Synthesis and Characterization of Nano-Tungsten Oxide Precipitated onto Natural Inorganic Clay for Humidity-Sensing Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Materials
2.2. Characterization of the Material
3. Results
3.1. Particle-Size Distribution
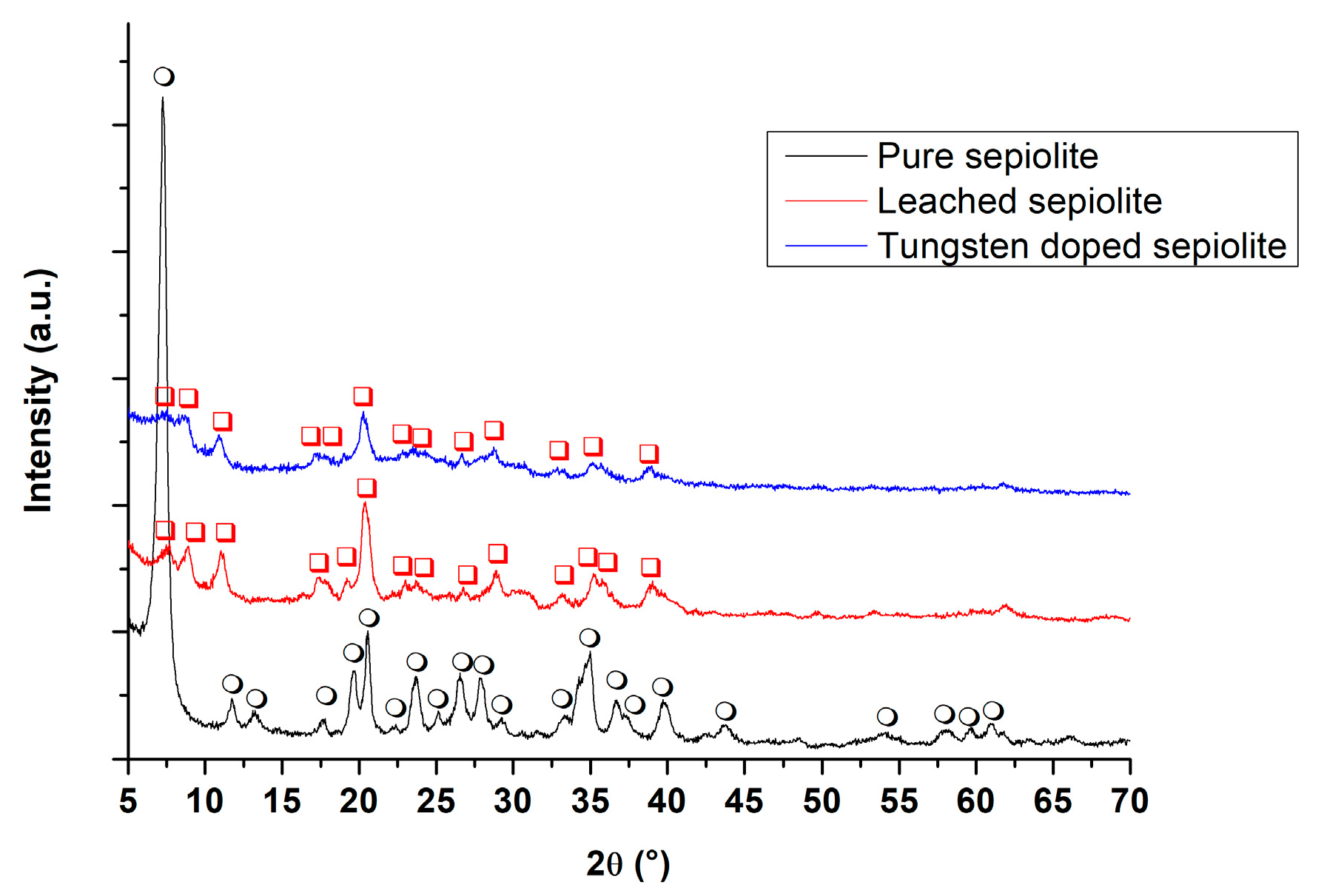
3.2. X-ray Diffraction
3.3. BET Measurements
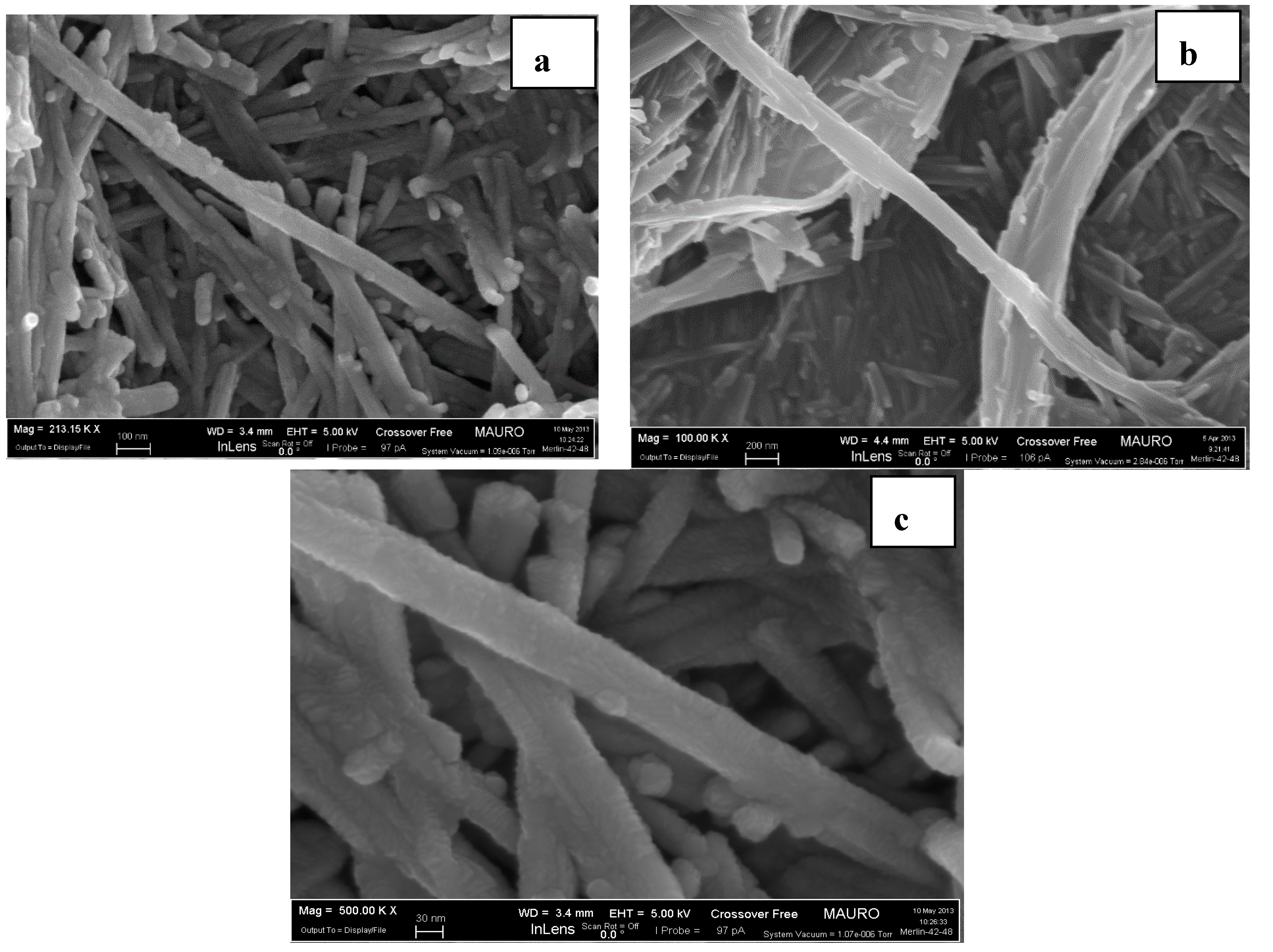
3.4. FE-SEM Observation
3.5. Energy Dispersive X-ray Spectroscopy (EDX)
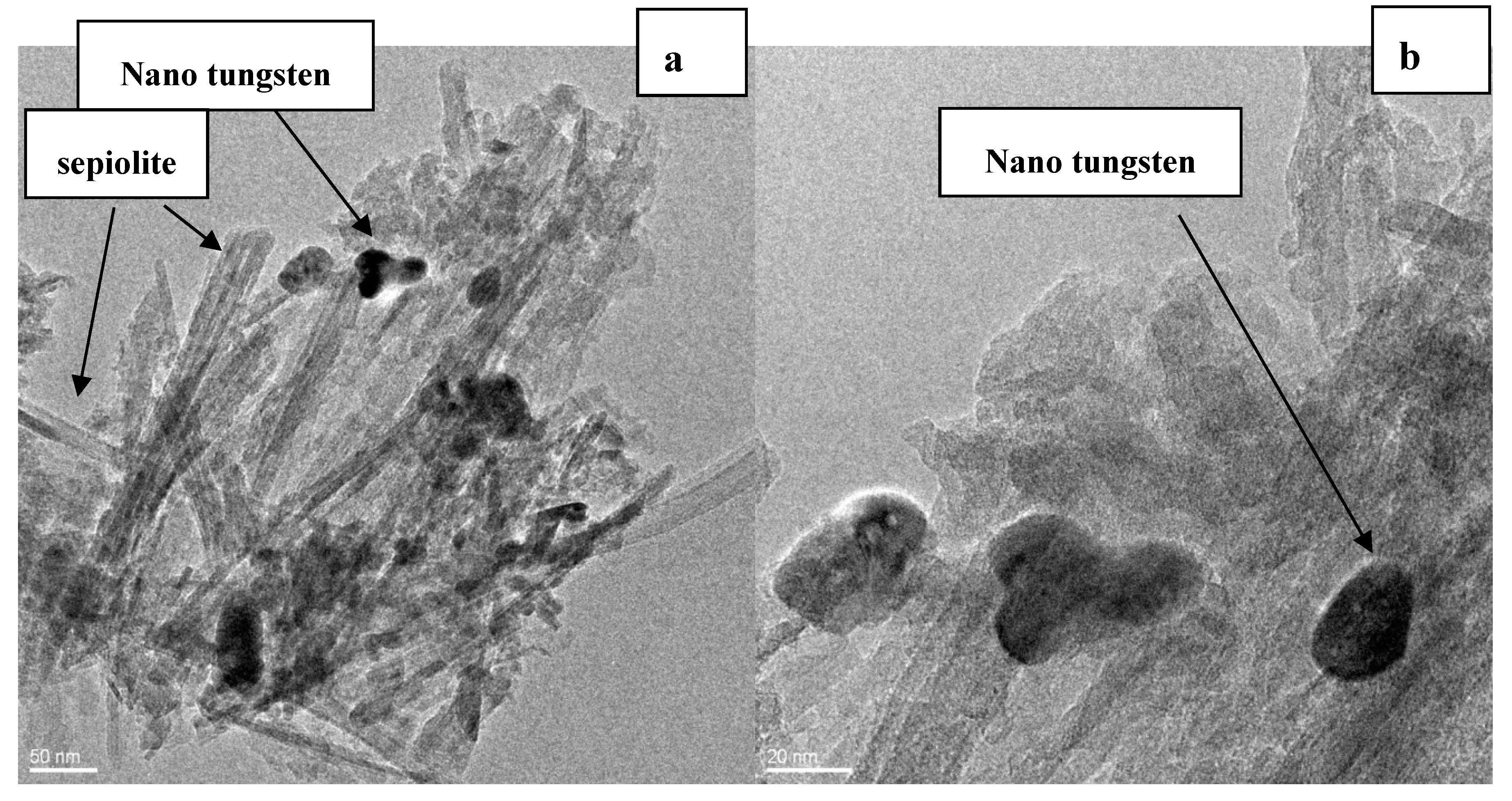
3.6. HRTEM Observation
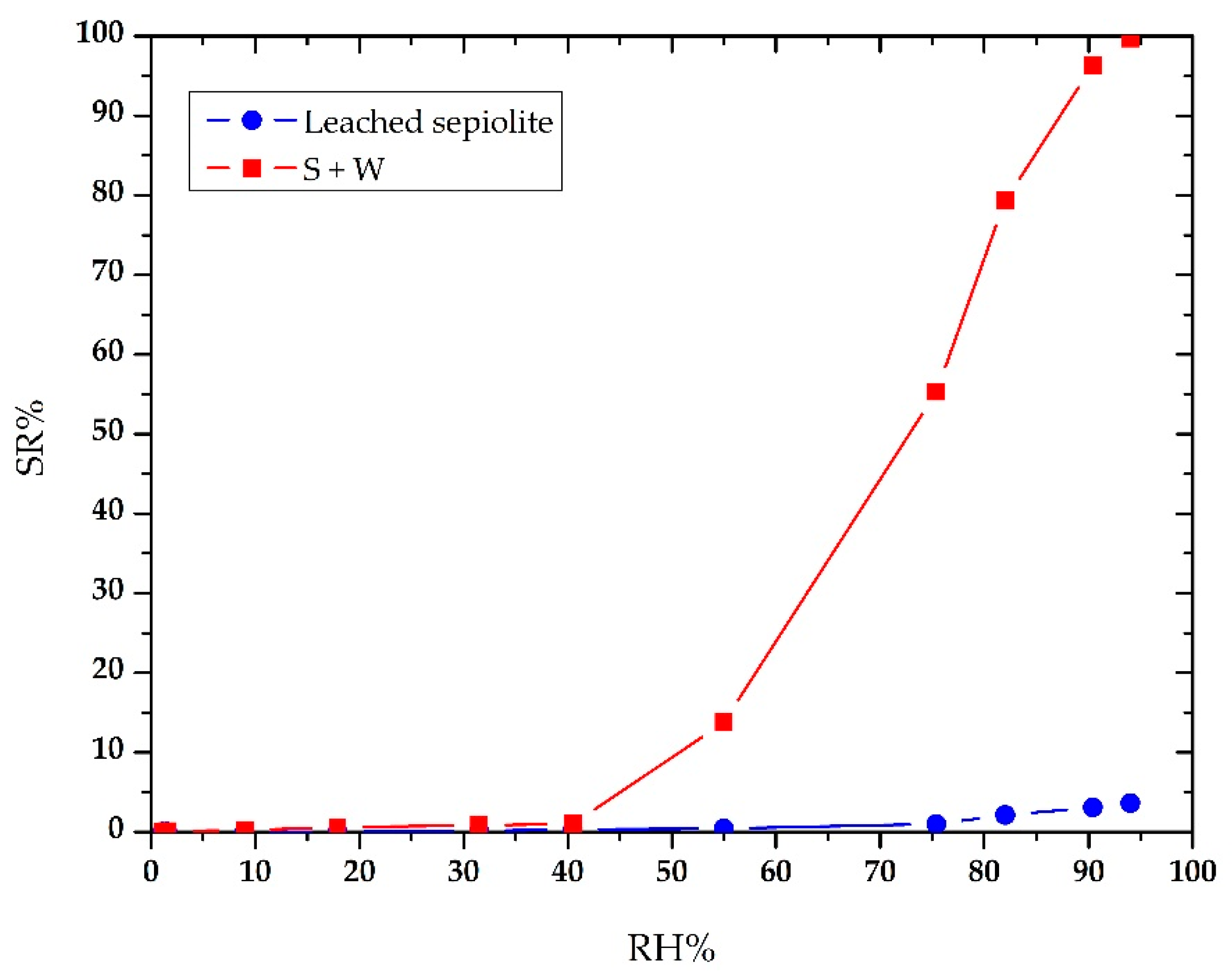
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Georg, A.; Graf, W.; Wittwer, V. Switchable windows with tungsten oxide. Vacuum 2008, 82, 730–735. [Google Scholar] [CrossRef]
- Meng, D.; Yamazaki, T.; Shen, Y.; Liu, Z.; Kikuta, T. Preparation of WO3 nanoparticles and application to NO2 sensor. Appl. Surf. Sci. 2009, 256, 1050–1053. [Google Scholar] [CrossRef]
- Tesfamichael, T.; Arita, M.; Bostrom, T.; Bell, J. Thin film deposition and characterization of pure and iron-doped electron-beam evaporated tungsten oxide for gas sensors. Thin Solid Films 2010, 518, 4791–4797. [Google Scholar] [CrossRef] [Green Version]
- Ghasempour, R. Hybrid multiwalled carbon nanotubes and trioxide tungsten nanoparticles for hydrogen gas sensing. J. Phys. D Appl. Phys. 2009, 42, 165105. [Google Scholar] [CrossRef]
- Pérez-Cadenas, A.; Moreno-Castilla, C.; Maldonado-Hódar, F.; Fierro, J. Tungsten oxide catalysts supported on activated carbons: Effect of tungsten precursor and pretreatment on dispersion, distribution, and surface acidity of catalysts. J. Catal. 2003, 217, 30–37. [Google Scholar] [CrossRef]
- Azimirad, R.; Khosravi, P.; Moshfegh, A. Synthesis of W17O47 nanothick plates with preferred orientation and their photocatalytic activity. Surf. Interface Anal. 2011, 43, 1397–1402. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Z.; Cao, H. Electrochromic properties of N-doped tungsten oxide thin films prepared by reactive DC-pulsed sputtering. Thin Solid Films 2011, 519, 3032–3036. [Google Scholar] [CrossRef]
- Schubert, W.; Lassner, E. Production and characterization of hydrogen-reduced submicron tungsten powders. Part I: State of the art in research, production and characterization of raw materials and tungsten powders. Int. J. Refract. Met. Hard Mater. 1991, 10, 133–141. [Google Scholar] [CrossRef]
- Aegerter, M.A.; Avellaneda, C.O.; Pawlicka, A.; Atik, M. Electrochromism in materials prepared by the sol-gel process. J. Sol-Gel Sci. Technol. 1997, 8, 689–696. [Google Scholar] [CrossRef]
- Santato, C.; Odziemkowski, M.; Ulmann, M.; Augustynski, J. Crystallographically oriented mesoporous WO3 films: Synthesis, characterization, and applications. J. Am. Chem. Soc. 2001, 123, 10639–10649. [Google Scholar] [CrossRef] [PubMed]
- Losier, P.; Ashrit, P. Flash evaporated tungsten oxide thin films for electrochromic applications. J. Mater. Sci. Lett. 2003, 22, 1095–1098. [Google Scholar] [CrossRef]
- Palgrave, R.G.; Parkin, I.P. Chemical vapour deposition of titanium chalcogenides and pnictides and tungsten oxide thin films. New J. Chem. 2006, 30, 505–514. [Google Scholar] [CrossRef]
- Regragui, M.; Addou, M.; Outzourhit, A.; Bernede, J.; El-Idrissi, E.; Benseddik, E.; Kachouane, A. Preparation and characterization of pyrolytic spray deposited electrochromic tungsten trioxide films. Thin Solid Films 2000, 358, 40–45. [Google Scholar] [CrossRef]
- Esteban-Cubillo, A.; Tulliani, J.-M.; Pecharromán, C.; Moya, J.S. Iron-oxide nanoparticles supported on sepiolite as a novel humidity sensor. J. Eur. Ceram. Soc. 2007, 27, 1983–1989. [Google Scholar] [CrossRef]
- Ding, Z.; Frost, R.L. Study of copper adsorption on montmorillonites using thermal analysis methods. J. Colloid Interface Sci. 2004, 269, 296–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, M.; Afify, A.S.S.; Ataalla, M.; Mohammed, A.; Staneva, A.; Dimitriev, Y.; Tulliani, J.M.C. Preparation and characterization of a zinc oxide nanopowder supported onto inorganic clay. J. Chem. Technol. Metall. 2016, 51, 168–172. [Google Scholar]
- Miura, A.; Nakazawa, K.; Takei, T.; Kumada, N.; Kinomura, N.; Ohki, R.; Koshiyama, H. Acid-, base-, and heat-induced degradation behavior of Chinese sepiolite. Ceram. Int. 2012, 38, 4677–4684. [Google Scholar] [CrossRef]
- Valentín, J.; López-Manchado, M.; Rodríguez, A.; Posadas, P.; Ibarra, L. Novel anhydrous unfolded structure by heating of acid pre-treated sepiolite. Appl. Clay Sci. 2007, 36, 245–255. [Google Scholar] [CrossRef]
- Hassan, M.; Afify, A.S.; Tulliani, J.M. Synthesis of ZnO nanoparticles onto sepiolite needles and determination of their sensitivity toward humidity, NO2 and H2. J. Mater. Sci. Technol. 2016, 32, 573–582. [Google Scholar] [CrossRef]
- Afify, A.S.; Hassan, M.; Piumetti, M.; Peter, I.; Bonelli, B.; Tulliani, J.-M. Elaboration and characterization of modified sepiolites and their humidity sensing features for environmental monitoring. Appl. Clay Sci. 2015, 115, 165–173. [Google Scholar] [CrossRef]
- Yebra-Rodriguez, A.; Martin-Ramos, J.D.; Del Rey, F.; Viseras, C.; Lopez-Galindo, A. Effect of acid treatment on the structure of sepiolite. Clay Miner. 2003, 38, 353–360. [Google Scholar] [CrossRef] [Green Version]
- Fuhr, J.R.; Wiese, W.L.; Lide, D.R. CRC Handbook of Chemistry and Physics, 90th ed.; Lide, D.R., Haynes, W.M., Eds.; CRC Press Publisher: Boca Raton, FL, USA, 2009; p. 1978. ISBN 978-1-4200-9084-0. [Google Scholar]
| Cumulative wt % | Sepiolite (µm) | W-Modified Sepiolite (µm) |
|---|---|---|
| 10% | 6.2 | 4.8 |
| 50% | 16.3 | 11.6 |
| 90% | 56.7 | 43.4 |
| Metals | Atom % |
|---|---|
| Mg | 16 |
| Si | 77.9 |
| W | 6.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afify, A.S.; Elsayed, A.; Hassan, M.; Ataalla, M.; Mohamed, A.; Hussain, A.; Abu-Khadra, A.S.; Tulliani, J.-M. Synthesis and Characterization of Nano-Tungsten Oxide Precipitated onto Natural Inorganic Clay for Humidity-Sensing Applications. Ceramics 2018, 1, 120-127. https://doi.org/10.3390/ceramics1010010
Afify AS, Elsayed A, Hassan M, Ataalla M, Mohamed A, Hussain A, Abu-Khadra AS, Tulliani J-M. Synthesis and Characterization of Nano-Tungsten Oxide Precipitated onto Natural Inorganic Clay for Humidity-Sensing Applications. Ceramics. 2018; 1(1):120-127. https://doi.org/10.3390/ceramics1010010
Chicago/Turabian StyleAfify, Ahmed S., Ahmed Elsayed, Mohamed Hassan, Mohamed Ataalla, Amr Mohamed, Azhar Hussain, Ahmad S. Abu-Khadra, and Jean-Marc Tulliani. 2018. "Synthesis and Characterization of Nano-Tungsten Oxide Precipitated onto Natural Inorganic Clay for Humidity-Sensing Applications" Ceramics 1, no. 1: 120-127. https://doi.org/10.3390/ceramics1010010
APA StyleAfify, A. S., Elsayed, A., Hassan, M., Ataalla, M., Mohamed, A., Hussain, A., Abu-Khadra, A. S., & Tulliani, J.-M. (2018). Synthesis and Characterization of Nano-Tungsten Oxide Precipitated onto Natural Inorganic Clay for Humidity-Sensing Applications. Ceramics, 1(1), 120-127. https://doi.org/10.3390/ceramics1010010






