Interactions of Arbuscular Mycorrhizal Fungi with Hyphosphere Microbial Communities in a Saline Soil: Impacts on Phosphorus Availability and Alkaline Phosphatase Gene Abundance
Abstract
:1. Introduction
2. Materials and methods
2.1. Soil
2.2. AMF Inoculum and Plant Host
2.3. Experimental Design and Growth Conditions
2.4. Root Staining and AMF Colonization
2.5. Soil Extractable P and Plant Shoot P Concentration
2.6. Phosphatase Gene Quantitation in Hyphosphere Soil
2.7. Alkaline Phosphatase Enzyme Assay
2.8. Statistical Analysis
3. Results
3.1. Root Colonization Effects of Experimental Treatments
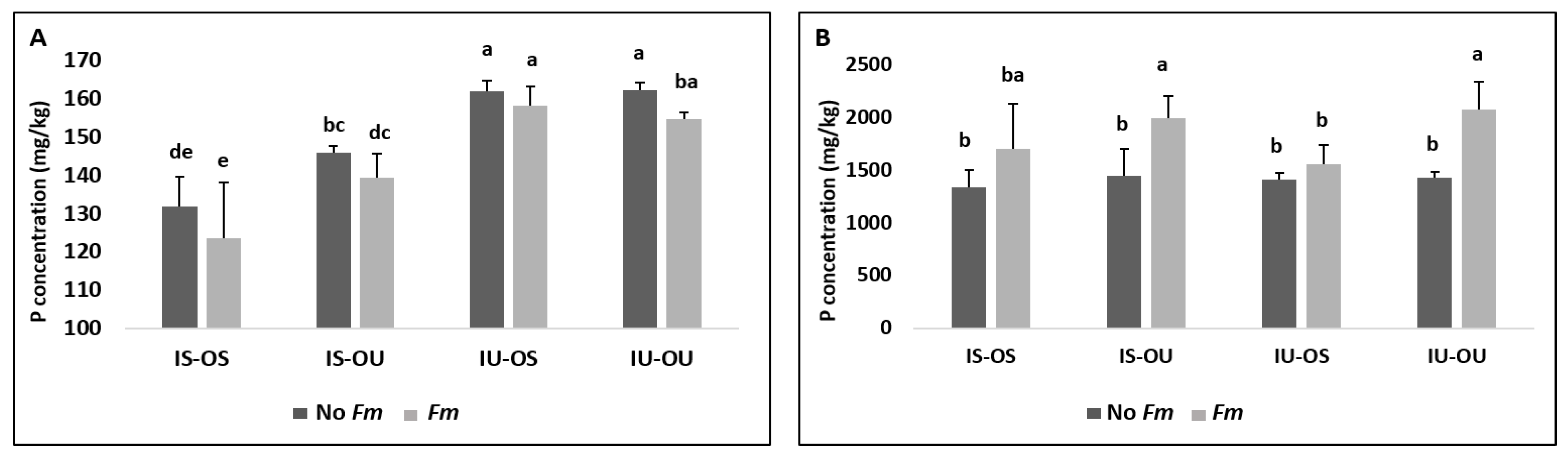
3.2. Plant Available P in Hyphosphere Soil and Its Uptake by Plants
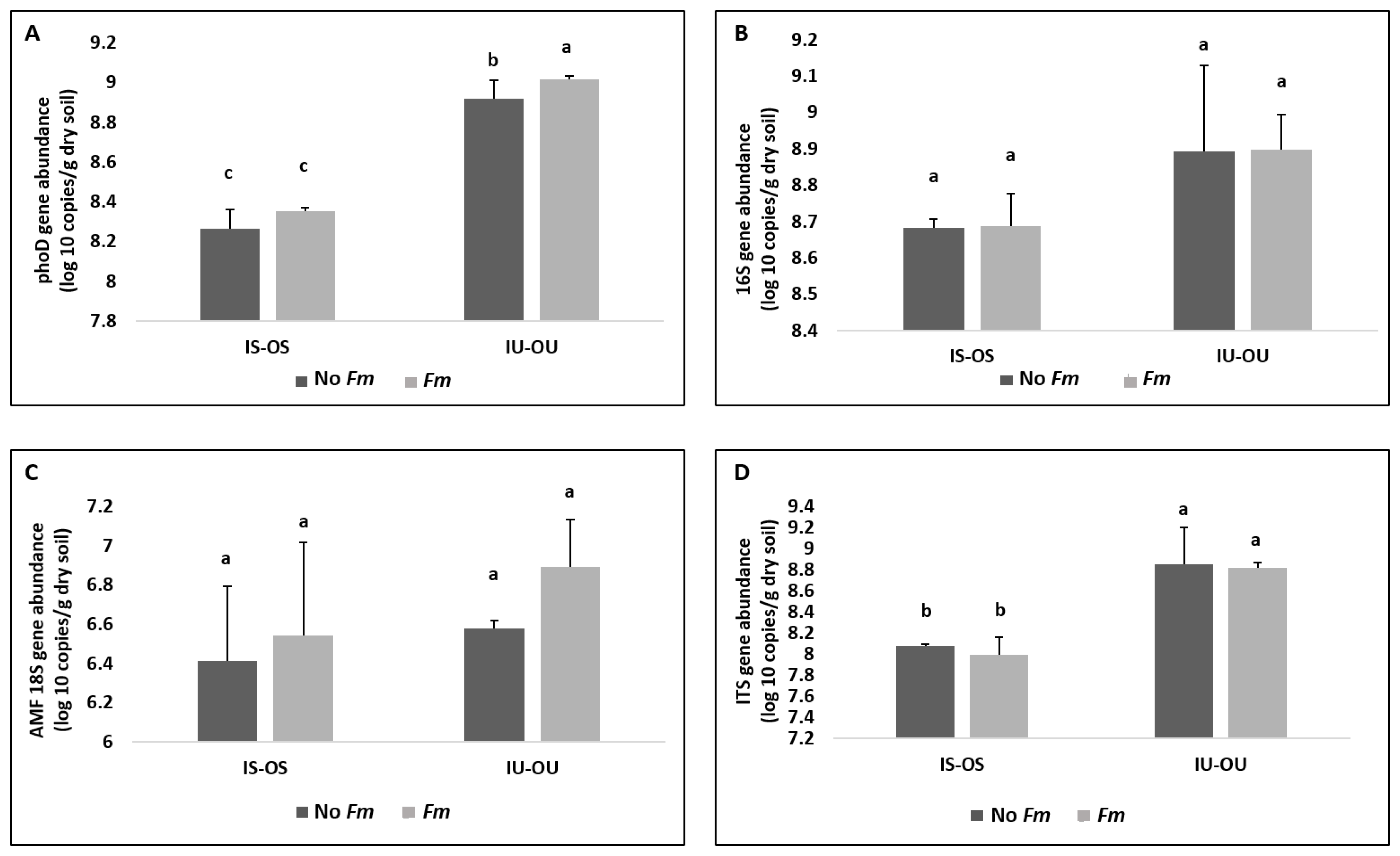
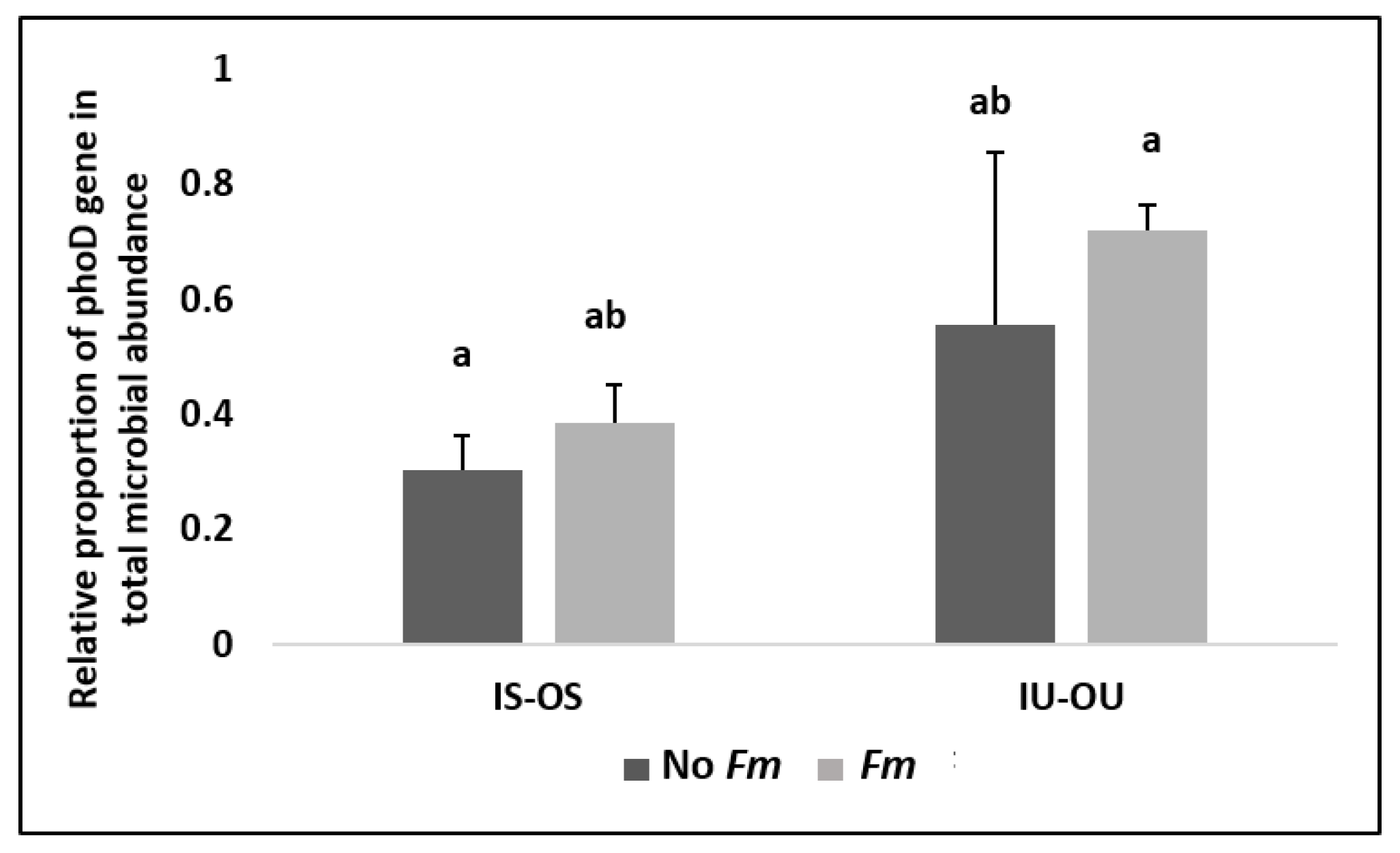
3.3. Alkaline Phosphatase Gene (phoD) and Microbial Community Abundance in the Hyphosphere
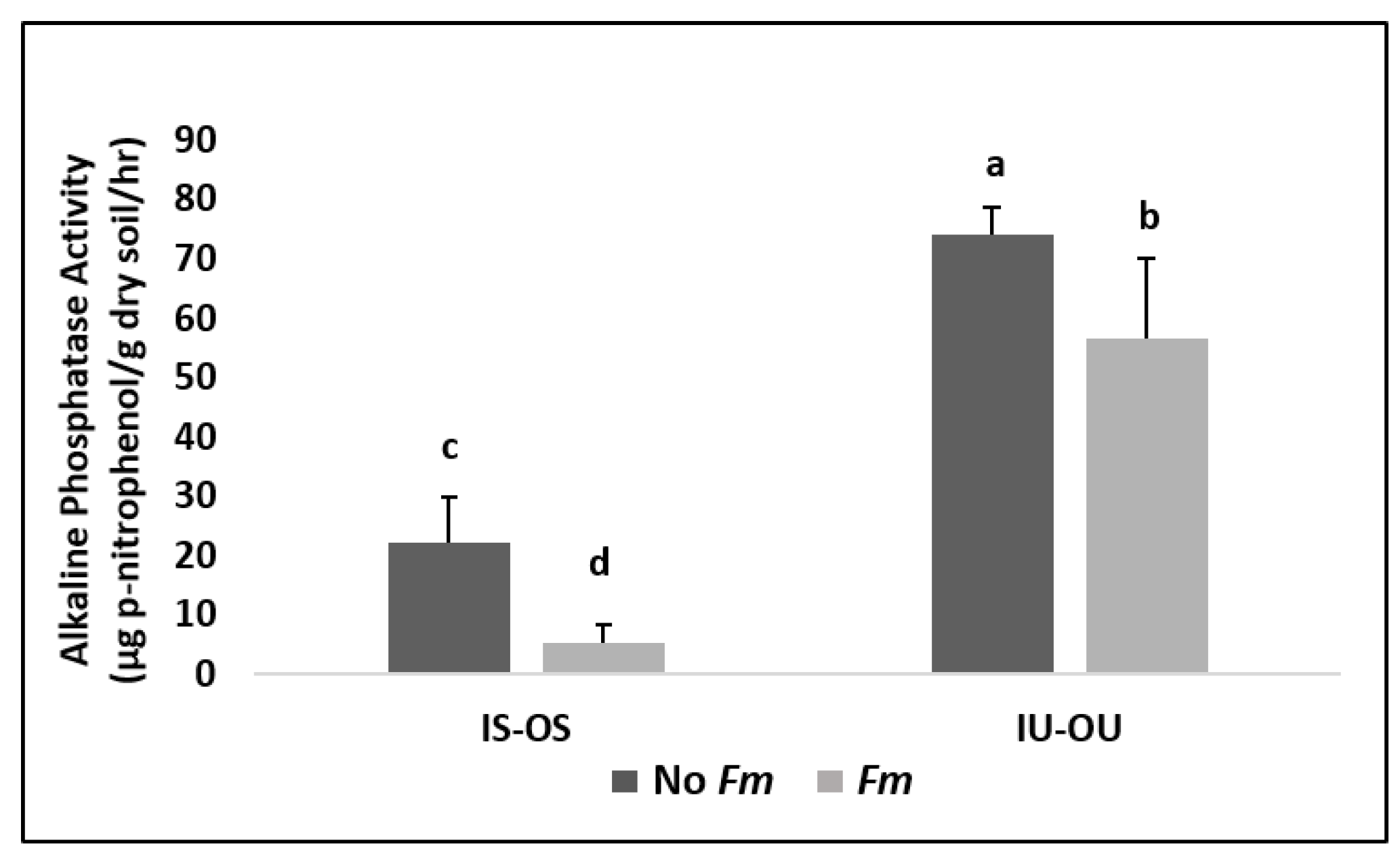
3.4. Alkaline Phosphatase Enzyme Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Szabolcs, I. Salt affected soils as the ecosystem for halophytes. In Halophytes as a Resource for Livestock and for Rehabilitation of Degraded Lands; Springer: Dordrecht, The Netherlands, 1994; pp. 19–24. [Google Scholar]
- Butcher, K.; Wick, A.F.; DeSutter, T.; Chatterjee, A.; Harmon, J. Soil Salinity: A Threat to Global Food Security. Agron. J. 2016, 108, 2189–2200. [Google Scholar] [CrossRef]
- De Aguilar, C.A.-G.; Azcón, R.; Barea, J.M. Endomycorrhizal fungi and Rhizobium as biological fertilisers for Medicago sativa in normal cultivation. Nat. Cell Biol. 1979, 279, 325–327. [Google Scholar] [CrossRef]
- Richardson, A.E.; Hocking, P.J.; Simpson, R.J.; George, T.S. Plant mechanisms to optimise access to soil phosphorus. Crop. Pasture Sci. 2009, 60, 124–143. [Google Scholar] [CrossRef]
- Jansa, J.; Finlay, R.D.; Wallander, H.; Smith, F.A.; Smith, S.E. Role of Mycorrhizal Symbioses in Phosphorus Cycling. In Phosphorus in Action; Springer: Berlin, Germay, 2011; pp. 137–168. [Google Scholar]
- Miransari, M. Arbuscular Mycorrhizal Fungi and Soil Salinity. In Mycorrhizal Mediation of Soil; Elsevier: Amsterdam, The Netherlands, 2017; pp. 263–277. [Google Scholar]
- Ruiz-Lozano, J.M.; Azcón, R. Symbiotic efficiency and infectivity of an autochthonous arbuscular mycorrhizal Glomus sp. from saline soils and Glomus deserticola under salinity. Mycorrhiza 2000, 10, 137–143. [Google Scholar] [CrossRef]
- Kumar, A.; Dames, J.F.; Gupta, A.; Sharma, S.; Gilbert, J.A.; Ahmad, P. Current developments in arbuscular mycorrhizal fungi research and its role in salinity stress alleviation: A biotechnological perspective. Crit. Rev. Biotechnol. 2015, 35, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Evelin, H.; Kapoor, R.; Giri, B. Arbuscular mycorrhizal fungi in alleviation of salt stress: A review. Ann. Bot. 2009, 104, 1263–1280. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.; Zahir, Z.A.; Nazli, F.; Akram, F.; Arshad, M.; Khalid, M. Effectiveness of halo-tolerant, auxin producing Pseudomonas and Rhizobium strains to improve osmotic stress tolerance in mung bean (Vigna radiata L.). Braz. J. Microbiol. 2013, 44, 1341–1348. [Google Scholar] [CrossRef] [Green Version]
- Shukla, P.S.; Agarwal, P.K.; Jha, B. Improved Salinity Tolerance of Arachis hypogaea (L.) by the Interaction of Halotolerant Plant-Growth-Promoting Rhizobacteria. J. Plant Growth Regul. 2012, 31, 195–206. [Google Scholar] [CrossRef]
- Tank, N.; Saraf, M. Salinity-resistant plant growth promoting rhizobacteria ameliorates sodium chloride stress on tomato plants. J. Plant Interact. 2010, 5, 51–58. [Google Scholar] [CrossRef]
- Calvo, P.; Watts, D.B.; Kloepper, J.W.; Torbert, H.A. Effect of microbial-based inoculants on nutrient concentrations and early root morphology of corn (Zea mays). J. Plant Nutr. Soil Sci. 2017, 180, 636. [Google Scholar] [CrossRef] [Green Version]
- Köhler, J.; Caravaca, F.; Roldan, A. An AM fungus and a PGPR intensify the adverse effects of salinity on the stability of rhizosphere soil aggregates of Lactuca sativa. Soil Biol. Biochem. 2010, 42, 429–434. [Google Scholar] [CrossRef]
- Giri, B.; Mukerji, K.G. Mycorrhizal inoculant alleviates salt stress in Sesbania aegyptiaca and Sesbania grandiflora under field conditions: Evidence for reduced sodium and improved magnesium uptake. Mycorrhiza 2004, 14, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Hidri, R.; Mahmoud, O.M.-B.; Farhat, N.; Cordero, I.; Pueyo, J.J.; Debez, A.; Barea, J.-M.; Abdelly, C.; Azcon, R.; Debbez, A. Arbuscular mycorrhizal fungus and rhizobacteria affect the physiology and performance of Sulla coronaria plants subjected to salt stress by mitigation of ionic imbalance. J. Plant Nutr. Soil Sci. 2019, 182, 451–462. [Google Scholar] [CrossRef]
- Giri, B.; Kapoor, R.; Mukerji, K.G. Improved Tolerance of Acacia nilotica to Salt Stress by Arbuscular Mycorrhiza, Glomus fasciculatum may be Partly Related to Elevated K/Na Ratios in Root and Shoot Tissues. Microb. Ecol. 2007, 54, 753–760. [Google Scholar] [CrossRef]
- Ryan, M.H.; Graham, J.H. Little evidence that farmers should consider abundance or diversity of arbuscular mycorrhizal fungi when managing crops. New Phytol. 2018, 220, 1092–1107. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.-S.; Sharma, S.; Gosal, S.K.K.; Choudhary, R.; Singh, R.; Adholeya, A.; Singh, B.-S. Synergistic Use of Plant Growth-Promoting Rhizobacteria, Arbuscular Mycorrhizal Fungi, and Spectral Properties for Improving Nutrient Use Efficiencies in Wheat (Triticum aestivum L.). Commun. Soil Sci. Plant Anal. 2020, 51, 14–27. [Google Scholar] [CrossRef]
- Martin, F.M.; Harrison, M.J.; Lennon, S.; Lindahl, B.; Öpik, M.; Polle, A.; Requena, N.; Selosse, M.-A. Cross-scale integration of mycorrhizal function. New Phytol. 2018, 220, 941–946. [Google Scholar] [CrossRef] [Green Version]
- Talaat, N.B.; Shawky, B. Microbe-Mediated Induced Abiotic Stress Tolerance Responses in Plants. In Plant-Microbe Interactions in Agro-Ecological Perspectives; Springer: Berlin, Germany, 2017; pp. 101–133. [Google Scholar]
- Johansson, J.F.; Paul, L.R.; Finlay, R.D. Microbial interactions in the mycorrhizosphere and their significance for sustainable agriculture. FEMS Microbiol. Ecol. 2004, 48, 1–13. [Google Scholar] [CrossRef]
- Bonfante, P.; Anca, I.-A. Plants, Mycorrhizal Fungi, and Bacteria: A Network of Interactions. Annu. Rev. Microbiol. 2009, 63, 363–383. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Xu, M.; Liu, Y.; Zhang, F.; Hodge, A.; Feng, G. Carbon and phosphorus exchange may enable cooperation between an arbuscular mycorrhizal fungus and a phosphate-solubilizing bacterium. New Phytol. 2016, 210, 1022–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battini, F.; Grønlund, M.; Agnolucci, M.; Giovannetti, M.; Jakobsen, I. Facilitation of phosphorus uptake in maize plants by mycorrhizosphere bacteria. Sci. Rep. 2017, 7, 4686. [Google Scholar] [CrossRef] [PubMed]
- Ezawa, T.; Saito, K. How do arbuscular mycorrhizal fungi handle phosphate? New insight into fine-tuning of phosphate metabolism. New Phytol. 2018, 220, 1116–1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayachandran, K.; Hetrick, B.A.D.; Schwab, A.P. Mycorrhizal Mediation of Phosphorus Availability: Synthetic Iron Chelate Effects on Phosphorus Solubilization. Soil Sci. Soc. Am. J. 1989, 53, 1701–1706. [Google Scholar] [CrossRef]
- Koide, R.T.; Kabir, Z. Extraradical hyphae of the mycorrhizal fungus Glomus intraradices can hydrolyse organic phosphate. New Phytol. 2000, 148, 511–517. [Google Scholar] [CrossRef]
- Song, Y.; Li, X.; Feng, G.; Zhang, F.; Peter, C. Rapid assessment of acid phosphatase activity in the mycorrhizosphere and in arbuscular mycorrhizal fungal hyphae. Chin. Sci. Bull. 2000, 45, 1187–1191. [Google Scholar] [CrossRef]
- Ding, X.; Fu, L.; Liu, C.; Chen, F.; Hoffland, E.; Shen, J.; Zhang, F.; Feng, G. Positive feedback between acidification and organic phosphate mineralization in the rhizosphere of maize (Zea mays L.). Plant Soil 2011, 349, 13–24. [Google Scholar] [CrossRef]
- Ragot, S.A.; Kertesz, M.A.; Bunemann, E.K. phoD Alkaline Phosphatase Gene Diversity in Soil. Appl. Environ. Microbiol. 2015, 81, 7281–7289. [Google Scholar] [CrossRef] [Green Version]
- Fraser, T.; Lynch, D.H.; Entz, M.H.; Dunfield, K.E. Linking alkaline phosphatase activity with bacterial phoD gene abundance in soil from a long-term management trial. Geoderma 2015, 257, 115–122. [Google Scholar] [CrossRef]
- Kageyama, H.; Tripathi, K.; Rai, A.K.; Cha-Um, S.; Waditee-Sirisattha, R.; Takabe, T. An Alkaline Phosphatase/Phosphodiesterase, PhoD, Induced by Salt Stress and Secreted Out of the Cells of Aphanothece halophytica, a Halotolerant Cyanobacterium. Appl. Environ. Microbiol. 2011, 77, 5178–5183. [Google Scholar] [CrossRef] [Green Version]
- Rabie, G.H.; Almadini, A.M. Role of bioinoculants in development of salt-tolerance of Vicia faba plants under salinity stress. Afr. J. Biotechnol. 2005, 4, 210–222. [Google Scholar]
- Kohler, J.; Hernández, J.A.; Caravaca, F.; Roldán, A. Induction of antioxidant enzymes is involved in the greater effectiveness of a PGPR versus AM fungi with respect to increasing the tolerance of lettuce to severe salt stress. Environ. Exp. Bot. 2009, 65, 245–252. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, X.; Li, G.; Qin, P.-Y. Interactions between arbuscular mycorrhizal fungi and phosphate-solubilizing fungus (Mortierella sp.) and their effects on Kostelelzkya virginica growth and enzyme activities of rhizosphere and bulk soils at different salinities. Biol. Fertil. Soils 2011, 47, 543–554. [Google Scholar] [CrossRef] [Green Version]
- Bouyoucos, G.J. Hydrometer Method Improved for Making Particle Size Analyses of Soils1. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Garg, N.; Pandey, R. Effectiveness of native and exotic arbuscular mycorrhizal fungi on nutrient uptake and ion homeostasis in salt-stressed Cajanus cajan L. (Millsp.) genotypes. Mycorrhiza 2015, 25, 165–180. [Google Scholar] [CrossRef]
- Namdari, A.; Arani, A.B.; Moradi, A. Arbuscular mycorrhizal (Funneliformis mosseae) improves alfalfa (Medicago sativa L.) re-growth ability in saline soil through enhanced nitrogen remobilization and improved nutritional balance. J. Central Eur. Agric. 2018, 19, 166–183. [Google Scholar] [CrossRef] [Green Version]
- Masrahi, A. Interactions of Arbuscular Mycorrhizal Fungi with Rhizosphere Communities in Saline Soils. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, 2018. [Google Scholar]
- Noggle, G.R.; Wynd, F.L. The determination of selected chemical characteristics of soil which affect the growth and composition of plants. Plant Physiol. 1941, 16, 39–60. [Google Scholar] [CrossRef] [Green Version]
- Phillips, J.; Hayman, D. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Tabatabai, M.; Bremner, J. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Li, Y.; Wu, A.; Huang, J. Effects of arbuscular mycorrhizal fungi on growth and nitrogen uptake of Chrysanthemum morifolium under salt stress. PLoS ONE 2018, 13, e0196408. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Gong, J. Differential effects of abiotic factors and host plant traits on diversity and community composition of root-colonizing arbuscular mycorrhizal fungi in a salt-stressed ecosystem. Mycorrhiza 2014, 24, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Frey-Klett, P.; Garbaye, J.; Tarkka, M. The mycorrhiza helper bacteria revisited. New Phytol. 2007, 176, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Svenningsen, N.B.; Watts-Williams, S.J.; Joner, E.J.; Battini, F.; Efthymiou, A.; Cruz-Paredes, C.; Nybroe, O.; Jakobsen, I. Suppression of the activity of arbuscular mycorrhizal fungi by the soil microbiota. ISME J. 2018, 12, 1296–1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ordoñez, Y.M.; Fernandez, B.R.; Lara, L.S.; Rodríguez, A.; Uribe-Velez, D.; Sanders, I.R. Bacteria with Phosphate Solubilizing Capacity Alter Mycorrhizal Fungal Growth Both Inside and Outside the Root and in the Presence of Native Microbial Communities. PLoS ONE 2016, 11, e0154438. [Google Scholar] [CrossRef]
- Grzybowska, B. Arbuscular mycorrhiza of herbs colonizing a salt affected area near Kraków (Poland). Acta Soc. Bot. Pol. 2004, 73, 247–253. [Google Scholar] [CrossRef]
- Zimmerman, A.E.; Martiny, A.C.; Allison, S.D. Microdiversity of extracellular enzyme genes among sequenced prokaryotic genomes. ISME J. 2013, 7, 1187–1199. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.; Barret, M.; Mooij, M.J.; Rice, O.; Morrissey, J.P.; Dobson, A.; Griffiths, B.; O’Gara, F. Long-term phosphorus fertilisation increased the diversity of the total bacterial community and the phoD phosphorus mineraliser group in pasture soils. Biol. Fertil. Soils 2013, 49, 661–672. [Google Scholar] [CrossRef] [Green Version]

| Parameter | Value |
|---|---|
| pH | 8.5 |
| EC (saturated extract, dS/m) | 6.32 |
| P (Mehlich-3, mg/kg) Nitrate-N (mg/kg) Potassium (mg/kg) | 45 26 614 |
| Organic matter content (%) | 0.34 |
| Clay (%) | 46.7 |
| Silt (%) | 20.7 |
| Sand (%) | 32.6 |
| AMF Inoculum | Treatment | % Root Colonized by AMF |
|---|---|---|
| None | IS-OS | 0 ± 0 e |
| IS-OU | 2.91 ± 2.6 ecd | |
| IU-OS | 0.05 ± 0.08 e | |
| IU-OU | 1.95 ± 1.5 ed | |
| Fm | IS-OS | 25.9 ± 15.1 ba |
| IS-OU | 15.30 ± 6.2 bc | |
| IU-OS | 14.47 ± 7.6 bcd | |
| IU-OU | 30.82 ± 10.7 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masrahi, A.; Somenahally, A.; Gentry, T. Interactions of Arbuscular Mycorrhizal Fungi with Hyphosphere Microbial Communities in a Saline Soil: Impacts on Phosphorus Availability and Alkaline Phosphatase Gene Abundance. Soil Syst. 2020, 4, 63. https://doi.org/10.3390/soilsystems4040063
Masrahi A, Somenahally A, Gentry T. Interactions of Arbuscular Mycorrhizal Fungi with Hyphosphere Microbial Communities in a Saline Soil: Impacts on Phosphorus Availability and Alkaline Phosphatase Gene Abundance. Soil Systems. 2020; 4(4):63. https://doi.org/10.3390/soilsystems4040063
Chicago/Turabian StyleMasrahi, Abdurrahman, Anil Somenahally, and Terry Gentry. 2020. "Interactions of Arbuscular Mycorrhizal Fungi with Hyphosphere Microbial Communities in a Saline Soil: Impacts on Phosphorus Availability and Alkaline Phosphatase Gene Abundance" Soil Systems 4, no. 4: 63. https://doi.org/10.3390/soilsystems4040063





