich Tuff. Water 2020, 12, 2944. [Google Scholar] [CrossRef]
- Ferretti, G.; Keiblinger, K.M.; Zimmermann, M.; Di Giuseppe, D.; Faccini, B.; Colombani, N.; Mentler, A.; Zechmeister-Boltenstern, S.; Coltorti, M.; Mastrocicco, M. High resolution short-term investigation of soil CO2, N2O, NOx and NH3 emissions after different chabazite zeolite amendments. Appl. Soil Ecol. 2017, 119, 138–144. [Google Scholar] [CrossRef]
- Ferretti, G.; Keiblinger, K.M.; Di Giuseppe, D.; Faccini, B.; Colombani, N.; Zechmeister-Boltenstern, S.; Coltorti, M.; Mastrocicco, M. Short-Term Response of Soil Microbial Biomass to Different Chabazite Zeolite Amendments. Pedosphere 2018, 28, 277–287. [Google Scholar] [CrossRef]
- Ferretti, G.; Galamini, G.; Deltedesco, E.; Gorfer, M.; Fritz, J.; Faccini, B.; Mentler, A.; Zechmeister-Boltenstern, S.; Coltorti, M.; Keiblinger, K.M. Gross Ammonification and Nitrification Rates in Soil Amended with Natural and NH4-Enriched Chabazite Zeolite and Nitrification Inhibitor DMPP. Appl. Sci. 2021, 11, 2605. [Google Scholar] [CrossRef]
- Ferretti, G.; Faccini, B.; Antisari, L.V.; Di Giuseppe, D.; Coltorti, M. 15N natural abundance, nitrogen and carbon pools in soil-sorghum system amended with natural and NH4+-enriched zeolitites. Appl. Sci. 2019, 9, 4524. [Google Scholar] [CrossRef]
- Galamini, G.; Ferretti, G.; Rosinger, C.; Huber, S.; Medoro, V.; Mentler, A.; Diaz-Pines, E.; Gorfer, M.; Faccini, B.; Keiblinger, K.M. Recycling nitrogen from liquid digestate via novel reactive struvite and zeolite minerals to mitigate agricultural pollution. Chemosphere 2023, 317, 137881. [Google Scholar] [CrossRef]
- Bateman, E.J.; Baggs, E.M. Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol. Fertil. Soils 2005, 41, 379–388. [Google Scholar] [CrossRef]
- Salehi, M.H.; Beni, O.H.; Harchegani, H.B.; Borujeni, I.E.; Motaghian, H.R. Refining Soil Organic Matter Determination by Loss-on-Ignition. Pedosphere 2011, 21, 473–482. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, Y.; Wu, Z.; Yan, X.; Gunina, A.; Kuzyakov, Y.; Xiong, Z. Effects of six-year biochar amendment on soil aggregation, crop growth, and nitrogen and phosphorus use efficiencies in a rice-wheat rotation. J. Clean. Prod. 2020, 242, 118435. [Google Scholar] [CrossRef]
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. R Package Version 1.2-0. 2014. Available online: http://CRAN.R-project.org/package=agricolae (accessed on 10 January 2024).
- Ginestet, C. ggplot2: Elegant Graphics for Data Analysis. J. R. Stat. Soc. Ser. A Stat. Soc. 2011, 174, 245–246. [Google Scholar] [CrossRef]
- Oshunsanya, S. (Ed.) Soil pH for Nutrient Availability and Crop Performance; IntechOpen: London, UK, 2019; ISBN 978-1-78985-015-4. [Google Scholar]
- Latifah, O.; Ahmed, O.H.; Majid, N.M.A. Soil pH Buffering Capacity and Nitrogen Availability Following Compost Application in a Tropical Acid Soil. Compost Sci. Util. 2018, 26, 1–15. [Google Scholar] [CrossRef]
- García-Gil, J.C.; Ceppi, S.B.; Velasco, M.I.; Polo, A.; Senesi, N. Long-term effects of amendment with municipal solid waste compost on the elemental and acidic functional group composition and pH-buffer capacity of soil humic acids. Geoderma 2004, 121, 135–142. [Google Scholar] [CrossRef]
- Terman, G.L. Volatilization Losses of Nitrogen as Ammonia from Surface-Applied Fertilizers, Organic Amendments, and Crop Residues. Adv. Agron. 1980, 31, 189–223. [Google Scholar] [CrossRef]
- Ernst, J.W.; Massey, H.F. The Effects of Several Factors on Volatilization of Ammonia Formed from Urea in the Soil. Soil Sci. Soc. Am. J. 1960, 24, 87–90. [Google Scholar] [CrossRef]
- Cantarella, H.; Otto, R.; Soares, J.R.; de Brito Silva, A.G. Agronomic efficiency of NBPT as a urease inhibitor: A review. J. Adv. Res. 2018, 13, 19–27. [Google Scholar] [CrossRef]
- Wang, J.; Tu, X.; Zhang, H.; Cui, J.; Ni, K.; Chen, J.; Cheng, Y.; Zhang, J.; Chang, S.X. Effects of ammonium-based nitrogen addition on soil nitrification and nitrogen gas emissions depend on fertilizer-induced changes in pH in a tea plantation soil. Sci. Total Environ. 2020, 747, 141340. [Google Scholar] [CrossRef]
- Zaman, M.; Nguyen, M.L.; Matheson, F.; Blennerhassett, J.D.; Quin, B.F. Can soil amendments (zeolite or lime) shift the balance between nitrous oxide and dinitrogen emissions from pasture and wetland soils receiving urine or urea-N? Aust. J. Soil Res. 2007, 45, 543–553. [Google Scholar] [CrossRef]
- Ghorbani, M.; Amirahmadi, E.; Konvalina, P.; Moudrý, J.; Bárta, J.; Kopecký, M.; Teodorescu, R.I.; Bucur, R.D. Comparative Influence of Biochar and Zeolite on Soil Hydrological Indices and Growth Characteristics of Corn (Zea mays L.). Water 2022, 14, 3506. [Google Scholar] [CrossRef]
- Tsadilas, C.D.; Dimoyiannis, D.; Samaras, V. Effect of zeolite application and soil pH on cadmium sorption in soils. Commun. Soil Sci. Plant Anal. 1997, 28, 1591–1602. [Google Scholar] [CrossRef]
- De Boer, W.; Kowalchuk, G.A. Nitrification in acid soils: Micro-organisms and mechanisms. Soil Biol. Biochem. 2001, 33, 853–866. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Li, Y. Zeolite as slow release fertilizer on spinach yields and quality in a greenhouse test. J. Plant Nutr. 2013, 36, 1496–1505. [Google Scholar] [CrossRef]
- Cataldo, E.; Salvi, L.; Paoli, F.; Fucile, M.; Masciandaro, G.; Manzi, D.; Masini, C.M.; Mattii, G.B. Application of Zeolites in Agriculture and Other Potential Uses: A Review. Agronomy 2021, 11, 1547. [Google Scholar] [CrossRef]
- De Campos Bernardi, A.C.; Oliviera, P.P.A.; De Melo Monte, M.B.; Souza-Barros, F. Brazilian sedimentary zeolite use in agriculture. Microporous Mesoporous Mater. 2013, 167, 16–21. [Google Scholar] [CrossRef]
- Ramesh, V.; Jyothi, J.S.; Shibli, S.M.A. Effect of zeolites on soil quality, plant growth and nutrient uptake efficiency in sweet potato (Ipomoea batatas L.). J. Root Crops 2015, 41, 25–31. [Google Scholar]
- Ravali, C.; Rao, J.K.; Anjaiah, T.; Suresh, K. Effect of zeolite on soil physical and physico-chemical properties. J. Sci. Agric. Eng. 2020, 10, 776–781. [Google Scholar]
- Norton, J.M. Nitrification in Agricultural Soils. In Nitrogen in Agricultural Systems; Wiley: Hoboken, New Jersey, USA, 2015; pp. 173–199. ISBN 9780891181910. [Google Scholar]
- Król, M. Natural vs. Synthetic Zeolites. Crystals 2020, 10, 622. [Google Scholar] [CrossRef]
- Bundan, L.; Majid, N.M.A.; Ahmed, O.H.; Jiwan, M.; Kundat, F.R. Ammonia volatilization from urea at different levels of zeolite. Int. J. Phys. Sci. 2011, 6, 7717–7720. [Google Scholar] [CrossRef]
- Ahmed, O.H.; Yap, C.H.B.; Muhamad, A.M.N. Minimizing ammonia loss from urea through mixing with zeolite and acid sulphate soil. Int. J. Phys. Sci. 2010, 5, 2198–2202. [Google Scholar]
- Bi, Q.-F.; Chen, Q.-H.; Yang, X.-R.; Li, H.; Zheng, B.-X.; Zhou, W.-W.; Liu, X.-X.; Dai, P.-B.; Li, K.-J.; Lin, X.-Y. Effects of combined application of nitrogen fertilizer and biochar on the nitrification and ammonia oxidizers in an intensive vegetable soil. AMB Express 2017, 7, 198. [Google Scholar] [CrossRef]
- Hoogsteen, M.J.J.; Lantinga, E.A.; Bakker, E.J.; Groot, J.C.J.; Tittonell, P.A. Estimating soil organic carbon through loss on ignition: Effects of ignition conditions and structural water loss. Eur. J. Soil Sci. 2015, 66, 320–328. [Google Scholar] [CrossRef]
- Nakatsuka, A.; Kawata, N.; Fujiwara, K. Dehydration behavior of a natural hydrated Ca-chabazite studied by in-situ high-temperature single-crystal X-ray diffraction. Microporous Mesoporous Mater. 2020, 311, 110648. [Google Scholar] [CrossRef]


| PSD | Diameter (µm) | Weight % | CEC | (mEq/kg) |
|---|---|---|---|---|
| Very coarse sand | 2000–1000 | 9.50 ± 0.41 | Na+ | 0.35 ± 0.03 |
| Coarse sand | 1000–630 | 10.4 ± 1.6 | Mg2+ | 9.56 ± 0.42 |
| Medium sand | 630–200 | 20.0 ± 1.6 | K+ | 4.6 ± 0.02 |
| Fine sand | 200–63 | 16.5 ± 0.1 | Ca2+ | 23.3 ± 0.90 |
| Coarse silt | 63–20 | 24.8 ± 0.0 | ||
| Silt | 20–2 | 9.28 ± 1.00 | ||
| Clay | <2 | 9.50 ± 0.60 | Total CEC | 37.9 ± 1.40 |
| pH | 5.27 ± 0.03 | |||
| EC (mS/cm) | 0.59 ± 0.07 | |||
| Total N (g/kg) | 1.17 ± 0.07 | |||
| Organic C (g/kg) | 8.77 ± 0.25 |
| N Sources | TN (g/kg) | NH4+-N (g/kg) | TC (g/kg) |
|---|---|---|---|
| M | 38.3 | 0.207 | 305 |
| UREA | 460 * | / | 200 |
| CZ | 6.02 | 4.02 | 1.70 ¥ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferretti, G.; Alberghini, M.; Galamini, G.; Medoro, V.; Faccini, B.; Balzan, S.; Coltorti, M. Exploring the Combined Effects of Different Nitrogen Sources and Chabazite Zeolite-Tuff on Nitrogen Dynamics in an Acidic Sandy-Loam Soil. Soil Syst. 2024, 8, 16. https://doi.org/10.3390/soilsystems8010016
Ferretti G, Alberghini M, Galamini G, Medoro V, Faccini B, Balzan S, Coltorti M. Exploring the Combined Effects of Different Nitrogen Sources and Chabazite Zeolite-Tuff on Nitrogen Dynamics in an Acidic Sandy-Loam Soil. Soil Systems. 2024; 8(1):16. https://doi.org/10.3390/soilsystems8010016
Chicago/Turabian StyleFerretti, Giacomo, Matteo Alberghini, Giulio Galamini, Valeria Medoro, Barbara Faccini, Silvia Balzan, and Massimo Coltorti. 2024. "Exploring the Combined Effects of Different Nitrogen Sources and Chabazite Zeolite-Tuff on Nitrogen Dynamics in an Acidic Sandy-Loam Soil" Soil Systems 8, no. 1: 16. https://doi.org/10.3390/soilsystems8010016







