A Comprehensive Review of High-Pressure Laser-Induced Materials Processing, Part I: Laser-Heated Diamond Anvil Cells
Abstract
:1. Introduction
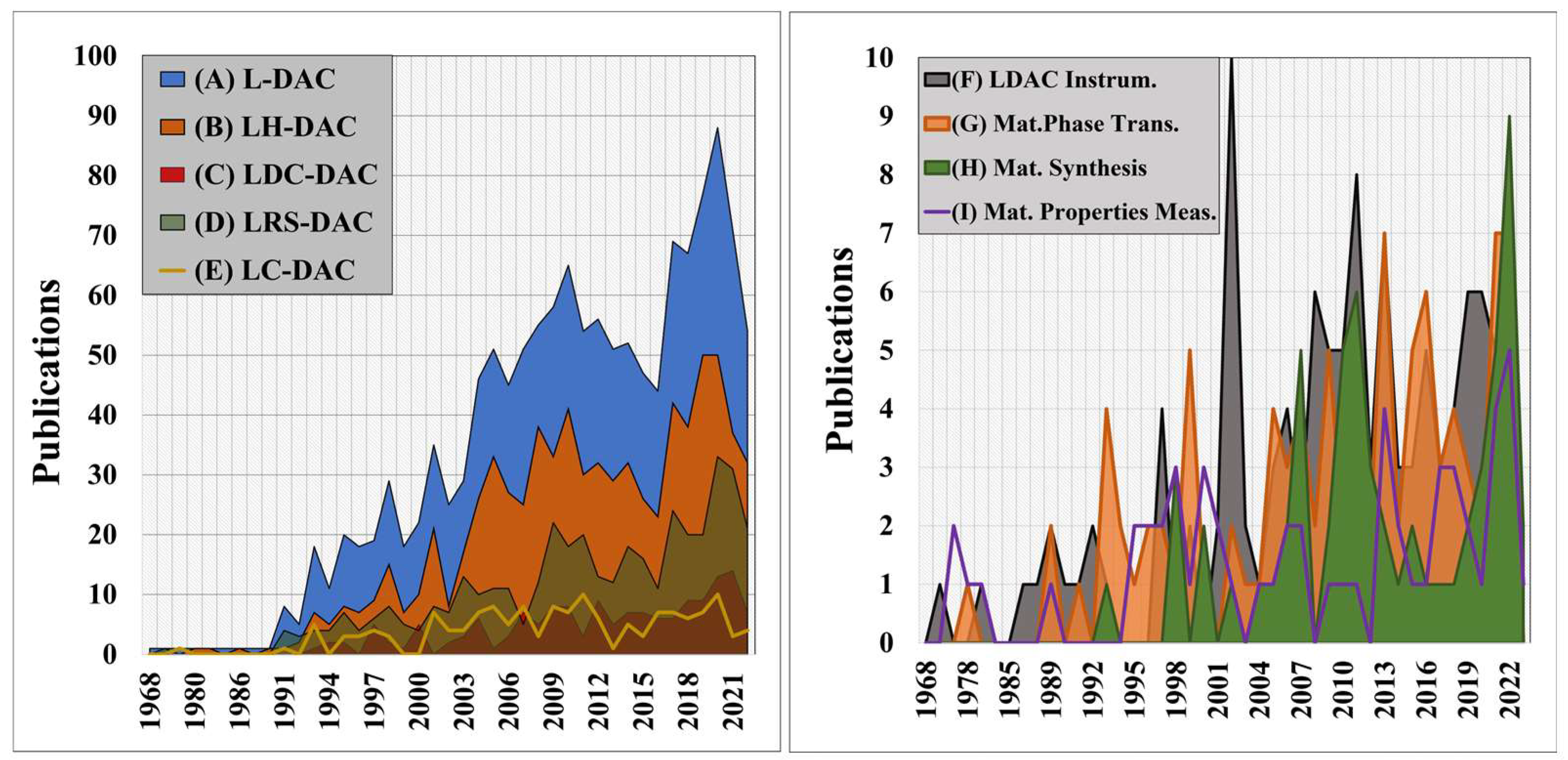
2. Methodology
3. Overview of Laser Processing in Diamond Anvil Cells (L-DACs)
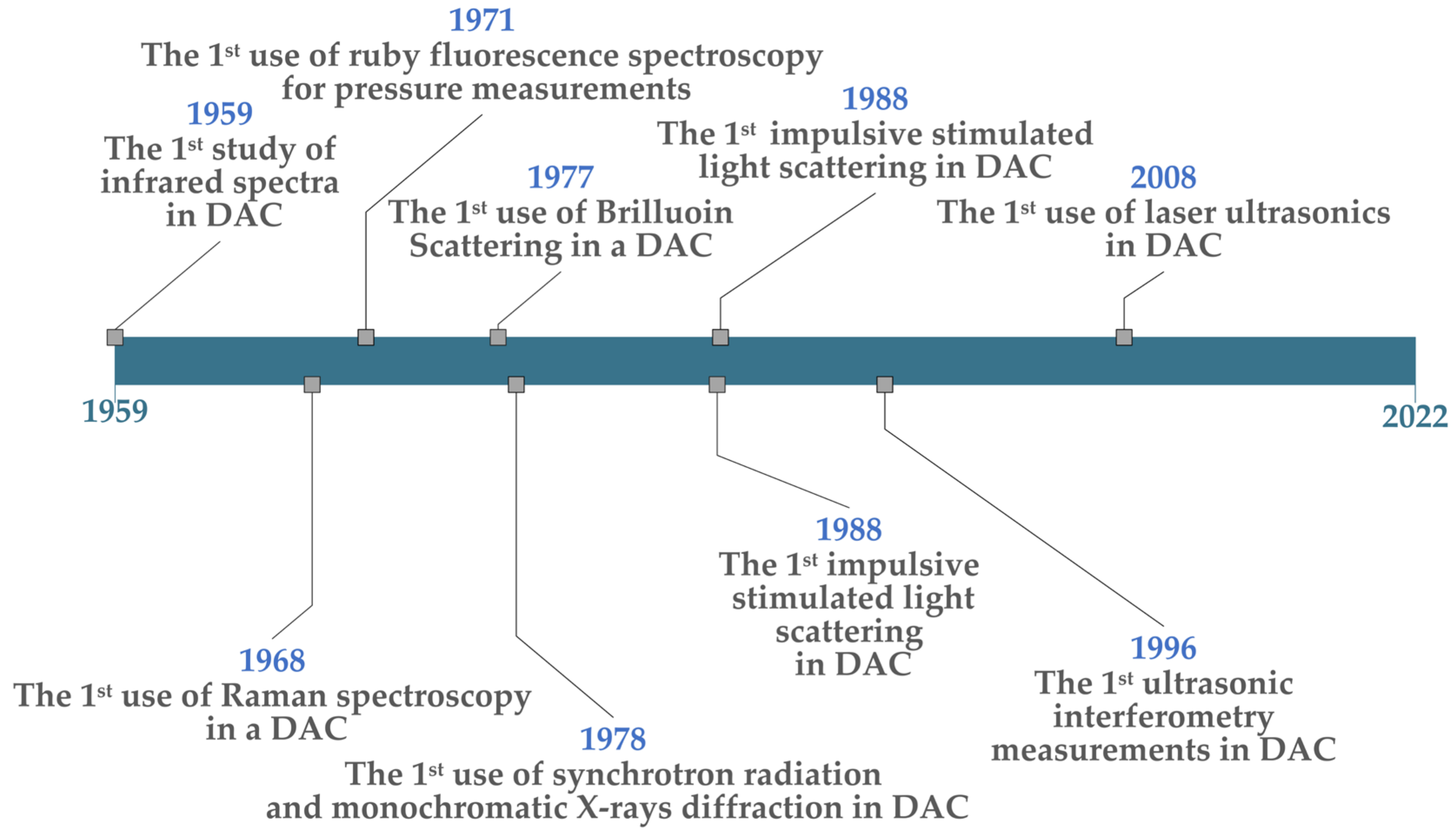
4. DACs: Fundamentals and Historical Development
5. LH-DACs: Fundamentals, Historical Development, and Key Experiments
5.1. LH-DACs: Physical Processes
5.2. LH-DAC: Historical Development
5.3. LH-DAC: Key Experiments
6. LC-DACs: Fundamentals and Historical Development
6.1. Spectroscopic Pressure Measurements
6.2. Optical Temperature Measurements by Micro-Scale Multi-Band Pyrometry
6.3. Laser-based Spectroscopy and Characterisation
7. Conclusions and Future Work
“Just when I think I have heard of all the analytical methods that can be applied to samples in a diamond anvil cell, I read a paper describing yet another new method. There is no apparent end in sight. The future of diamond anvil cell research appears to be very bright indeed …” [2].
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, G.; Mao, H.K. High-pressure studies with X-rays using diamond anvil cells. Rep. Prog. Phys. 2016, 80, 016101. [Google Scholar] [CrossRef] [PubMed]
- Bassett, W.A. Diamond anvil cell, 50th birthday. High Press. Res. 2009, 29, 163–186. [Google Scholar] [CrossRef]
- Serghiou, G.; Miehe, G.; Tschauner, O.; Zerr, A.; Boehler, R. Synthesis of a cubic Ge3N4 phase at high pressures and temperatures. J. Chem. Phys. 1999, 111, 4659–4662. [Google Scholar] [CrossRef]
- Bassett, W.A. The birth and development of laser heating in diamond anvil cells. Rev. Sci. Instrum. 2001, 72, 1270–1272. [Google Scholar] [CrossRef]
- Saxena, S.K.; Dubrovinsky, L.S.; Häggkvist, P.; Cerenius, Y.; Shen, G.; Mao, H.K. Synchrotron X-Ray Study of Iron at High Pressure and Temperature. Science 1995, 269, 1703–1704. [Google Scholar] [CrossRef]
- Andrault, D.; Fiquet, G. Synchrotron radiation and laser heating in a diamond anvil cell. Rev. Sci. Instrum. 2001, 72, 1283–1288. [Google Scholar] [CrossRef]
- Hemley, R.J.; Mao, H.-K.; Struzhkin, V.V. Synchrotron radiation and high pressure: New light on materials under extreme conditions. J. Synchrotron Radiat. 2005, 12, 135–154. [Google Scholar] [CrossRef]
- Kavner, A.; Panero, W.R. Temperature gradients and evaluation of thermoelastic properties in the synchrotron-based laser-heated diamond cell. Phys. Earth Planet. Inter. 2004, 143–144, 527–539. [Google Scholar] [CrossRef]
- Duffy, T.S. Synchrotron facilities and the study of the Earth’s deep interior. Rep. Prog. Phys. 2005, 68, 1811–1859. [Google Scholar] [CrossRef]
- Boehler, R.; Musshoff, H.G.; Ditz, R.; Aquilanti, G.; Trapananti, A. Portable laser-heating stand for synchrotron applications. Rev. Sci. Instrum. 2009, 80, 045103. [Google Scholar] [CrossRef] [Green Version]
- Piermarini, G.J. High pressure X-ray crystallography with the diamond cell at NIST/NBS. J. Res. Nat. Inst. Stand. Technol. 2001, 106, 889. [Google Scholar] [CrossRef]
- O’Bannon, E.F.; Jenei, Z.; Cynn, H.; Lipp, M.J.; Jeffries, J.R. Contributed Review: Culet diameter and the achievable pressure of a diamond anvil cell: Implications for the upper pressure limit of a diamond anvil cell. Rev. Sci. Instrum. 2018, 89, 111501. [Google Scholar] [CrossRef]
- Ren, D.; Li, H. A Review of High-Temperature and High-Pressure Experimental Apparatus Capable of Generating Differential Stress. Front. Earth Sci. 2022, 10, 852403. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, M.; Xiao, G.; Zou, B. Thinking about the Development of High-Pressure Experimental Chemistry. J. Phys. Chem. Lett. 2020, 11, 7297–7306. [Google Scholar] [CrossRef]
- Akimoto, S.-I. High-Pressure Research in Geophysics: Past, Present and Future. In High-Pressure Research in Mineral Physics: A Volume in Honor of Syun-iti Akimoto; Terra Scientific Publishing Company: Tokyo, Japan, 1987; pp. 1–13. [Google Scholar]
- Brown, J.M.; Slutsky, L.J.; Nelson, K.A.; Cheng, L.-T. Velocity of Sound and Equations of State for Methanol and Ethanol in a Diamond-Anvil Cell. Science 1988, 241, 65–67. [Google Scholar] [CrossRef]
- Deemyad, S.; Silvera, I.F. Melting Line of Hydrogen at High Pressures. Phys. Rev. Lett. 2008, 100, 155701. [Google Scholar] [CrossRef]
- Kavner, A.; Jeanloz, R. High-pressure melting curve of platinum. J. Appl. Phys. 1998, 83, 7553–7559. [Google Scholar] [CrossRef]
- Kavner, A.; Jeanloz, R. The high-pressure melting curve of Allende meteorite. Geophys. Res. Lett. 1998, 25, 4161–4164. [Google Scholar] [CrossRef]
- Yoo, C.S.; Akella, J.; Cynn, H.; Nicol, M. Direct elementary reactions of boron and nitrogen at high pressures and temperatures. Phys. Rev. B Condens. Matter 1997, 56, 140–146. [Google Scholar] [CrossRef]
- Young, A.F.; Sanloup, C.; Gregoryanz, E.; Scandolo, S.; Hemley, R.J.; Mao, H.-K. Synthesis of Novel Transition Metal Nitrides IrN2 and OsN2. Phys. Rev. Lett. 2006, 96, 155501. [Google Scholar] [CrossRef] [Green Version]
- Friedrich, A.; Winkler, B.; Bayarjargal, L.; Morgenroth, W.; Juarez-Arellano, E.A.; Milman, V.; Refson, K.; Kunz, M.; Chen, K. Novel Rhenium Nitrides. Phys. Rev. Lett. 2010, 105, 085504. [Google Scholar] [CrossRef]
- Kumar, N.R.S.; Shekar, N.V.C.; Chandra, S.; Basu, J.; Divakar, R.; Sahu, P.C. Synthesis of novel Ru2C under high pressure–high temperature conditions. J. Phys. Condens. Matter 2012, 24, 362202. [Google Scholar] [CrossRef]
- Walsh, J.P.S.; Freedman, D.E. High-Pressure Synthesis: A New Frontier in the Search for Next-Generation Intermetallic Compounds. Acc. Chem. Res. 2018, 51, 1315–1323. [Google Scholar] [CrossRef]
- Aslandukova, A.; Aslandukov, A.; Yuan, L.; Laniel, D.; Khandarkhaeva, S.; Fedotenko, T.; Steinle-Neumann, G.; Glazyrin, K.; Dubrovinskaia, N.; Dubrovinsky, L. Novel High-Pressure Yttrium Carbide γ-Y4C5 Containing [C2] and Nonlinear [C3] Units with Unusually Large Formal Charges. Phys. Rev. Lett. 2021, 127, 135501. [Google Scholar] [CrossRef]
- Brygoo, S.; Loubeyre, P.; Millot, M.; Rygg, J.R.; Celliers, P.M.; Eggert, J.H.; Jeanloz, R.; Collins, G.W. Evidence of hydrogen−helium immiscibility at Jupiter-interior conditions. Nature 2021, 593, 517–521. [Google Scholar] [CrossRef]
- Loubeyre, P.; Occelli, F.; Dumas, P. Synchrotron infrared spectroscopic evidence of the probable transition to metal hydrogen. Nature 2020, 577, 631–635. [Google Scholar] [CrossRef]
- Caldwell, W.A.; Nguyen, J.H.; Pfrommer, B.G.; Mauri, F.; Louie, S.G.; Jeanloz, R. Structure, bonding, and geochemistry of xenon at high pressures. Science 1997, 277, 930–933. [Google Scholar] [CrossRef]
- Yusa, H.; Takemura, K.; Matsui, Y.; Morishima, H.; Watanabe, K.; Yamawaki, H.; Aoki, K. Direct transformation of graphite to cubic diamond observed in a laser-heated diamond anvil cell. Appl. Phys. Lett. 1998, 72, 1843–1845. [Google Scholar] [CrossRef]
- Ming, L.C.; Bassett, W.A. Laser heating in the diamond anvil press up to 2000 °C sustained and 3000 °C pulsed at pressures up to 260 kilobars. Rev. Sci. Instrum. 1974, 45, 1115–1118. [Google Scholar] [CrossRef]
- Grande, Z.M.; Pham, C.H.; Smith, D.; Boisvert, J.H.; Huang, C.; Smith, J.S.; Goldman, N.; Belof, J.L.; Tschauner, O.; Steffen, J.H.; et al. Pressure-driven symmetry transitions in dense H2O ice. Phys. Rev. B Condens. Matter 2022, 105, 104109. [Google Scholar] [CrossRef]
- Liu, L.-G. A new high-pressure phase of spinel. Earth Planet. Sci. Lett. 1978, 41, 398–404. [Google Scholar] [CrossRef]
- Bassett, W.A.; Weathers, M.S. Temperature measurement in laser heated diamond anvil cells. Phys. B+C 1986, 139–140, 900–902. [Google Scholar] [CrossRef]
- Yang, L. How to detect melting in laser heating diamond anvil cell. Chin. Phys. B 2016, 25, 076201. [Google Scholar] [CrossRef]
- Boehler, R.; Ross, M.; Boercker, D.B. Melting of LiF and NaCl to 1 Mbar: Systematics of Ionic Solids at Extreme Conditions. Phys. Rev. Lett. 1997, 78, 4589–4592. [Google Scholar] [CrossRef]
- Boehler, R.; Von Bargen, N.; Chopelas, A. Melting, thermal expansion, and phase transitions of iron at high pressures. J. Geophys. Res. Solid Earth 1990, 95, 21731–21736. [Google Scholar] [CrossRef]
- Golberg, D.; Bando, Y.; Eremets, M.; Takemura, K.; Kurashima, K.; Yusa, H. Nanotubes in boron nitride laser heated at high pressure. Appl. Phys. Lett. 1996, 69, 2045–2047. [Google Scholar] [CrossRef]
- Jeanloz, R.; Kavner, A.; Jephcoat, A.P.; Angel, R.J.; O’Nions, R.K. Melting criteria and imaging spectroradiometry in laser-heated diamond-cell experiments. Philos. Trans. R. Soc. Lond. Ser. A 1996, 354, 1279–1305. [Google Scholar] [CrossRef]
- Chigarev, N.; Zinin, P.; Ming, L.-C.; Amulele, G.; Bulou, A.; Gusev, V. Laser generation and detection of longitudinal and shear acoustic waves in a diamond anvil cell. Appl. Phys. Lett. 2008, 93, 181905. [Google Scholar] [CrossRef]
- Shen, G.; Wang, L.; Ferry, R.; Mao, H.-K.; Hemley, R.J. A portable laser heating microscope for high pressure research. J. Phys. Conf. Ser. 2010, 215, 012191. [Google Scholar] [CrossRef]
- Subramanian, N.; Struzhkin, V.V.; Goncharov, A.F.; Hemley, R.J. A virtual experiment control and data acquisition system for in situ laser heated diamond anvil cell Raman spectroscopy. Rev. Sci. Instrum. 2010, 81, 093906. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Ming, L.C.; Manghnani, M.H. High-pressure phase transformations in a natural crystalline diopside and a synthetic CaMgSi2O6 glass. Phys. Earth Planet. Inter. 1994, 83, 67–79. [Google Scholar] [CrossRef]
- Liu, L.-G. High pressure NaAlSiO4: The first silicate calcium ferrite isotype. Geophys. Res. Lett. 1977, 4, 183–186. [Google Scholar] [CrossRef]
- Coppari, F.; Smith, R.F.; Eggert, J.H.; Wang, J.; Rygg, J.R.; Lazicki, A.; Hawreliak, J.A.; Collins, G.W.; Duffy, T.S. Experimental evidence for a phase transition in magnesium oxide at exoplanet pressures. Nat. Geosci. 2013, 6, 926–929. [Google Scholar] [CrossRef]
- Jeanloz, R.; Celliers, P.M.; Collins, G.W.; Eggert, J.H.; Lee, K.K.M.; McWilliams, R.S.; Brygoo, S.; Loubeyre, P. Achieving high-density states through shock-wave loading of precompressed samples. Proc. Natl. Acad. Sci. USA 2007, 104, 9172–9177. [Google Scholar] [CrossRef]
- Eggert, J.H.; Celliers, P.M.; Hicks, D.G.; Rygg, J.R.; Collins, G.W.; Brygoo, S.; Loubeyre, P.; McWilliams, R.S.; Spaulding, D.; Jeanloz, R.; et al. Shock Experiments on Pre-Compressed Fluid Helium. AIP Conf. Proc. 2009, 1161, 26–31. [Google Scholar] [CrossRef]
- Eggert, J.; Brygoo, S.; Loubeyre, P.; McWilliams, R.S.; Celliers, P.M.; Hicks, D.G.; Boehly, T.R.; Jeanloz, R.; Collins, G.W. Hugoniot Data for Helium in the Ionization Regime. Phys. Rev. Lett. 2008, 100, 124503. [Google Scholar] [CrossRef]
- Yusa, H. Laser-heated diamond anvil cell system for photochemical reaction measurements. Rev. Sci. Instrum. 2001, 72, 1309–1312. [Google Scholar] [CrossRef]
- Daviau, K.; Meng, Y.; Lee, K.K.M. SiO2-SiC Mixtures at High Pressures and Temperatures: Implications for Planetary Bodies Containing SiC. J. Geophys. Res. Planets 2019, 124, 2294–2305. [Google Scholar] [CrossRef]
- Goarant, F.; Guyot, F.; Peyronneau, J.; Poirier, J.-P. High-pressure and high-temperature reactions between silicates and liquid iron alloys, in the diamond anvil cell, studied by analytical electron microscopy. J. Geophys. Res. Solid Earth 1992, 97, 4477–4487. [Google Scholar] [CrossRef]
- Sahu, P.C.; Takemura, K.; Yusa, H. Synthesis experiments on B-Sb, Ge-Sb, and Xe-Pd systems using a laser heated diamond anvil cell. High Press. Res. 2001, 21, 41–50. [Google Scholar] [CrossRef]
- Hemley, R.J.; Bell, P.M.; Mao, H.K. Laser Techniques in High-Pressure Geophysics. Science 1987, 237, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Brasch, J.W.; Melveger, A.J.; Lippincott, E.R. Laser excited Raman spectra of samples under very high pressures. Chem. Phys. Lett. 1968, 2, 99–100. [Google Scholar] [CrossRef]
- Shuker, P.; Melchior, A.; Assor, Y.; Belker, D.; Sterer, E. IR pyrometry in diamond anvil cell above 400K. Rev. Sci. Instrum. 2008, 79, 073908. [Google Scholar] [CrossRef] [PubMed]
- Hrubiak, R.; Sinogeikin, S.; Rod, E.; Shen, G. The laser micro-machining system for diamond anvil cell experiments and general precision machining applications at the High Pressure Collaborative Access Team. Rev. Sci. Instrum. 2015, 86, 072202. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Sasaki, T.; Gaida, N.A.; Niwa, K.; Hasegawa, M. Crystal and Electronic Structure of U7Te12-Type Tungsten Nitride Synthesized under High Pressure. Inorg. Chem. 2021, 60, 13278–13283. [Google Scholar] [CrossRef] [PubMed]
- Laniel, D.; Aslandukova, A.A.; Aslandukov, A.N.; Fedotenko, T.; Chariton, S.; Glazyrin, K.; Prakapenka, V.B.; Dubrovinsky, L.S.; Dubrovinskaia, N. High-Pressure Synthesis of the β-Zn3N2 Nitride and the α-ZnN4 and β-ZnN4 Polynitrogen Compounds. Inorg. Chem. 2021, 60, 14594–14601. [Google Scholar] [CrossRef] [PubMed]
- Prakapenka, V.B.; Holtgrewe, N.; Lobanov, S.S.; Goncharov, A.F. Structure and properties of two superionic ice phases. Nat. Phys. 2021, 17, 1233–1238. [Google Scholar] [CrossRef]
- Yusa, H.; Yagi, T.; Arashi, H. Pressure dependence of Sm:YAG fluorescence to 50 GPa: A new calibration as a high pressure scale. J. Appl. Phys. 1994, 75, 1463–1466. [Google Scholar] [CrossRef]
- Sui, Z.; Hu, S.; Chen, H.; Gao, C.; Su, H.; Rahman, A.; Dai, R.; Wang, Z.; Zheng, X.; Zhang, Z. Laser effects on phase transition for cubic Sb2O3 microcrystals under high pressure. J. Mater. Chem. C 2017, 5, 5451–5457. [Google Scholar] [CrossRef]
- Duvaut, T. Comparison between multiwavelength infrared and visible pyrometry: Application to metals. Infrared Phys. Technol. 2008, 51, 292–299. [Google Scholar] [CrossRef]
- Dadashev, A.; Pasternak, M.P.; Rozenberg, G.K.; Taylor, R.D. Applications of perforated diamond anvils for very high-pressure research. Rev. Sci. Instrum. 2001, 72, 2633–2637. [Google Scholar] [CrossRef]
- Armstrong, M.R.; Crowhurst, J.C.; Bastea, S.; Zaug, J.M. Ultrafast observation of shocked states in a precompressed material. J. Appl. Phys. 2010, 108, 023511. [Google Scholar] [CrossRef]
- Meng, Y.; Hrubiak, R.; Rod, E.; Boehler, R.; Shen, G. New developments in laser-heated diamond anvil cell with in situ synchrotron X-ray diffraction at High Pressure Collaborative Access Team. Rev. Sci. Instrum. 2015, 86, 072201. [Google Scholar] [CrossRef]
- Aria, M.; Cuccurullo, C. bibliometrix: An R-tool for comprehensive science mapping analysis. J. Inform. 2017, 11, 959–975. [Google Scholar] [CrossRef]
- Prakapenka, V.B.; Kubo, A.; Kuznetsov, A.; Laskin, A.; Shkurikhin, O.; Dera, P.; Rivers, M.L.; Sutton, S.R. Advanced flat top laser heating system for high pressure research at GSECARS: Application to the melting behavior of germanium. High Press. Res. 2008, 28, 225–235. [Google Scholar] [CrossRef]
- Tateno, S.; Hirose, K.; Ohishi, Y.; Tatsumi, Y. The Structure of Iron in Earth’s Inner Core. Science 2010, 330, 359–361. [Google Scholar] [CrossRef]
- Dubrovinsky, L.S.; Saxena, S.K.; Lazor, P.; Ahuja, R.; Eriksson, O.; Wills, J.M.; Johansson, B. Experimental and theoretical identification of a new high-pressure phase of silica. Nature 1997, 388, 362–365. [Google Scholar] [CrossRef]
- Mao, H.K.; Xu, J.; Bell, P.M. Calibration of the ruby pressure gauge to 800 kbar under quasi-hydrostatic conditions. J. Geophys. Res. Solid Earth 1986, 91, 4673–4676. [Google Scholar] [CrossRef]
- Hammersley, A.P.; Svensson, S.O.; Hanfland, M.; Fitch, A.N.; Hausermann, D. Two-dimensional detector software: From real detector to idealised image or two-theta scan. High Press. Res. 1996, 14, 235–248. [Google Scholar] [CrossRef]
- Shen, G.; Rivers, M.L.; Wang, Y.; Sutton, S.R. Laser heated diamond cell system at the Advanced Photon Source for in situ X-ray measurements at high pressure and temperature. Rev. Sci. Instrum. 2001, 72, 1273. [Google Scholar] [CrossRef]
- Shen, G.; Mao, H.-K.; Hemley, R.J.; Duffy, T.S.; Rivers, M.L. Melting and crystal structure of iron at high pressures and temperatures. Geophys. Res. Lett. 1998, 25, 373–376. [Google Scholar] [CrossRef]
- Fei, Y.; Ricolleau, A.; Frank, M.; Mibe, K.; Shen, G.; Prakapenka, V. Toward an internally consistent pressure scale. Proc. Natl. Acad. Sci. USA 2007, 104, 9182–9186. [Google Scholar] [CrossRef]
- Mao, H.K.; Bell, P.M.; Shaner, J.W.; Steinberg, D.J. Specific volume measurements of Cu, Mo, Pd, and Ag and calibration of the ruby R1 fluorescence pressure gauge from 0.06 to 1 Mbar. J. Appl. Phys. 1978, 49, 3276–3283. [Google Scholar] [CrossRef]
- Boehler, R. Temperatures in the Earth’s core from melting-point measurements of iron at high static pressures. Nature 1993, 363, 534–536. [Google Scholar] [CrossRef]
- Prescher, C.; Prakapenka, V.B. DIOPTAS: A program for reduction of two-dimensional X-ray diffraction data and data exploration. High Press. Res. 2015, 35, 223–230. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Murakami, M.; Hirose, K.; Kawamura, K.; Sata, N.; Ohishi, Y. Post-Perovskite Phase Transition in MgSiO3. Science 2004, 304, 855–858. [Google Scholar] [CrossRef]
- Dziewonski, A.M.; Anderson, D.L. Preliminary reference Earth model. Phys. Earth Planet. Inter. 1981, 25, 297–356. [Google Scholar] [CrossRef]
- Jayaraman, A. Ultrahigh pressures. Rev. Sci. Instrum. 1986, 57, 1013–1031. [Google Scholar] [CrossRef]
- Soignard, E.; McMillan, P.F. An Introduction to Diamond Anvil Cells and Loading Techniques. In High-Pressure Crystallogr; Springer: Dordrecht, The Netherlands, 2004; pp. 81–100. [Google Scholar]
- Webb, A.A.; Qadri, S.B. Pneumatic driver for diamond anvil cells. Rev. Sci. Instrum. 1982, 53, 1796–1797. [Google Scholar] [CrossRef]
- Girard, E.; Fourme, R.; Ciurko, R.; Joly, J.; Bouis, F.; Legrand, P.; Jacobs, J.; Dhaussy, A.-C.; Ferrer, J.-L.; Mezouar, M.; et al. Macromolecular crystallography at high pressure with pneumatic diamond anvil cells handled by a six-axis robotic arm. J. Appl. Crystallogr. 2010, 43, 762–768. [Google Scholar] [CrossRef]
- Evans, W.J.; Yoo, C.-S.; Lee, G.W.; Cynn, H.; Lipp, M.J.; Visbeck, K. Dynamic diamond anvil cell (dDAC): A novel device for studying the dynamic-pressure properties of materials. Rev. Sci. Instrum. 2007, 78, 073904. [Google Scholar] [CrossRef] [PubMed]
- Dunstan, D.J.; Spain, I.L. Technology of diamond anvil high-pressure cells: I. Principles, design and construction. J. Phys. E Sci. Instrum. 1989, 22, 913–923. [Google Scholar] [CrossRef]
- Dubrovinskaia, N.; Dubrovinsky, L.; Natalia, A.S.; Abakumov, A.; Turner, S.; Hanfland, M.; Bykova, E.; Bykov, M.; Prescher, C.; Vitali, B.P.; et al. Terapascal static pressure generation with ultrahigh yield strength nanodiamond. Sci. Adv. 2016, 2, e1600341. [Google Scholar] [CrossRef]
- Xu, J.-A.; Mao, H.-K.; Hemley, R.J. The gem anvil cell: High-pressure behaviour of diamond and related materials. J. Phys. Condens. Matter 2002, 14, 11549–11552. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, H.; Xiao, W.; Zeng, Y. High-Temperature and High-Pressure Cubic Zirconia Anvil Cell for Raman Spectroscopy. Appl. Spectrosc. 2003, 57, 1295–1299. [Google Scholar] [CrossRef]
- Bonetti, M.; Calmettes, P. Sapphire-anvil cell for small-angle neutron scattering measurements in large-volume liquid samples up to 530 MPa. Rev. Sci. Instrum. 2005, 76, 043903. [Google Scholar] [CrossRef]
- Xu, J.-A.; Mao, H.-K. Moissanite: A Window for High-Pressure Experiments. Science 2000, 290, 783–785. [Google Scholar] [CrossRef]
- Blank, V.; Popov, M.; Pivovarov, G.; Lvova, N.; Gogolinsky, K.; Reshetov, V. Ultrahard and superhard phases of fullerite C60: Comparison with diamond on hardness and wear. Diam. Relat. Mater. 1998, 7, 427–431. [Google Scholar] [CrossRef]
- Callister, W.D. Materials Science and Engineering, 9th ed.; SI version; Callister, W.D., Jr., Rethwisch, D.G., Eds.; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Zaitsev, A.M. Optical Electronic Transitions. In Optical Properties of Diamond: A Data Handbook; Zaitsev, A.M., Ed.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 125–376. [Google Scholar]
- Bini, R.A.S.; Vincenzo. Materials under Extreme Conditions: Molecular Crystals at High Pressure; Imperial College Press: London, UK, 2014; Volume 1. [Google Scholar]
- Zaitsev, A.M. Refraction. In Optical Properties of Diamond: A Data Handbook; Zaitsev, A.M., Ed.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 1–11. [Google Scholar]
- Subramanian, N.; Shekar, N.V.C.; Kumar, N.R.S.; Sahu, P.C. Development of laser-heated diamond anvil cell facility for synthesis of novel materials. Curr. Sci. Assoc. 2006, 91, 175–182. [Google Scholar]
- Shekar, N.; Sahu, P.C.; Rajan, K. Laser-heated diamond-anvil cell (LHDAC) in materials science research. J. Mater. Sci. Technol. 2003, 19, 518–525. [Google Scholar]
- Feng, W.; Yang, D.; Zhu, X.; Guo, Y.; Liao, W. Simulation of laser-generated longitudinal and shear ultrasonic waves in a diamond anvil cell by the finite element method. J. Appl. Phys. 2012, 111, 013107. [Google Scholar] [CrossRef]
- Andrault, D.; Fiquet, G.; Itié, J.-P.; Richet, P.; Gillet, P.; Häusermann, D.; Hanfland, M. Thermal pressure in the laser-heated diamond-anvil cell: An X-ray diffraction study. Eur. J. Miner. 1998, 10, 931–940. [Google Scholar] [CrossRef]
- Andrault, D.; Morard, G.; Bolfan-Casanova, N.; Ohtaka, O.; Fukui, H.; Arima, H.; Guignot, N.; Funakoshi, K.; Lazor, P.; Mezouar, M. Study of partial melting at high-pressure using in situ X-ray diffraction. High Press. Res. 2006, 26, 267–276. [Google Scholar] [CrossRef]
- Anzellini, S.; Dewaele, A.; Mezouar, M.; Loubeyre, P.; Morard, G. Melting of Iron at Earth’s Inner Core Boundary Based on Fast X-ray Diffraction. Science 2013, 340, 464–466. [Google Scholar] [CrossRef]
- Anzellini, S.; Monteseguro, V.; Bandiello, E.; Dewaele, A.; Burakovsky, L.; Errandonea, D. In situ characterization of the high pressure–high temperature melting curve of platinum. Sci. Rep. 2019, 9, 13034. [Google Scholar] [CrossRef]
- Aprilis, G.; Strohm, C.; Kupenko, I.; Linhardt, S.; Laskin, A.; Vasiukov, D.M.; Cerantola, V.; Koemets, E.G.; McCammon, C.; Kurnosov, A.; et al. Portable double-sided pulsed laser heating system for time-resolved geoscience and materials science applications. Rev. Sci. Instrum. 2017, 88, 084501. [Google Scholar] [CrossRef]
- Aprilis, G.; Kantor, I.; Kupenko, I.; Cerantola, V.; Pakhomova, A.; Collings, I.E.; Torchio, R.; Fedotenko, T.; Chariton, S.; Bykov, M.; et al. Comparative study of the influence of pulsed and continuous wave laser heating on the mobilization of carbon and its chemical reaction with iron in a diamond anvil cell. J. Appl. Phys. 2019, 125, 095901. [Google Scholar] [CrossRef]
- Baron, M.A.; Lord, O.T.; Myhill, R.; Thomson, A.R.; Wang, W.; Trønnes, R.G.; Walter, M.J. Experimental constraints on melting temperatures in the MgO–SiO2 system at lower mantle pressures. Earth Planet. Sci. Lett. 2017, 472, 186–196. [Google Scholar] [CrossRef]
- Boccato, S.; Torchio, R.; Kantor, I.; Morard, G.; Anzellini, S.; Giampaoli, R.; Briggs, R.; Smareglia, A.; Irifune, T.; Pascarelli, S. The Melting Curve of Nickel Up to 100 GPa Explored by XAS. J. Geophys. Res. Solid Earth 2017, 122, 9921–9930. [Google Scholar] [CrossRef]
- Boehler, R. High-pressure experiments and the phase diagram of lower mantle and core materials. Rev. Geophys. 2000, 38, 221–245. [Google Scholar] [CrossRef]
- Boehler, R.; Santamaría-Pérez, D.; Errandonea, D.; Mezouar, M. Melting, density, and anisotropy of iron at core conditions: New X-ray measurements to 150 GPa. J. Phys. Conf. Ser. 2008, 121, 022018. [Google Scholar] [CrossRef]
- Briggs, R.; Daisenberger, D.; Lord, O.T.; Salamat, A.; Bailey, E.; Walter, M.J.; McMillan, P.F. High-pressure melting behavior of tin up to 105 GPa. Phys. Rev. B Condens. Matter 2017, 95, 054102. [Google Scholar] [CrossRef]
- Errandonea, D. High-pressure melting curves of the transition metals Cu, Ni, Pd, and Pt. Phys. Rev. B Condens. Matter 2013, 87, 054108. [Google Scholar] [CrossRef]
- Karandikar, A.; Boehler, R. Flash melting of tantalum in a diamond cell to 85 GPa. Phys. Rev. B Condens. Matter 2016, 93, 054107. [Google Scholar] [CrossRef]
- Zerr, A.; Miehe, G.; Serghiou, G.; Schwarz, M.; Kroke, E.; Riedel, R.; Fueß, H.; Kroll, P.; Boehler, R. Synthesis of cubic silicon nitride. Nature 1999, 400, 340–342. [Google Scholar] [CrossRef]
- Knittle, E.; Jeanloz, R. Synthesis and Equation of State of (Mg,Fe) SiO3 Perovskite to Over 100 Gigapascals. Science 1987, 235, 668–670. [Google Scholar] [CrossRef]
- Liu, L.-G. Silicate perovskite from phase transformations of pyrope-garnet at high pressure and temperature. Geophys. Res. Lett. 1974, 1, 277–280. [Google Scholar] [CrossRef]
- Mao, H.K.; Mao, W.L. Theory and Practice: Diamond-Anvil Cells and Probes for High-P-T Mineral Physics Studies; Elsevier Inc.: Cambridge, MA, USA, 2015; Volume 2, pp. 263–291. [Google Scholar]
- Benedetti, L.R.; Loubeyre, P. Temperature gradients, wavelength-dependent emissivity, and accuracy of high and very-high temperatures measured in the laser-heated diamond cell. High Press. Res. 2004, 24, 423–445. [Google Scholar] [CrossRef]
- Dubrovinsky, L.; Khandarkhaeva, S.; Fedotenko, T.; Laniel, D.; Bykov, M.; Giacobbe, C.; Bright, E.L.; Sedmak, P.; Chariton, S.; Prakapenka, V.; et al. Materials synthesis at terapascal static pressures. Nature 2022, 605, 274–278. [Google Scholar] [CrossRef]
- Duffy, T.S.; Smith, R.F. Ultra-High Pressure Dynamic Compression of Geological Materials. Front. Earth Sci. 2019, 7, 23. [Google Scholar] [CrossRef]
- Yoo, C.-S.; Wei, H.; Dias, R.; Shen, G.; Smith, J.; Chen, J.-Y.; Evans, W. Time-Resolved Synchrotron X-ray Diffraction on Pulse Laser Heated Iron in Diamond Anvil Cell. J. Phys. Conf. Ser. 2012, 377, 012108. [Google Scholar] [CrossRef] [Green Version]
- Dewaele, A.; Mezouar, M.; Guignot, N.; Loubeyre, P. High melting points of tantalum in a laser-heated diamond anvil cell. Phys. Rev. Lett. 2010, 104, 255701. [Google Scholar] [CrossRef] [PubMed]
- Andrault, D.; Fiquet, G.; Kunz, M.; Visocekas, F.; Häusermann, D. The Orthorhombic Structure of Iron: An in Situ Study at High-Temperature and High-Pressure. Science 1997, 278, 831–834. [Google Scholar] [CrossRef]
- Armstrong, L.S.; Walter, M.J. Tetragonal almandine pyrope phase (TAPP): Retrograde Mg-perovskite from subducted oceanic crust? Eur. J. Miner. 2012, 24, 587–597. [Google Scholar] [CrossRef]
- Armstrong, L.S.; Walter, M.J.; Tuff, J.R.; Lord, O.T.; Lennie, A.R.; Kleppe, A.K.; Clark, S.M. Perovskite Phase Relations in the System CaO–MgO–TiO2–SiO2 and Implications for Deep Mantle Lithologies. J. Petrol. 2012, 53, 611–635. [Google Scholar] [CrossRef]
- Boehler, R. Melting of the FeFeO and the FeFeS systems at high pressure: Constraints on core temperatures. Earth Planet. Sci. Lett. 1992, 111, 217–227. [Google Scholar] [CrossRef]
- Boehler, R.; Ross, M.; Boercker, D.B. High-pressure melting curves of alkali halides. Phys. Rev. B Condens. Matter 1996, 53, 556–563. [Google Scholar] [CrossRef]
- Boehler, R.; Chopelas, A. A new approach to laser heating in high pressure mineral physics. Geophys. Res. Lett. 1991, 18, 1147–1150. [Google Scholar] [CrossRef]
- Bolfan-Casanova, N.; Andrault, D.; Amiguet, E.; Guignot, N. Equation of state and post-stishovite transformation of Al-bearing silica up to 100GPa and 3000K. Phys. Earth Planet. Inter. 2009, 174, 70–77. [Google Scholar] [CrossRef]
- Meade, C.; Mao, H.K.; Hu, J. High-Temperature Phase Transition and Dissociation of (Mg, Fe)SiO3 Perovskite at Lower Mantle Pressures. Science 1995, 268, 1743–1745. [Google Scholar] [CrossRef] [PubMed]
- Dewaele, A.; Mezouar, M.; Guignot, N.; Loubeyre, P. Melting of lead under high pressure studied using second-scale time-resolved X-ray diffraction. Phys. Rev. B Condens. Matter 2007, 76, 144106. [Google Scholar] [CrossRef]
- Dewaele, A.; Belonoshko, A.B.; Garbarino, G.; Occelli, F.; Bouvier, P.; Hanfland, M.; Mezouar, M. High-pressure--high-temperature equation of state of KCl and KBr. Phys. Rev. B Condens. Matter 2012, 85, 214105. [Google Scholar] [CrossRef]
- Dobson, D.P.; Hunt, S.A.; Ahmed, J.; Lord, O.T.; Wann, E.T.H.; Santangeli, J.; Wood, I.G.; Vočadlo, L.; Walker, A.M.; Thomson, A.R.; et al. The phase diagram of NiSi under the conditions of small planetary interiors. Phys. Earth Planet. Inter. 2016, 261, 196–206. [Google Scholar] [CrossRef]
- Errandonea, D.; Schwager, B.; Ditz, R.; Gessmann, C.; Boehler, R.; Ross, M. Systematics of transition-metal melting. Phys. Rev. B Condens. Matter 2001, 63, 132104. [Google Scholar] [CrossRef]
- Errandonea, D.; Somayazulu, M.; Häusermann, D.; Mao, H.K. Melting of tantalum at high pressure determined by angle dispersive X-ray diffraction in a double-sided laser-heated diamond-anvil cell. J. Phys. Condens. Matter 2003, 15, 7635–7649. [Google Scholar] [CrossRef]
- Errandonea, D.; Boehler, R.; Japel, S.; Mezouar, M.; Benedetti, L.R. Structural transformation of compressed solid Ar: An X-ray diffraction study to114 GPa. Phys. Rev. B Condens. Matter 2006, 73, 092106. [Google Scholar] [CrossRef]
- Errandonea, D.; MacLeod, S.G.; Burakovsky, L.; Santamaria-Perez, D.; Proctor, J.E.; Cynn, H.; Mezouar, M. Melting curve and phase diagram of vanadium under high-pressure and high-temperature conditions. Phys. Rev. B Condens. Matter 2019, 100, 094111. [Google Scholar] [CrossRef]
- Fedotenko, T.; Dubrovinsky, L.; Aprilis, G.; Koemets, E.; Snigirev, A.; Snigireva, I.; Barannikov, A.; Ershov, P.; Cova, F.; Hanfland, M.; et al. Laser heating setup for diamond anvil cells for in situ synchrotron and in house high and ultra-high pressure studies. Rev. Sci. Instrum. 2019, 90, 104501. [Google Scholar] [CrossRef]
- Fiquet, G.; Andrault, D. Powder X-ray diffraction under extreme conditions of pressure and temperature. J. Synchrotron Radiat. 1999, 6, 81–86. [Google Scholar] [CrossRef]
- Fiquet, G.; Andrault, D.; Itié, J.P.; Gillet, P.; Richet, P. X-ray diffraction of periclase in a laser-heated diamond-anvil cell. Phys. Earth Planet. Inter. 1996, 95, 1–17. [Google Scholar] [CrossRef]
- Fiquet, G.; Dewaele, A.; Andrault, D.; Kunz, M.; Le Bihan, T. Thermoelastic properties and crystal structure of MgSiO3 perovskite at lower mantle pressure and temperature conditions. Geophys. Res. Lett. 2000, 27, 21–24. [Google Scholar] [CrossRef]
- Friedrich, A.; Morgenroth, W.; Bayarjargal, L.; Juarez-Arellano, E.A.; Winkler, B.; Konôpková, Z. In situ study of the high pressure high-temperature stability field of TaN and of the compressibilities of ϑ-TaN and TaON. High Press. Res. 2013, 33, 633–641. [Google Scholar] [CrossRef]
- Heinz, D.L.; Sweeney, J.S.; Miller, P. A laser heating system that stabilizes and controls the temperature: Diamond anvil cell applications. Rev. Sci. Instrum. 1991, 62, 1568–1575. [Google Scholar] [CrossRef]
- Hrubiak, R.; Meng, Y.; Shen, G. Microstructures define melting of molybdenum at high pressures. Nat. Commun. 2017, 8, 14562. [Google Scholar] [CrossRef]
- Huang, X.; Li, F.; Zhou, Q.; Meng, Y.; Litasov, K.D.; Wang, X.; Liu, B.; Cui, T. Thermal equation of state of Molybdenum determined from in situ synchrotron X-ray diffraction with laser-heated diamond anvil cells. Sci. Rep. 2016, 6, 19923. [Google Scholar] [CrossRef]
- Japel, S.; Schwager, B.; Boehler, R.; Ross, M. Melting of copper and nickel at high pressure: The role of d electrons. Phys. Rev. Lett. 2005, 95, 167801. [Google Scholar] [CrossRef]
- Kavner, A.; Duffy, T.S. Pressure–volume–temperature paths in the laser-heated diamond anvil cell. J. Appl. Phys. 2001, 89, 1907–1914. [Google Scholar] [CrossRef]
- Kesson, S.E.; Gerald, J.D.F. Partitioning of MgO, FeO, NiO, MnO and Cr2O3 between magnesian silicate perovskite and magnesiowüstite: Implications for the origin of inclusions in diamond and the composition of the lower mantle. Earth Planet. Sci. Lett. 1992, 111, 229–240. [Google Scholar] [CrossRef]
- Kesson, S.E.; Gerald, J.D.F.; Shelley, J.M.G. Mineral chemistry and density of subducted basaltic crust at lower-mantle pressures. Nature 1994, 372, 767–769. [Google Scholar] [CrossRef]
- Kiefer, B.; Duffy, T.S. Finite element simulations of the laser-heated diamond-anvil cell. J. Appl. Phys. 2005, 97, 114902. [Google Scholar] [CrossRef]
- Kimura, T.; Kuwayama, Y.; Yagi, T. Melting temperatures of H2O up to 72 GPa measured in a diamond anvil cell using CO2 laser heating technique. J. Chem. Phys. 2014, 140, 074501. [Google Scholar] [CrossRef]
- Ko, Y.-H.; Oh, K.H.; Kim, K.J. Installation and Operation of a Double-Sided Laser Heating System for the Synthesis of Novel Materials Under Extreme Conditions. New Phys. Sae Mulli 2019, 69, 1107–1114. [Google Scholar] [CrossRef]
- Kumar, N.R.; Shekar, N.V.; Sekar, M.; Subramanian, N.; Mohan, P.C.; Srinivasan, M.P.; Parameswaran, P.; Sahu, P.C. Diamond and diamond-like carbon in laser heated diamond anvil cell at 16.5 GPa and above 2000 K from pyrolitic graphite. Indian J. Pure Appl. Phys. 2008, 46, 783–787. [Google Scholar]
- Kupenko, I.; Strohm, C.; McCammon, C.; Cerantola, V.; Glazyrin, K.; Petitgirard, S.; Vasiukov, D.; Aprilis, G.; Chumakov, A.I.; Rüffer, R.; et al. Time differentiated nuclear resonance spectroscopy coupled with pulsed laser heating in diamond anvil cells. Rev. Sci. Instrum. 2015, 86, 114501. [Google Scholar] [CrossRef]
- Lavina, B.; Dera, P.; Kim, E.; Meng, Y.; Robert, T.D.; Philippe, F.W.; Stephen, R.S.; Zhao, Y. Discovery of the recoverable high-pressure iron oxide Fe4O5. Proc. Natl. Acad. Sci. USA 2011, 108, 17281–17285. [Google Scholar] [CrossRef]
- Lazicki, A.; Dewaele, A.; Loubeyre, P.; Mezouar, M. High-pressure--temperature phase diagram and the equation of state of beryllium. Phys. Rev. B Condens. Matter 2012, 86, 174118. [Google Scholar] [CrossRef]
- Lin, J.-F.; Santoro, M.; Struzhkin, V.V.; Mao, H.-K.; Hemley, R.J. In situ high pressure-temperature Raman spectroscopy technique with laser-heated diamond anvil cells. Rev. Sci. Instrum. 2004, 75, 3302–3306. [Google Scholar] [CrossRef]
- Lin, J.-F.; Sturhahn, W.; Zhao, J.; Shen, G.; Mao, H.-K.; Hemley, R.J. Absolute temperature measurement in a laser-heated diamond anvil cell. Geophys. Res. Lett. 2004, 31, L14611. [Google Scholar] [CrossRef]
- Lin, Y.; Hu, Q.; Zhu, L.; Meng, Y. Structure and Stability of Iron Fluoride at High Pressure–Temperature and Implication for a New Reservoir of Fluorine in the Deep Earth. Minerals 2020, 10, 783. [Google Scholar] [CrossRef]
- Liu, J.; Wang, C.; Lv, C.; Su, X.; Liu, Y.; Tang, R.; Chen, J.; Hu, Q.; Mao, H.-K.; Mao, W.L. Evidence for oxygenation of Fe-Mg oxides at mid-mantle conditions and the rise of deep oxygen. Natl. Sci. Rev. 2020, 8, nwaa096. [Google Scholar] [CrossRef] [PubMed]
- Lord, O.T.; Wann, E.T.H.; Hunt, S.A.; Walker, A.M.; Santangeli, J.; Walter, M.J.; Dobson, D.P.; Wood, I.G.; Vočadlo, L.; Morard, G.; et al. The NiSi melting curve to 70 GPa. Phys. Earth Planet. Inter. 2014, 233, 13–23. [Google Scholar] [CrossRef]
- Lord, O.T.; Wood, I.G.; Dobson, D.P.; Vočadlo, L.; Wang, W.; Thomson, A.R.; Wann, E.T.H.; Morard, G.; Mezouar, M.; Walter, M.J. The melting curve of Ni to 1 Mbar. Earth Planet. Sci. Lett. 2014, 408, 226–236. [Google Scholar] [CrossRef] [Green Version]
- Meng, Y.; Shen, G.; Mao, H.K. Double-sided laser heating system at HPCAT for in situ X-ray diffraction at high pressures and high temperatures. J. Phys. Condens. Matter 2006, 18, S1097–S1103. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, L.; Kanitpanyacharoen, W.; Raju, S.V.; Kaercher, P.; Knight, J.; MacDowell, A.; Wenk, H.-R.; Williams, Q.; Alarcon, E.Z. Combined resistive and laser heating technique for in situ radial X-ray diffraction in the diamond anvil cell at high pressure and temperature. Rev. Sci. Instrum. 2013, 84, 025118. [Google Scholar] [CrossRef] [PubMed]
- Nabiei, F.; Badro, J.; Boukaré, C.; Hébert, C.; Cantoni, M.; Borensztajn, S.; Wehr, N.; Gillet, P. Investigating Magma Ocean Solidification on Earth Through Laser-Heated Diamond Anvil Cell Experiments. Geophys. Res. Lett. 2021, 48, e2021GL092446. [Google Scholar] [CrossRef] [PubMed]
- Ohfuji, H.; Irifune, T.; Okada, T.; Yagi, T.; Sumiya, H. Laser heating in nano-polycrystalline diamond anvil cell. J. Phys. Conf. Ser. 2010, 215, 012192. [Google Scholar] [CrossRef]
- Ohtaka, O.; Andrault, D.; Bouvier, P.; Schultz, E.; Mezouar, M. Phase relations and equation of state of ZrO2 to 100 GPa. J. Appl. Crystallogr. 2005, 38, 727–733. [Google Scholar] [CrossRef]
- Panero, W.R.; Jeanloz, R. Temperature gradients in the laser-heated diamond anvil cell. J. Geophys. Res. Solid Earth 2001, 106, 6493–6498. [Google Scholar] [CrossRef]
- Santamaría-Pérez, D.; Boehler, R. FeSi melting curve up to 70 GPa. Earth Planet. Sci. Lett. 2008, 265, 743–747. [Google Scholar] [CrossRef]
- Pigott, J.S.; Reaman, D.M.; Panero, W.R. Microfabrication of controlled-geometry samples for the laser-heated diamond-anvil cell using focused ion beam technology. Rev. Sci. Instrum. 2011, 82, 115106. [Google Scholar] [CrossRef]
- Pigott, J.S.; Velisavljevic, N.; Moss, E.K.; Draganic, N.; Jacobsen, M.K.; Meng, Y.; Hrubiak, R.; Sturtevant, B.T. Experimental melting curve of zirconium metal to 37 GPa. J. Phys. Condens. Matter 2020, 32, 355402. [Google Scholar] [CrossRef]
- Polvani, D.A.; Meng, J.F.; Hasegawa, M.; Badding, J.V. Measurement of the thermoelectric power of very small samples at ambient and high pressures. Rev. Sci. Instrum. 1999, 70, 3586–3589. [Google Scholar] [CrossRef]
- Prakapenka, V.B.; Shen, G.; Dubrovinsky, L.S. Carbon transport in diamond anvil cells. High Temp. High Press. 2003, 35–36, 237–249. [Google Scholar] [CrossRef]
- Raju, S.V.; Hrubiak, R.; Drozd, V.; Saxena, S. Laser-assisted processing of Ni-Al-Co-Ti under high pressure. Mater. Manuf. Process. 2017, 32, 1606–1611. [Google Scholar] [CrossRef]
- Ross, M.; Boehler, R.; Söderlind, P. Xenon Melting Curve to 80 GPa and 5p-d Hybridization. Phys. Rev. Lett. 2005, 95, 257801. [Google Scholar] [CrossRef]
- Runge, C.E.; Kubo, A.; Kiefer, B.; Meng, Y.; Prakapenka, V.B.; Shen, G.; Cava, R.J.; Duffy, T.S. Equation of state of MgGeO3 perovskite to 65 GPa: Comparison with the post-perovskite phase. Phys. Chem. Miner. 2006, 33, 699–709. [Google Scholar] [CrossRef]
- Sadovyi, B.; Wierzbowska, M.; Stelmakh, S.; Boccato, S.; Gierlotka, S.; Irifune, T.; Porowski, S.; Grzegory, I. Experimental and theoretical evidence of the temperature-induced wurtzite to rocksalt phase transition in GaN under high pressure. Phys. Rev. B Condens. Matter 2020, 102, 235109. [Google Scholar] [CrossRef]
- Saha, P.; Mukherjee, G.D. Temperature measurement in double-sided laser-heated diamond anvil cell and reaction of carbon. Indian J. Phys. 2021, 95, 621–628. [Google Scholar] [CrossRef]
- Saha, P.; Mazumder, A.; Mukherjee, G.D. Thermal conductivity of dense hcp iron: Direct measurements using laser heated diamond anvil cell. Geosci. Front. 2020, 11, 1755–1761. [Google Scholar] [CrossRef]
- Salem, R.; Matityahu, S.; Melchior, A.; Nikolaevsky, M.; Noked, O.; Sterer, E. Image analysis of speckle patterns as a probe of melting transitions in laser-heated diamond anvil cell experiments. Rev. Sci. Instrum. 2015, 86, 093907. [Google Scholar] [CrossRef]
- Santamaría-Pérez, D.; Ross, M.; Errandonea, D.; Mukherjee, G.D.; Mezouar, M.; Boehler, R. X-ray diffraction measurements of Mo melting to 119 GPa and the high pressure phase diagram. J. Chem. Phys. 2009, 130, 124509. [Google Scholar] [CrossRef]
- Schultz, E.; Mezouar, M.; Crichton, W.; Bauchau, S.; Blattmann, G.; Andrault, D.; Fiquet, G.; Boehler, R.; Rambert, N.; Sitaud, B.; et al. Double-sided laser heating system for in situ high pressure–high temperature monochromatic X-ray diffraction at the esrf. High Press. Res. 2005, 25, 71–83. [Google Scholar] [CrossRef]
- Shen, G.; Prakapenka, V.B.; Rivers, M.L.; Sutton, S.R. Structure of Liquid Iron at Pressures up to 58 GPa. Phys. Rev. Lett. 2004, 92, 185701. [Google Scholar] [CrossRef]
- Shen, G.; Mao, H.-K.; Hemley, R. Laser-Heated Diamond Anvil Cell Technique: Double-Sided Heating with Multimode Nd:YAG Laser. In Advanced Materials ’96 New Trends in High Pressure Research; Akaishi, M., Ed.; The Institute: Tsukuba, Japan, 1996. [Google Scholar]
- Shieh, S.R.; Duffy, T.S.; Shen, G. X-ray diffraction study of phase stability in SiO2 at deep mantle conditions. Earth Planet. Sci. Lett. 2005, 235, 273–282. [Google Scholar] [CrossRef]
- Shim, S.H.; Duffy, T.S.; Jeanloz, R.; Shen, G. Stability and crystal structure of MgSiO3 perovskite to the core-mantle boundary. Geophys. Res. Lett. 2004, 31. [Google Scholar] [CrossRef]
- Shukla, B.; Shekar, N.V.C.; Kumar, N.R.S.; Ravindran, T.R.; Sahoo, P.; Dhara, S.; Sahu, P.C. Twin chamber sample assembly in DAC and HPHT studies on GaN nano-particles. J. Phys. Conf. Ser. 2012, 377, 012014. [Google Scholar] [CrossRef]
- Singh, A.K.; Andrault, D.; Bouvier, P. X-ray diffraction from stishovite under nonhydrostatic compression to 70 GPa: Strength and elasticity across the tetragonal→orthorhombic transition. Phys. Earth Planet. Inter. 2012, 208–209, 1–10. [Google Scholar] [CrossRef]
- Sinmyo, R.; Hirose, K. The Soret diffusion in laser-heated diamond-anvil cell. Phys. Earth Planet. Inter. 2010, 180, 172–178. [Google Scholar] [CrossRef]
- Sinmyo, R.; Pesce, G.; Greenberg, E.; McCammon, C.; Dubrovinsky, L. Lower mantle electrical conductivity based on measurements of Al, Fe-bearing perovskite under lower mantle conditions. Earth Planet. Sci. Lett. 2014, 393, 165–172. [Google Scholar] [CrossRef]
- Spiekermann, G.; Kupenko, I.; Petitgirard, S.; Harder, M.; Nyrow, A.; Weis, C.; Albers, C.; Biedermann, N.; Libon, L.; Sahle, C.J.; et al. A portable on-axis laser-heating system for near-90degrees X-ray spectroscopy: Application to ferropericlase and iron silicide. J. Synchrotron Radiat. 2020, 27, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Stutzmann, V.; Dewaele, A.; Bouchet, J.; Bottin, F.; Mezouar, M. High-pressure melting curve of titanium. Phys. Rev. B Condens. Matter 2015, 92, 224110. [Google Scholar] [CrossRef]
- Auzende, A.-L.; Gillot, J.; Coquet, A.; Hennet, L.; Ona-Nguema, G.; Bonnin, D.; Esteve, I.; Roskosz, M.; Fiquet, G. Synthesis of amorphous MgO-rich peridotitic starting material for laser-heated diamond anvil cell experiments–application to iron partitioning in the mantle. High Press. Res. 2011, 31, 199–213. [Google Scholar] [CrossRef]
- Watanuki, T.; Shimomura, O.; Yagi, T.; Kondo, T.; Isshiki, M. Construction of laser-heated diamond anvil cell system for in situ X-ray diffraction study at SPring-8. Rev. Sci. Instrum. 2001, 72, 1289–1292. [Google Scholar] [CrossRef]
- Weck, G.; Recoules, V.; Queyroux, J.-A.; Datchi, F.; Bouchet, J.; Ninet, S.; Garbarino, G.; Mezouar, M.; Loubeyre, P. Determination of the melting curve of gold up to 110 GPa. Phys. Rev. B Condens. Matter 2020, 101, 014106. [Google Scholar] [CrossRef]
- Yagi, T.; Susaki, J.-I. A Laser Heating System for Diamond Anvil Using CO2 Laser. In High-Pressure Research: Application to Earth and Planetary Sciences; Terra Scientific Publishing Company: Tokyo, Japan, 1992; pp. 51–54. [Google Scholar]
- Yang, L.; Karandikar, A.; Boehler, R. Flash heating in the diamond cell: Melting curve of rhenium. Rev. Sci. Instrum. 2012, 83, 063905. [Google Scholar] [CrossRef]
- Yoo, C.S.; Akella, J.; Moriarty, J.A. High-pressure melting temperatures of uranium: Laser-heating experiments and theoretical calculations. Phys. Rev. B Condens. Matter 1993, 48, 15529–15534. [Google Scholar] [CrossRef]
- Yoo, C.S.; Akella, J.; Campbell, A.J.; Mao, H.K.; Hemley, R.J. Phase Diagram of Iron by in Situ X-ray Diffraction: Implications for Earth’s Core. Science 1995, 270, 1473–1475. [Google Scholar] [CrossRef]
- Yoo, C.S.; Söderlind, P.; Moriarty, J.A.; Cambell, A.J. dhcp as a possible new ϵ′ phase of iron at high pressures and temperatures. Phys. Lett. A 1996, 214, 65–70. [Google Scholar] [CrossRef]
- Zerr, A.; Boehier, R. Melting of (Mg, Fe)SiO3-Perovskite to 625 Kilobars: Indication of a High Melting Temperature in the Lower Mantle. Science 1993, 262, 553–555. [Google Scholar] [CrossRef]
- Zhang, L.; Meng, Y.; Mao, H.-K. Unit cell determination of coexisting post-perovskite and H-phase in (Mg,Fe)SiO3 using multigrain XRD: Compositional variation across a laser heating spot at 119 GPa. Prog. Earth Planet. Sci. 2016, 3, 13. [Google Scholar] [CrossRef]
- Zhang, D.; Jackson, J.M.; Zhao, J.; Sturhahn, W.; Alp, E.E.; Hu, M.Y.; Toellner, T.S.; Murphy, C.A.; Prakapenka, V.B. Temperature of Earth’s core constrained from melting of Fe and Fe0.9Ni0.1 at high pressures. Earth Planet. Sci. Lett. 2016, 447, 72–83. [Google Scholar] [CrossRef]
- Zinin, P.V.; Prakapenka, V.B.; Burgess, K.; Odake, S.; Chigarev, N.; Sharma, S.K. Combined laser ultrasonics, laser heating, and Raman scattering in diamond anvil cell system. Rev. Sci. Instrum. 2016, 87, 123908. [Google Scholar] [CrossRef]
- Zou, G.; Ma, Y.; Mao, H.-K.; Hemley, R.J.; Gramsch, S.A. A diamond gasket for the laser-heated diamond anvil cell. Rev. Sci. Instrum. 2001, 72, 1298–1301. [Google Scholar] [CrossRef]
- Weathers, M.S.; Bassett, W.A. Melting of carbon at 50 to 300 kbar. Phys. Chem. Miner. 1987, 15, 105–112. [Google Scholar] [CrossRef]
- Huang, D.; Siebert, J.; Badro, J. High pressure partitioning behavior of Mo and W and late sulfur delivery during Earth’s core formation. Geochim. Cosmochim. Acta 2021, 310, 19–31. [Google Scholar] [CrossRef]
- Kurnosov, A.; Marquardt, H.; Dubrovinsky, L.; Potapkin, V. A waveguide-based flexible CO2-laser heating system for diamond-anvil cell applications. Comptes Rendus Geosci. 2019, 351, 280–285. [Google Scholar] [CrossRef]
- Chidester, B.A.; Thompson, E.C.; Fischer, R.A.; Heinz, D.L.; Prakapenka, V.B.; Meng, Y.; Campbell, A.J. Experimental thermal equation of state of B2− KCl. Phys. Rev. B Condens. Matter 2021, 104, 094107. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, Y.; Geng, H.Y.; Salke, N.P.; Gao, Z.; Li, J.; Sekine, T.; Wang, Q.; Greenberg, E.; Prakapenka, V.B.; et al. Melting curve of vanadium up to 256 GPa: Consistency between experiments and theory. Phys. Rev. B Condens. Matter 2020, 102, 214104. [Google Scholar] [CrossRef]
- Gaida, N.A.; Niwa, K.; Sasaki, T.; Hasegawa, M. Phase relations and thermoelasticity of magnesium silicide at high pressure and temperature. J. Chem. Phys. 2021, 154, 144701. [Google Scholar] [CrossRef]
- Anzellini, S.; Burakovsky, L.; Turnbull, R.; Bandiello, E.; Errandonea, D. P–V–T Equation of State of Iridium Up to 80 GPa and 3100 K. Crystals 2021, 11, 452. [Google Scholar] [CrossRef]
- Nishiyama, N.; Langer, J.; Sakai, T.; Kojima, Y.; Holzheid, A.; Gaida, N.A.; Kulik, E.; Hirao, N.; Kawaguchi, S.I.; Irifune, T.; et al. Phase relations in silicon and germanium nitrides up to 98 GPa and 2400 °C. J. Am. Ceram. Soc. 2019, 102, 2195–2202. [Google Scholar] [CrossRef]
- Bassett, W.A.; Li-Chung, M. Disproportionation of Fe2SiO4 to 2FeO+SiO2 at pressures up to 250kbar and temperatures up to 3000 °C. Phys. Earth Planet. Inter. 1972, 6, 154–160. [Google Scholar] [CrossRef]
- Wakamatsu, T.; Ohta, K.; Yagi, T.; Hirose, K.; Ohishi, Y. Measurements of sound velocity in iron–nickel alloys by femtosecond laser pulses in a diamond anvil cell. Phys. Chem. Miner. 2018, 45, 589–595. [Google Scholar] [CrossRef]
- Deemyad, S.; Sterer, E.; Barthel, C.; Rekhi, S.; Tempere, J.; Silvera, I.F. Pulsed laser heating and temperature determination in a diamond anvil cell. Rev. Sci. Instrum. 2005, 76, 125104. [Google Scholar] [CrossRef]
- Nissim, N.; Eliezer, S.; Werdiger, M. The sound velocity throughout the P-ρ phase-space with application to laser induced shock wave in matter precompressed by a diamond anvil cell. J. Appl. Phys. 2014, 115, 213503. [Google Scholar] [CrossRef]
- Brygoo, S.; Millot, M.; Loubeyre, P.; Lazicki, A.E.; Hamel, S.; Qi, T.; Celliers, P.M.; Coppari, F.; Eggert, J.H.; Fratanduono, D.E.; et al. Analysis of laser shock experiments on precompressed samples using a quartz reference and application to warm dense hydrogen and helium. J. Appl. Phys. 2015, 118, 195901. [Google Scholar] [CrossRef]
- Loubeyre, P.; Celliers, P.M.; Hicks, D.G.; Henry, E.; Dewaele, A.; Pasley, J.; Eggert, J.; Koenig, M.; Occelli, F.; Lee, K.M.; et al. Coupling static and dynamic compressions: First measurements in dense hydrogen. High Press. Res. 2004, 24, 25–31. [Google Scholar] [CrossRef]
- Loubeyre, P.; Brygoo, S.; Eggert, J.; Celliers, P.M.; Spaulding, D.K.; Rygg, J.R.; Boehly, T.R.; Collins, G.W.; Jeanloz, R. Extended data set for the equation of state of warm dense hydrogen isotopes. Phys. Rev. B Condens. Matter 2012, 86, 144115. [Google Scholar] [CrossRef]
- Nissim, N.; Eliezer, S.; Werdiger, M.; Perelmutter, L. Approaching the “cold curve” in laser-driven shock wave experiment of a matter precompressed by a partially perforated diamond anvil. Laser Part. Beams 2013, 31, 73–79. [Google Scholar] [CrossRef]
- Sorb, Y.A.; Subramanian, N.; Ravindran, T.R.; Sahu, P.C. High Pressure in situ Micro-Raman Spectroscopy of Ge-Sn System Synthesized in a Laser Heated Diamond Anvil Cell. AIP Conf. Proc. 2011, 1349, 1305–1306. [Google Scholar] [CrossRef]
- Fedotenko, T.; Dubrovinsky, L.; Khandarkhaeva, S.; Chariton, S.; Koemets, E.; Koemets, I.; Hanfland, M.; Dubrovinskaia, N. Synthesis of palladium carbides and palladium hydride in laser heated diamond anvil cells. J. Alloy. Compd. 2020, 844, 156179. [Google Scholar] [CrossRef]
- Gréaux, S.; Andrault, D.; Gautron, L.; Bolfan-Casanova, N.; Mezouar, M. Compressibility of Ca3Al2Si3O12 perovskite up to 55 GPa. Phys. Chem. Miner. 2014, 41, 419–429. [Google Scholar] [CrossRef]
- Zerr, A.; Serghiou, G.; Boehler, R.; Ross, M. Decomposition of alkanes at high pressures and temperatures. High Press. Res. 2006, 26, 23–32. [Google Scholar] [CrossRef]
- Vance, S.; Harnmeijer, J.; Kimura, J.; Hussmann, H.; DeMartin, B.; Brown, J.M. Hydrothermal Systems in Small Ocean Planets. Astrobiology 2007, 7, 987–1005. [Google Scholar] [CrossRef]
- Helled, R.; Anderson, J.D.; Podolak, M.; Schubert, G. Interior Models of Uranus and Neptune. Astrophys. J. 2010, 726, 15. [Google Scholar] [CrossRef]
- Faure, G.; Mensing, T. Introduction to Planetary Science; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Guillot, T.; Gautier, D. 10.13-Giant Planets. In Treatise on Geophysics; Schubert, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 439–464. [Google Scholar]
- Aprilis, G.; Pakhomova, A.; Chariton, S.; Khandarkhaeva, S.; Melai, C.; Bykova, E.; Bykov, M.; Fedotenko, T.; Koemets, E.; McCammon, C.; et al. The Effect of Pulsed Laser Heating on the Stability of Ferropericlase at High Pressures. Minerals 2020, 10, 542. [Google Scholar] [CrossRef]
- Patel, N.N.; Sunder, M.; Sharma, S.M. Laser heated diamond anvil cell facility for high temperature high pressure research: Application to material synthesis and melting studies. Indian J. Phys. 2018, 92, 1259–1269. [Google Scholar] [CrossRef]
- Friedrich, A.; Winkler, B.; Juarez-Arellano, E.A.; Bayarjargal, L. Synthesis of Binary Transition Metal Nitrides, Carbides and Borides from the Elements in the Laser-Heated Diamond Anvil Cell and Their Structure-Property Relations. Materials 2011, 4, 1648–1692. [Google Scholar] [CrossRef]
- Walker, D.; Lord, O.T.; Walter, M.J.; Clark, S.M. X-ray absorption contrast images of binary chemical reactions. Chem. Geol. 2009, 260, 211–220. [Google Scholar] [CrossRef]
- Hemley, R.J.; Percy, W. Bridgman’s second century. High Press. Res. 2010, 30, 581–619. [Google Scholar] [CrossRef]
- Bridgman, P.W. Polymorphism, Principally of the Elements, up to 50,000 kg/cm2. Phys. Rev. 1935, 48, 893–906. [Google Scholar] [CrossRef]
- Hall, H.T. Some High-Pressure, High-Temperature Apparatus Design Considerations: Equipment for Use at 100,000 Atmospheres and 3000 °C. Rev. Sci. Instrum. 1958, 29, 267–275. [Google Scholar] [CrossRef]
- Khvostantsev, L.G.; Slesarev, V.N.; Brazhkin, V.V. Toroid type high-pressure device: History and prospects. High Press. Res. 2004, 24, 371–383. [Google Scholar] [CrossRef]
- Fang, J.; Bull, C.L.; Loveday, J.S.; Nelmes, R.J.; Kamenev, K.V. Strength analysis and optimisation of double-toroidal anvils for high-pressure research. Rev. Sci. Instrum. 2012, 83, 093902. [Google Scholar] [CrossRef]
- Dewaele, A.; Loubeyre, P.; Occelli, F.; Marie, O.; Mezouar, M. Toroidal diamond anvil cell for detailed measurements under extreme static pressures. Nat. Commun. 2018, 9, 2913. [Google Scholar] [CrossRef]
- Van Valkenburg, A. Visual Observations of High Pressure Transitions. Rev. Sci. Instrum. 1962, 33, 1462. [Google Scholar] [CrossRef]
- Kawai, N.; Endo, S. The Generation of Ultrahigh Hydrostatic Pressures by a Split Sphere Apparatus. Rev. Sci. Instrum. 1970, 41, 1178–1181. [Google Scholar] [CrossRef]
- Bassett, W.A.; Shen, A.H.; Bucknum, M.; Chou, I.M. A new diamond anvil cell for hydrothermal studies to 2.5 GPa and from −190 to 1200 °C. Rev. Sci. Instrum. 1993, 64, 2340–2345. [Google Scholar] [CrossRef]
- Anzellini, S.; Boccato, S. A Practical Review of the Laser-Heated Diamond Anvil Cell for University Laboratories and Synchrotron Applications. Crystals 2020, 10, 459. [Google Scholar] [CrossRef]
- Bodea, S.; Jeanloz, R. Model calculations of the temperature distribution in the laser-heated diamond cell. J. Appl. Phys. 1989, 65, 4688–4692. [Google Scholar] [CrossRef]
- Williams, Q.; Knittle, E.; Jeanloz, R. The high-pressure melting curve of iron: A technical discussion. J. Geophys. Res. Solid Earth 1991, 96, 2171–2184. [Google Scholar] [CrossRef]
- Manga, M.; Jeanloz, R. Axial temperature gradients in dielectric samples in the laser-heated diamond cell. Geophys. Res. Lett. 1996, 23, 1845–1848. [Google Scholar] [CrossRef]
- Kavner, A.; Nugent, C. Precise measurements of radial temperature gradients in the laser-heated diamond anvil cell. Rev. Sci. Instrum. 2008, 79, 024902. [Google Scholar] [CrossRef]
- Lord, O.T.; Wang, W. MIRRORS: A MATLAB® GUI for temperature measurement by multispectral imaging radiometry. Rev. Sci. Instrum. 2018, 89, 104903. [Google Scholar] [CrossRef]
- Campbell, A.J. Measurement of temperature distributions across laser heated samples by multispectral imaging radiometry. Rev. Sci. Instrum. 2008, 79, 015108. [Google Scholar] [CrossRef]
- Eremets, M.I. High Pressure Experimental Methods; Oxford University Press: Oxford, UK, 1996. [Google Scholar]
- Caldwell, W.A.; Kunz, M.; Celestre, R.S.; Domning, E.E.; Walter, M.J.; Walker, D.; Glossinger, J.; MacDowell, A.A.; Padmore, H.A.; Jeanloz, R.; et al. Laser-heated diamond anvil cell at the advanced light source beamline 12.2.2. Nucl. Instrum. Methods Phys. Res. Sect. A 2007, 582, 221–225. [Google Scholar] [CrossRef]
- Hébert, P.; Saint-Amans, C. Study of the laser-induced decomposition of energetic materials at static high-pressure by time-resolved absorption spectroscopy. J. Phys. Conf. Ser. 2014, 500, 022002. [Google Scholar] [CrossRef]
- Goncharov, A.F.; Struzhkin, V.V.; Jacobsen, S.D. Reduced Radiative Conductivity of Low-Spin (Mg,Fe)O in the Lower Mantle. Science 2006, 312, 1205–1208. [Google Scholar] [CrossRef]
- Hemley, R.J.; Goncharov, A.F.; Lu, R.; Struzhkin, V.V.; Li, M.; Mao, H.K. High-pressure synchrotron infrared spectroscopy at the national synchrotron light source. Nuovo Cim. Soc. Ital. Fis. D 1998, 20, 539–551. [Google Scholar] [CrossRef]
- Bassett, W.A.; Brody, E.M. Brillouin Scattering: A New Way to Measure Elastic Moduli at High Pressures. In High-Pressure Research; Manghnani, M.H., Akimoto, S.-I., Eds.; Academic Press: Cambridge, MA, USA, 1977; pp. 519–532. [Google Scholar]
- Chigarev, N.; Zinin, P.; Mounier, D.; Bulou, A.; Ming, L.C.; Acosta, T.; Gusev, V. Analysis of ultrasonic echoes induced by pulsed laser action on an iron film in a diamond anvil cell. High Press. Res. 2010, 30, 78–82. [Google Scholar] [CrossRef]
- Chigarev, N.; Zinin, P.; Mounier, D.; Bulou, A.; Zerr, A.; Ming, L.C.; Gusev, V. Laser ultrasonic measurements in a diamond anvil cell on Fe and the KBr pressure medium. J. Phys. Conf. Ser. 2011, 278, 012017. [Google Scholar] [CrossRef]
- Decremps, F.; Belliard, L.; Perrin, B.; Gauthier, M. Sound Velocity and Absorption Measurements under High Pressure Using Picosecond Ultrasonics in a Diamond Anvil Cell: Application to the Stability Study of AlPdMn. Phys. Rev. Lett. 2008, 100, 035502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poprawski, W.; Gnutek, Z.; Radojewska, E.B.; Poprawski, R. Investigation of black body radiation with the aid of a self-made pyroelectric infrared detector. Eur. J. Phys. 2015, 36, 065025. [Google Scholar] [CrossRef]
- Levendis, Y.A.; Estrada, K.R.; Hottel, H.C. Development of multicolor pyrometers to monitor the transient response of burning carbonaceous particles. Rev. Sci. Instrum. 1992, 63, 3608–3622. [Google Scholar] [CrossRef]
- Yen, C.E.; Williams, Q.; Kunz, M. Thermal Pressure in the Laser-Heated Diamond Anvil Cell: A Quantitative Study and Implications for the Density Versus Mineralogy Correlation of the Mantle. J. Geophys. Res. Solid Earth 2020, 125, e2020JB020006. [Google Scholar] [CrossRef]
- Kunz, M.; Yan, J.; Cornell, E.; Domning, E.E.; Yen, C.E.; Doran, A.; Beavers, C.M.; Treger, A.; Williams, Q.; MacDowell, A.A. Implementation and application of the peak scaling method for temperature measurement in the laser heated diamond anvil cell. Rev. Sci. Instrum. 2018, 89, 083903. [Google Scholar] [CrossRef]
- Zinin, P.V.; Bykov, A.A.; Machikhin, A.S.; Troyan, I.A.; Bulatov, K.M.; Mantrova, Y.V.; Batshev, V.I.; Gaponov, M.I.; Kutuza, I.B.; Rashchenko, S.V.; et al. Measurement of the temperature distribution on the surface of the laser heated specimen in a diamond anvil cell system by the tandem imaging acousto-optical filter. High Press. Res. 2019, 39, 131–149. [Google Scholar] [CrossRef]
- Giampaoli, R.; Kantor, I.; Mezouar, M.; Boccato, S.; Rosa, A.D.; Torchio, R.; Garbarino, G.; Mathon, O.; Pascarelli, S. Measurement of temperature in the laser heated diamond anvil cell: Comparison between reflective and refractive optics. High Press. Res. 2018, 38, 250–269. [Google Scholar] [CrossRef]
- Du, Z.; Amulele, G.; Benedetti, L.R.; Lee, K.K. Mapping temperatures and temperature gradients during flash heating in a diamond-anvil cell. Rev. Sci. Instrum. 2013, 84, 075111. [Google Scholar] [CrossRef]
- Manghnani, M.H.; Yagi, T. Properties of Earth and Planetary Materials at High Pressure and Temperature; American Geophysical Union: Washington, DC, USA, 1998. [Google Scholar]
- Watkins, E.B.; Huber, R.C.; Childs, C.M.; Salamat, A.; Pigott, J.S.; Chow, P.; Xiao, Y.; Coe, J.D. Diamond and methane formation from the chemical decomposition of polyethylene at high pressures and temperatures. Sci. Rep. 2022, 12, 631. [Google Scholar] [CrossRef]
- Gomez-Perez, N.; Rodriguez, J.F.; McWilliams, R.S. Finite element modeling of melting and fluid flow in the laser-heated diamond-anvil cell. J. Appl. Phys. 2017, 121, 145904. [Google Scholar] [CrossRef]
- Dorfman, S.M.; Nabiei, F.; Boukaré, C.-E.; Prakapenka, V.B.; Cantoni, M.; Badro, J.; Gillet, P. Composition and Pressure Effects on Partitioning of Ferrous Iron in Iron-Rich Lower Mantle Heterogeneities. Minerals 2021, 11, 512. [Google Scholar] [CrossRef]
- Geballe, Z.M.; Jeanloz, R. Origin of temperature plateaus in laser-heated diamond anvil cell experiments. J. Appl. Phys. 2012, 111, 123518. [Google Scholar] [CrossRef]
- Cengel, Y.; Boles, M.A.; Boles, M.A. Thermodynamics: An Engineering Approach: An Engineering Approach; McGraw-Hill: New York, NY, USA, 2018. [Google Scholar]
- Miyajima, N.; Fujino, K.; Funamori, N.; Kondo, T.; Yagi, T. Garnet-perovskite transformation under conditions of the Earth’s lower mantle: An analytical transmission electron microscopy study. Phys. Earth Planet. Inter. 1999, 116, 117–131. [Google Scholar] [CrossRef]
- Juarez-Arellano, E.A.; Avdeev, M.; Yakovlev, S.; Lopez-de-la-Torre, L.; Bayarjargal, L.; Winkler, B.; Friedrich, A.; Kharton, V.V. High-pressure behavior and equations of state of the cobaltates YBaCo4O7, YBaCo4O7+δ, YBaCoZn3O7 and BaCoO3−x. J. Solid State Chem. 2012, 196, 209–216. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics; CRC Press LLC: Boca Raton, FL, USA, 2011. [Google Scholar]
- Goldsmith, A.; Waterman, T.E.; Hirschhorn, H.J. Handbook of Thermophysical Properties of Solid Materials; Macmillan: New York, NY, USA, 1961. [Google Scholar]
- Tateno, S.; Hirose, K.; Komabayashi, T.; Ozawa, H.; Ohishi, Y. The structure of Fe-Ni alloy in Earth’s inner core. Geophys. Res. Lett. 2012, 39. [Google Scholar] [CrossRef]
- Sist, M.; Fischer, K.F.F.; Kasai, H.; Iversen, B.B. Low-Temperature Anharmonicity in Cesium Chloride (CsCl). Angew. Chem. Int. Ed. 2017, 56, 3625–3629. [Google Scholar] [CrossRef]
- Drebushchak, V.A.; Kovalevskaya, Y.A.; Paukov, I.E.; Surkov, N.V. Low-temperature heat capacity of monoclinic enstatite. J. Therm. Anal. Calorim. 2008, 94, 493–497. [Google Scholar] [CrossRef]
- Funamori, N.; Jeanloz, R.; Miyajima, N.; Fujino, K. Mineral assemblages of basalt in the lower mantle. J. Geophys. Res. Solid Earth 2000, 105, 26037–26043. [Google Scholar] [CrossRef]
- Zeng, Z.; Yang, L.; Zeng, Q.; Lou, H.; Sheng, H.; Wen, J.; Miller, D.J.; Meng, Y.; Yang, W.; Mao, W.L.; et al. Synthesis of quenchable amorphous diamond. Nat. Commun. 2017, 8, 322. [Google Scholar] [CrossRef] [PubMed]
- Zinin, P.V.; Ming, L.C.; Ishii, H.A.; Jia, R.; Acosta, T.; Hellebrand, E. Phase transition in BCx system under high-pressure and high-temperature: Synthesis of cubic dense BC3 nanostructured phase. J. Appl. Phys. 2012, 111, 114905. [Google Scholar] [CrossRef]
- Briggs, R.; Daisenberger, D.; Salamat, A.; Garbarino, G.; Mezouar, M.; Wilson, M.; McMillan, P.F. Melting of Sn to 1 Mbar. J. Phys. Conf. Ser. 2012, 377, 012035. [Google Scholar] [CrossRef] [Green Version]
- Irifune, T.; Susaki, J.-I.; Yagi, T.; Sawamoto, H. Phase transformations in diopside CaMgSi2O6 at pressures Up to 25 GPa. Geophys. Res. Lett. 1989, 16, 187–190. [Google Scholar] [CrossRef]
- Touloukian, Y.S.; Buyco, E.H. Thermophysical Properties of Matter: The TPRC Data Series; S.R.S. Sastry: New York, NY, USA; Washington, DC, USA, 1971; Volume 4, pp. 559–560. [Google Scholar]
- Hearne, G.; Bibik, A.; Zhao, J. CO2 laser-heated diamond-anvil cell methodology revisited. J. Phys. Condens. Matter 2002, 14, 11531–11535. [Google Scholar] [CrossRef]
- Heinz, D.L.; Jeanloz, R. Temperature Measurements in the Laser-Heated Diamond Cell. In High-Pressure Research in Mineral Physics: A Volume in Honor of Syun-iti Akimoto; Terra Scientific Publishing Company: Tokyo, Japan, 1987; pp. 113–127. [Google Scholar]
- Weckert, E. The potential of future light sources to explore the structure and function of matter. IUCrJ 2015, 2, 230–245. [Google Scholar] [CrossRef]
- Kimura, T.; Ohfuji, H.; Nishi, M.; Irifune, T. Melting temperatures of MgO under high pressure by micro-texture analysis. Nat. Commun. 2017, 8, 15735. [Google Scholar] [CrossRef]
- Britvin, S.N.; Vereshchagin, O.S.; Shilovskikh, V.V.; Krzhizhanovskaya, M.G.; Gorelova, L.A.; Vlasenko, N.S.; Pakhomova, A.S.; Zaitsev, A.N.; Zolotarev, A.A.; Bykov, M.; et al. Discovery of terrestrial allabogdanite (Fe,Ni)2P, and the effect of Ni and Mo substitution on the barringerite-allabogdanite high-pressure transition. Am. Miner. 2021, 106, 944–952. [Google Scholar] [CrossRef]
- Forman, R.A.; Piermarini, G.J.; Barnett, J.D.; Block, S. Pressure measurement made by the utilization of ruby sharp-line luminescence. Science 1972, 176, 284–285. [Google Scholar] [CrossRef]
- Syassen, K. Ruby under pressure. High Press. Res. 2008, 28, 75–126. [Google Scholar] [CrossRef]
- Shen, G.; Wang, Y.; Dewaele, A.; Wu, C.; Fratanduono, D.E.; Eggert, J.; Klotz, S.; Dziubek, K.F.; Loubeyre, P.; Fat’yanov, O.V.; et al. Toward an international practical pressure scale: A proposal for an IPPS ruby gauge (IPPS-Ruby2020). High Press. Res. 2020, 40, 299–314. [Google Scholar] [CrossRef]
- Tuschel, D. Photoluminescence spectroscopy using a Raman spectrometer. Spectroscopy 2016, 31, 14–21. [Google Scholar]
- Vos, W.L.; Schouten, J.A. On the temperature correction to the ruby pressure scale. J. Appl. Phys. 1991, 69, 6744–6746. [Google Scholar] [CrossRef]
- Yen, J.; Nicol, M. Temperature dependence of the ruby luminescence method for measuring high pressures. J. Appl. Phys. 1992, 72, 5535–5538. [Google Scholar] [CrossRef]
- Chijioke, A.D.; Nellis, W.J.; Soldatov, A.; Silvera, I.F. The ruby pressure standard to 150 GPa. J. Appl. Phys. 2005, 98, 114905. [Google Scholar] [CrossRef]
- Araújo, A. Multi-spectral pyrometry—A review. Meas. Sci. Technol. 2017, 28, 082002. [Google Scholar] [CrossRef]
- Hasegawa, A.; Yagi, T.; Ohta, K. Combination of pulsed light heating thermoreflectance and laser-heated diamond anvil cell for in-situ high pressure-temperature thermal diffusivity measurements. Rev. Sci. Instrum. 2019, 90, 074901. [Google Scholar] [CrossRef]
- Benedetti, L.R.; Guignot, N.; Farber, D.L. Achieving accuracy in spectroradiometric measurements of temperature in the laser-heated diamond anvil cell: Diamond is an optical component. J. Appl. Phys. 2007, 101, 013109. [Google Scholar] [CrossRef]
- Weir, C.E.; Lippincott, E.R.; Van Valkenburg, A.; Bunting, E.N. Infrared Studies in the 1- to 15-Micron Region to 30,000 Atmospheres. J. Res. Nat. Bur. Stand. A Phys. Chem. 1959, 63, 55–62. [Google Scholar] [CrossRef]
- Spetzler, H.; Shen, A.; Chen, G.; Herrmannsdoerfer, G.; Schulze, H.; Weigel, R. Ultrasonic measurements in a diamond anvil cell. Phys. Earth Planet. Inter. 1996, 98, 93–99. [Google Scholar] [CrossRef]
- Shiell, T.B.; McCulloch, D.G.; Bradby, J.E.; Haberl, B.; Boehler, R.; McKenzie, D.R. Nanocrystalline hexagonal diamond formed from glassy carbon. Sci. Rep. 2016, 6, 37232. [Google Scholar] [CrossRef]
- Peng, F.; Sun, Y.; Pickard, C.J.; Needs, R.J.; Wu, Q.; Ma, Y. Hydrogen Clathrate Structures in Rare Earth Hydrides at High Pressures: Possible Route to Room-Temperature Superconductivity. Phys. Rev. Lett. 2017, 119, 107001. [Google Scholar] [CrossRef]
- Drozdov, A.P.; Kong, P.P.; Minkov, V.S.; Besedin, S.P.; Kuzovnikov, M.A.; Mozaffari, S.; Balicas, L.; Balakirev, F.F.; Graf, D.E.; Prakapenka, V.B.; et al. Superconductivity at 250 K in lanthanum hydride under high pressures. Nature 2019, 569, 528–531. [Google Scholar] [CrossRef]
- Wang, H.; Tse, J.S.; Tanaka, K.; Iitaka, T.; Ma, Y. Superconductive sodalite-like clathrate calcium hydride at high pressures. Proc. Natl. Acad. Sci. USA 2012, 109, 6463–6466. [Google Scholar] [CrossRef] [Green Version]
- Kong, P.; Minkov, V.S.; Kuzovnikov, M.A.; Drozdov, A.P.; Besedin, S.P.; Mozaffari, S.; Balicas, L.; Balakirev, F.F.; Prakapenka, V.B.; Chariton, S.; et al. Superconductivity up to 243 K in the yttrium-hydrogen system under high pressure. Nat. Commun. 2021, 12, 5075. [Google Scholar] [CrossRef]
- Drozdov, A.P.; Eremets, M.I.; Troyan, I.A.; Ksenofontov, V.; Shylin, S.I. Conventional superconductivity at 203 kelvin at high pressures in the sulfur hydride system. Nature 2015, 525, 73–76. [Google Scholar] [CrossRef]
- Matsumoto, R.; Yamamoto, S.; Adachi, S.; Sakai, T.; Irifune, T.; Takano, Y. Diamond anvil cell with boron-doped diamond heater for high-pressure synthesis and in situ transport measurements. Appl. Phys. Lett. 2021, 119, 053502. [Google Scholar] [CrossRef]
- Ke, F.; Wang, C.; Jia, C.; Wolf, N.R.; Yan, J.; Niu, S.; Devereaux, T.P.; Karunadasa, H.I.; Mao, W.L.; Lin, Y. Preserving a robust CsPbI3 perovskite phase via pressure-directed octahedral tilt. Nat. Commun. 2021, 12, 461. [Google Scholar] [CrossRef]








| Material Type | Thermal Cond [W/m·k] | Heat Capacity [J/kg·K] | Phase (Form)/Prefer Laser | LH-DAC Refs. | Property Refs. |
|---|---|---|---|---|---|
| Ne | 1.667 | 1029.9 | Gas | [104,119,120,158] | [269] |
| Ar | 1.677 | 520.3 | Gas/CO2 | [75,104,120,126,127,132,138,158,168,180,183,184,193,195,270] | [269] |
| NaCl | 5.4–6.49 | 854 | Powder/YAG | [32,66,120,127,128,171,174,175,185,189,270,271] | [272] |
| Al2O3 | 26–34.3 | 753–878.6 | Powder or single crystals/YAG | [119,120,121,144,164,193,198] | [272,273] |
| SiO2 | 1.6–4.18 | 703–1000 | Powder or glass pellets/YAG | [121,171,274] | [272,273] |
| N2 | 1.4 | 1039 | Gas | [37,112,116] | [269] |
| He | 1.667 | 3115.6 | Gas | [130] | [269] |
| MgO | 18–36 | 877–1046 | Powder/YAG | [120,131,144,198] | [272,273] |
| CsCl | 1 | 317.9 | Powder/YAG | [144,167,171] | [275] |
| KCl | 6.3 | 690 | Powder/YAG | [104,120,152,167,171,175,208] | [272] |
| KBr | 4.8 | 435 | Powder/YAG | [29,167] | [272] |
| MgSiO3 | 5.3 | 811.1 | dry gel | [179] | [272,276] |
| LiF | 150, 11.3 | 1562 | Powder/YAG | [198] | [272] |
| Au | 317 | 129 | foil | [211] | [272,273] |
| Material Type | Typical Gasket Thickness [μm] | Thermal Cond [W/m·k] | Heat Capacity [J/kg·K] | LH-DAC Refs. | Property Refs. |
|---|---|---|---|---|---|
| Rhenium (Re) | 25–50 | 47.9 | 137 | [103,105,120,127,136,139,160,164,174,183,184,191,193,195,208,221,229,270,274,278] | [272] |
| Stainless Steel | 30–250 | 12.67 | 483 | [29,75,138,147,148,150,170,181,185,229,270,279] | [272] |
| Tungsten (W) | 30–60 | 155 | 138 | [140,167,179,191,229] | [92,272] |
| Beryllium (Be) | 30 | 200 | 1825 | [40,152] | [272] |
| Diamond | 40,100 | 3150 | 520 | [203,280] | [92] |
| Nichrome alloy (NiCr) | 125 | 10.4–14 | 418–460 | [42,281] | [272,273,282] |
| Boron-kapton composite | 50 | Data N/A | Data N/A | [162] | |
| Nickel (Ni) | 200 | 70 | 450 | [50] | [92] |
| Modified Materials | Original Form or Phase | Press [GPa] | Laser Type [nm or μm] | Laser Power/ Energy [W/J], Spots [μm], Ind. Temps [K] | Refs. |
|---|---|---|---|---|---|
| c-BC3 | BC3 | 39 | Nd:YAG, 1.064 μm | 200 W, 2200 K | [279] |
| BN-NTs | c-BN | 5.4, 8.4 | CO2, 10.6 μm | ≤240 W (60 s), 80 μm, >5000 K | [37] |
| c-Diamond | a: Graphite, b: Graphite w/o a catalyst | a: 20 b: 30, 45 | a: Ruby, 694 nm b: CO2, 10.6 μm | a: ≤7 J/pulse (1 ms), 20–50 μm b: 2–80 W, 50 μm | a: [29,30] b: [29] |
| DLC, c-Diamond | Pyrolytic Graphite | 16.5 | CO2, 10.6 μm | 120 W, ≈30 μm | [151] |
| a-Diamond | glassy carbon | 45–50 | Nd:YLF, 1064 nm | 90–150 μm, 1800 K | [278] |
| c-Si3N4 | β-Si3N4 | a: 15 b: 30 | a: Nd:YLF, 1053 nm b: CO2, 10.6 μm | a: 35W, 2200 K b: 120 W, (1–10 min), >2800 K | [112] |
| ϑ-TaN | ε-TaN | 23.5 | Yb-Fibre | 100 W, 30 μm, 3500 K | [140] |
| η-Ta2N3 | ε-TaN +N | 9.9 | Yb-Fibre | 100 W (1-sided), 30 μm, ≈2000 K | [140] |
| csp-Ge3N4 | Gecrystal +N | 14 | Nd:YLF, 1053 nm | cw, 55 W, >2000 K | [3] |
| rs-GaN | wz-GaN | 47 | Nd:YAG, 1.06 μm | 300 K | [175] |
| Modified Materials | Original Form or Phase | Press [GPa] | Laser Type [nm or μm] | Laser Power/Energy [W/J], Spots [μm], Ind. Temps [K] | Refs. |
|---|---|---|---|---|---|
| tP-H2O | ice-VII | 30.9 | CO2, 10.6 μm | ≈30 μm | [31] |
| CaCl2-type-SiO2 | a: SiO2, b: TiO2-type-SiO2 | a: 80–85 b: 23 | a: Nd:YAG, 1.06 μm b: Nd:YAG, 1.06 μm | a: ≈36–48 min, 60 μm, 2000 K b: cw, 40 W (2X) | a: [68] b: [127] |
| pv-MgSiO3 | MgSiO3 | ≤86 | CO2, 10.6 μm | 120 W, 2700 K | [137] |
| opv-MgSiO3 | (Ca0.95 Fe0.06Mg0.92 Na0.02 Al0.05)Si2O6 | ≈24 | Nd:YAG, 1.06 μm | 27 W, (5–10 min) | [281] |
| β-Mg2Si04 | Mg2SiO4 | 13 | Ruby, 694 nm | ≤7 J/pulse (1 ms), 20–50 μm | [30] |
| il-MgSiO3 + cpv-CaSiO3 | (Ca0.95 Fe0.06Mg0.92 Na0.02 Al0.05)Si2O6 | ≈23 | Nd:YAG 1.06 μm | 27 W, (5–10 min) | [281] |
| sp-Mg2SiO4 +st-SiO2 + cpv-CaSiO3 | (Ca0.95 Mg1.01) Si2O6 | ≈17 | Nd:YAG, 1.06 μm | (5–10 min) | [281] |
| ϵ-MgAl2O4 | sp-MgAl2O4 | 28 | Nd:YAG, 1.06 μm | cw, 1273.15 K | [32] |
| (CaFe2O4)-type-NaAlSiO4 | NaAlSiO4 | 28 | Nd:YAG, 1.06 μm | ≈1273.15 K | [43] |
| ≈(dhcp)-Fe | ϵ (hcp)-Fe | ≈35–40 | Nd:YAG, 1.06 μm | cw, ≤18 W, 1200–1500 K | [5] |
| opv-(Mg,Fe)SiO3 | (Mg,Fe)SiO3 | 38 | CO2, 10.6 μm | ≤120 W, 50–75 μm 1850 K | [128] |
| pv-(Mg0.88Fe0.12) SiO3 | (Mg0.88Fe0.12) SiO3 | 127 | Nd:YAG, 1.06 μm | cw, 2000 K | [113] |
| a: γ-Fe2SiO4b: 2FeO + SiO2 | Fe2SiO4 | a: 4 b: 26 | Ruby, 694 nm | Pulsed, ≤7 J | [30] |
| pv-Al-(Mg,Fe)-SiO3 + st-SiO2 + pv-CaSiO3+ F | MORB | 45, 80, 100 | Nd:YAG, 1.06 μm | 10–12 W, 0.9–1 mm | [147] |
| a: Brr- (Fe, Ni) ₂P b: Abg- (Fe, Ni) ₂P | (Fe, Ni) ₂P | a: 2–4 b: 28.4–39 | Yb:SiO2 1.06 μm | a: 200 W, 5 min, ~15 μm, ~1625 K b: 200 W, 8 min, ~15 μm, ~1675 K | [287] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alabdulkarim, M.E.; Maxwell, W.D.; Thapliyal, V.; Maxwell, J.L. A Comprehensive Review of High-Pressure Laser-Induced Materials Processing, Part I: Laser-Heated Diamond Anvil Cells. J. Manuf. Mater. Process. 2022, 6, 111. https://doi.org/10.3390/jmmp6050111
Alabdulkarim ME, Maxwell WD, Thapliyal V, Maxwell JL. A Comprehensive Review of High-Pressure Laser-Induced Materials Processing, Part I: Laser-Heated Diamond Anvil Cells. Journal of Manufacturing and Materials Processing. 2022; 6(5):111. https://doi.org/10.3390/jmmp6050111
Chicago/Turabian StyleAlabdulkarim, Mohamad E., Wendy D. Maxwell, Vibhor Thapliyal, and James L. Maxwell. 2022. "A Comprehensive Review of High-Pressure Laser-Induced Materials Processing, Part I: Laser-Heated Diamond Anvil Cells" Journal of Manufacturing and Materials Processing 6, no. 5: 111. https://doi.org/10.3390/jmmp6050111







