Hot Extrusion of ZnSb-Based Thermoelectric Materials; A Novel Approach for Scale-Up Production
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
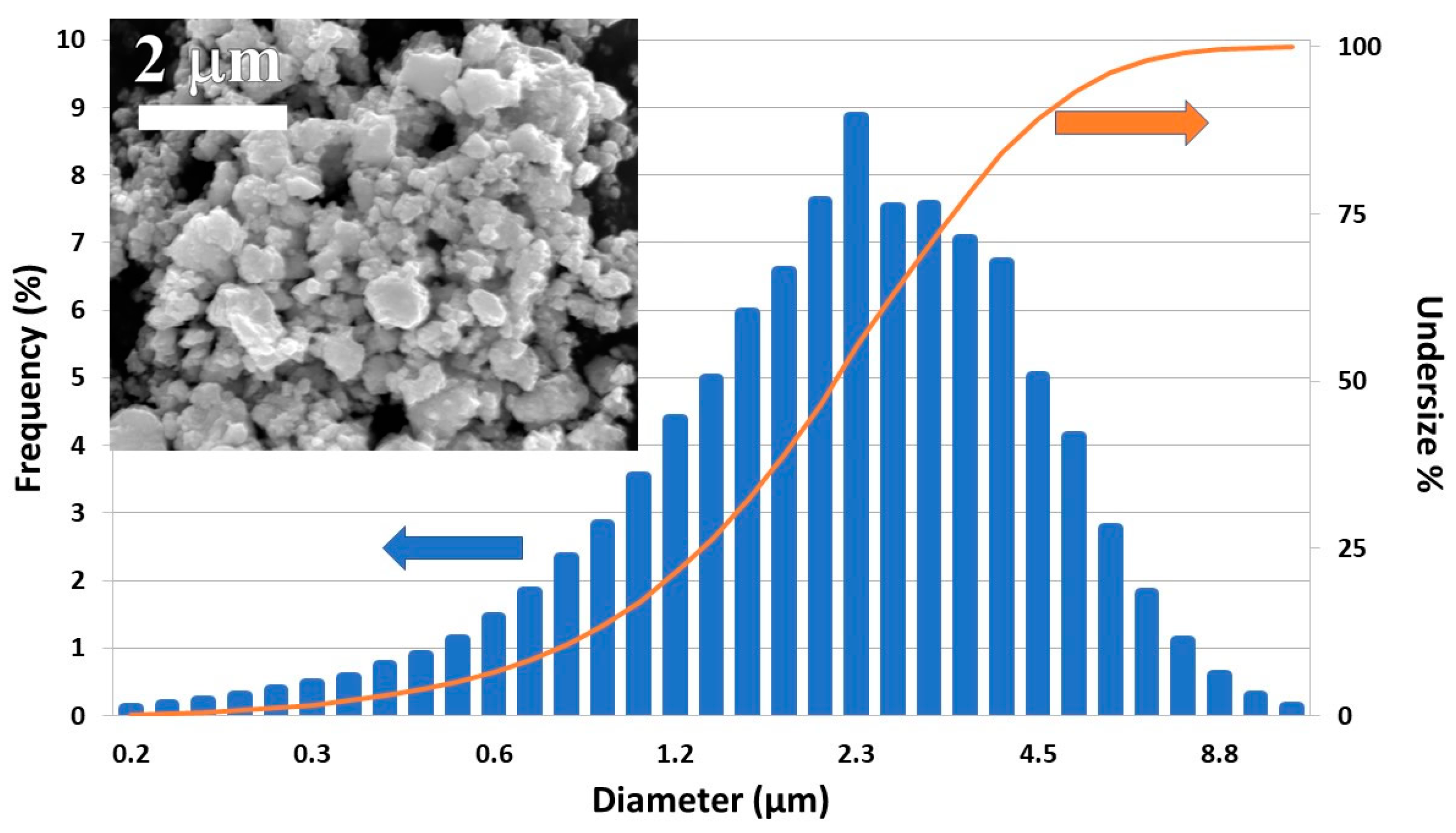
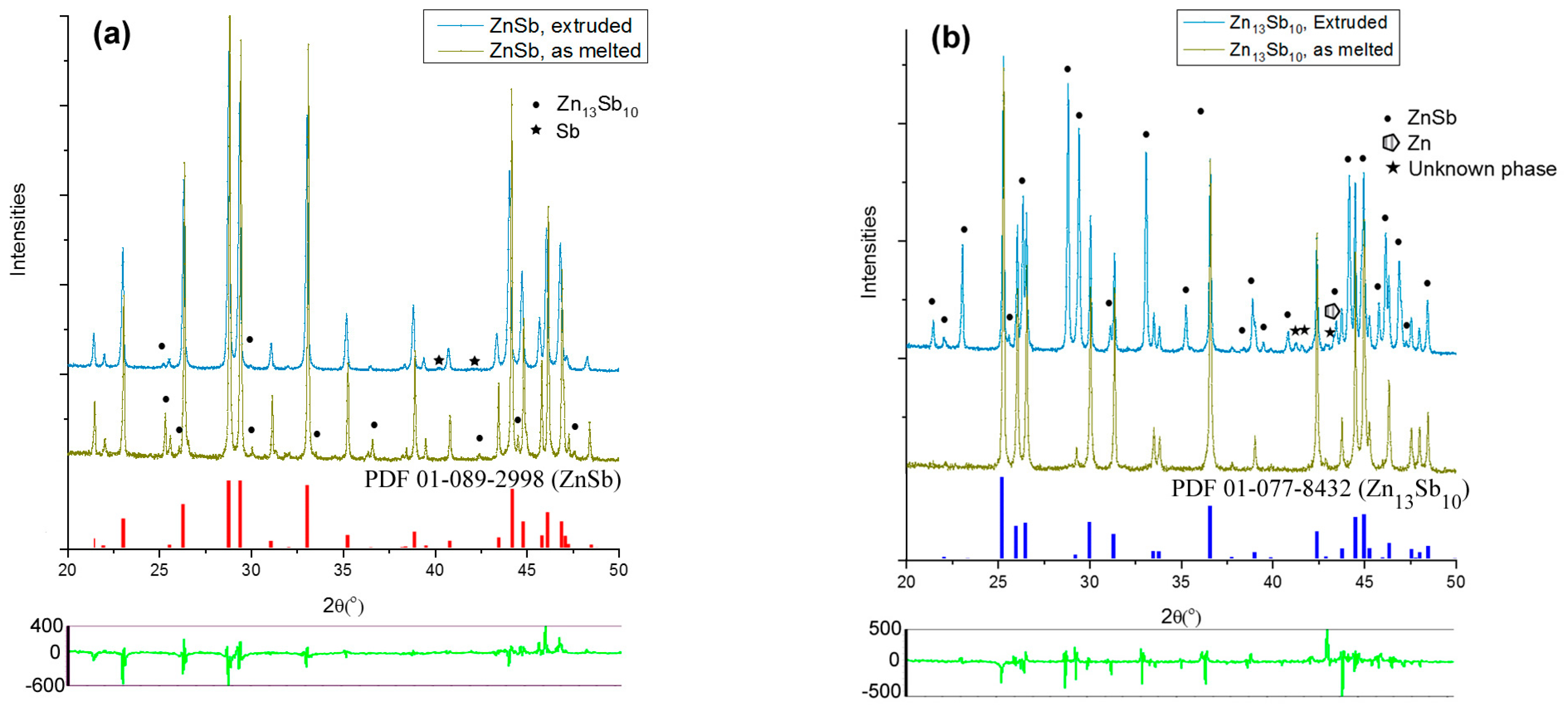
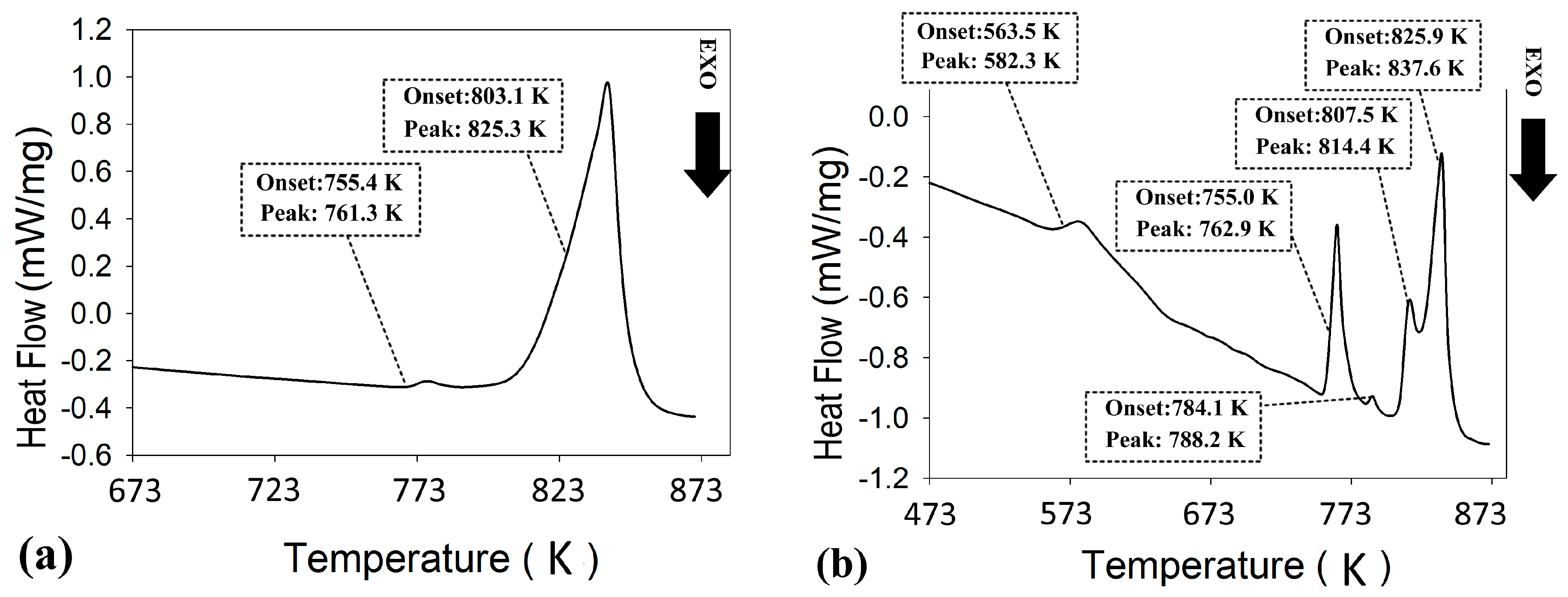
3.1. Pre-Extrusion Analyses of Materials
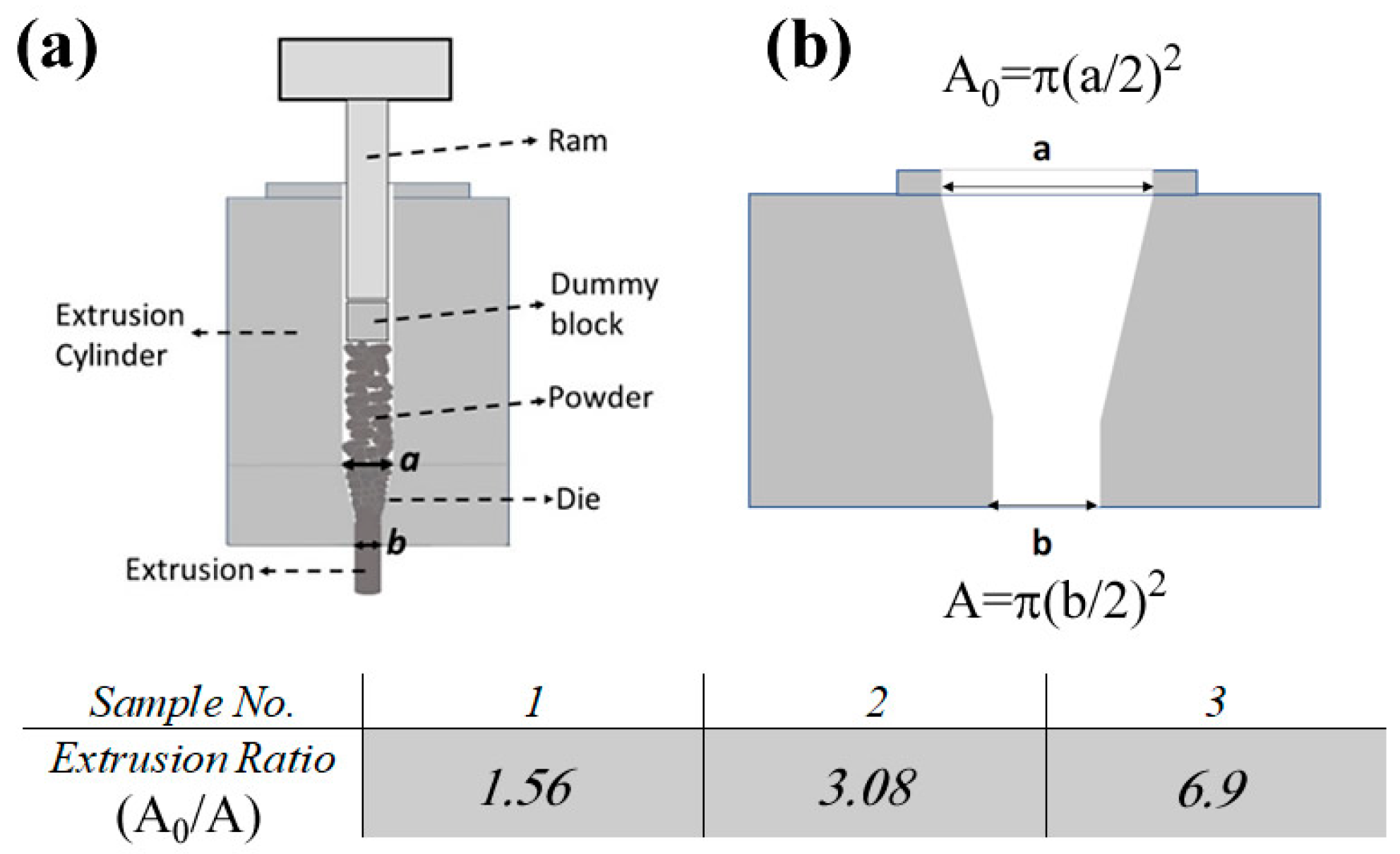
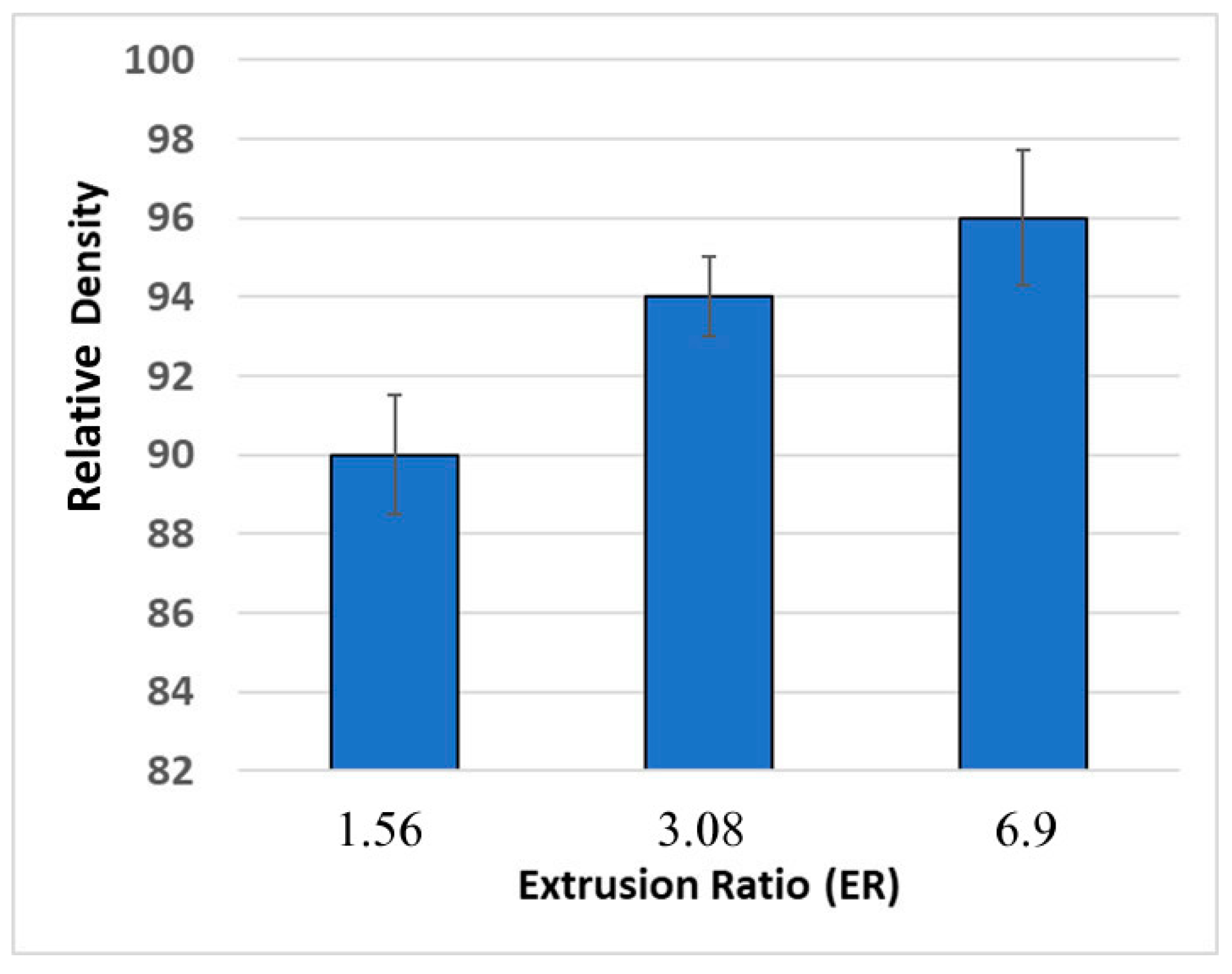
3.2. Optimization of the Hot Extrusion Process
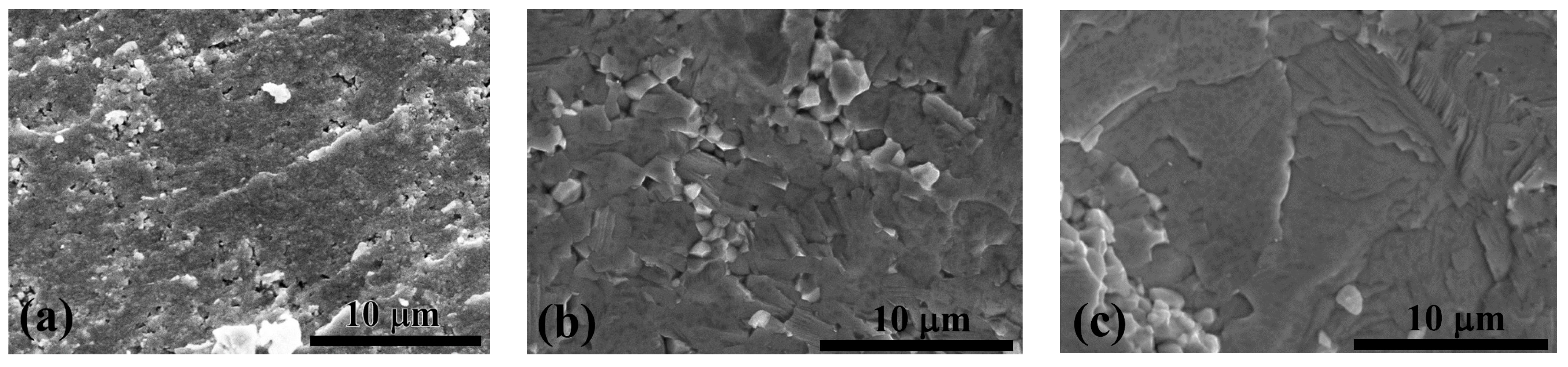
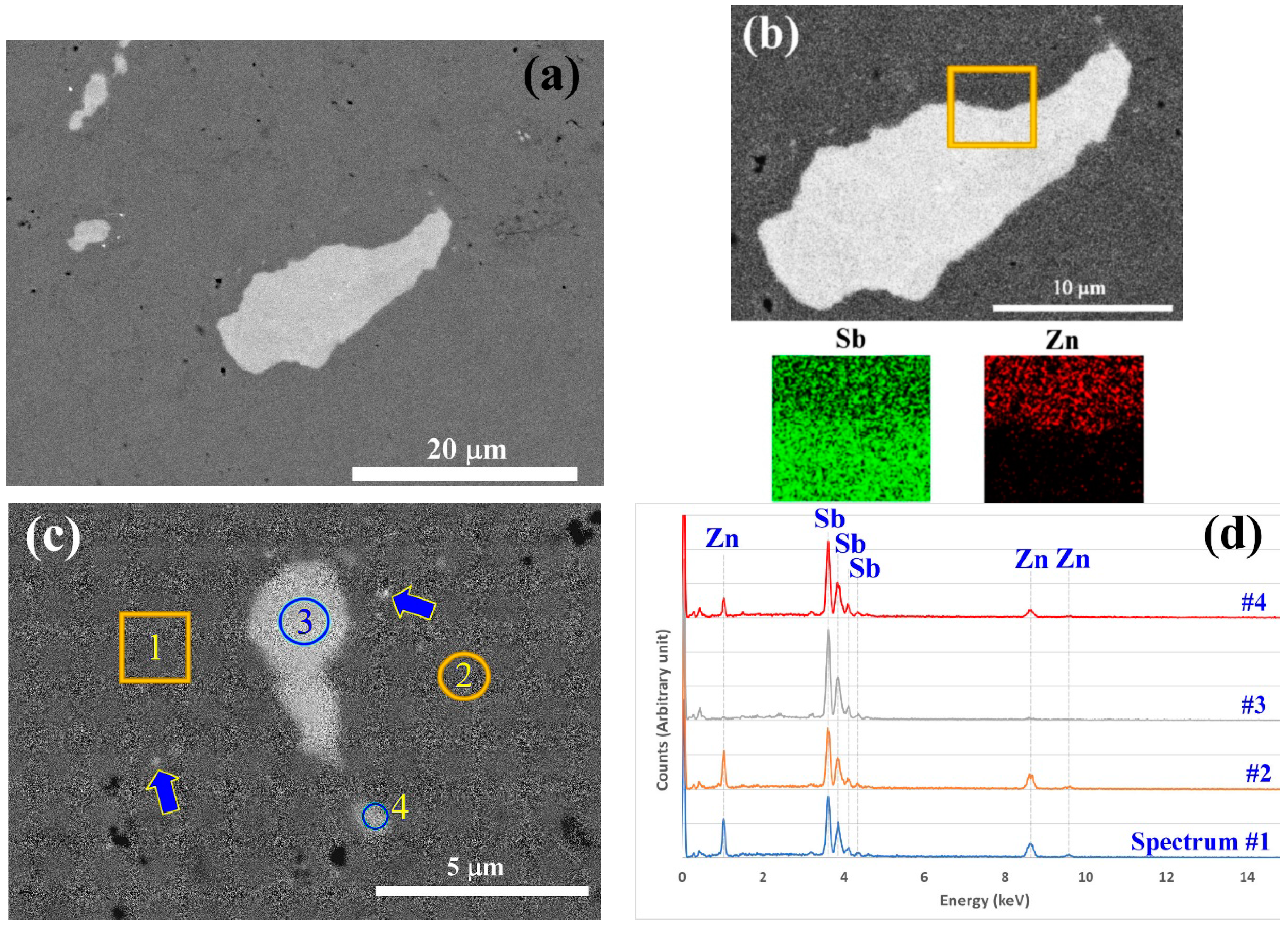
3.3. Microstructural Analysis
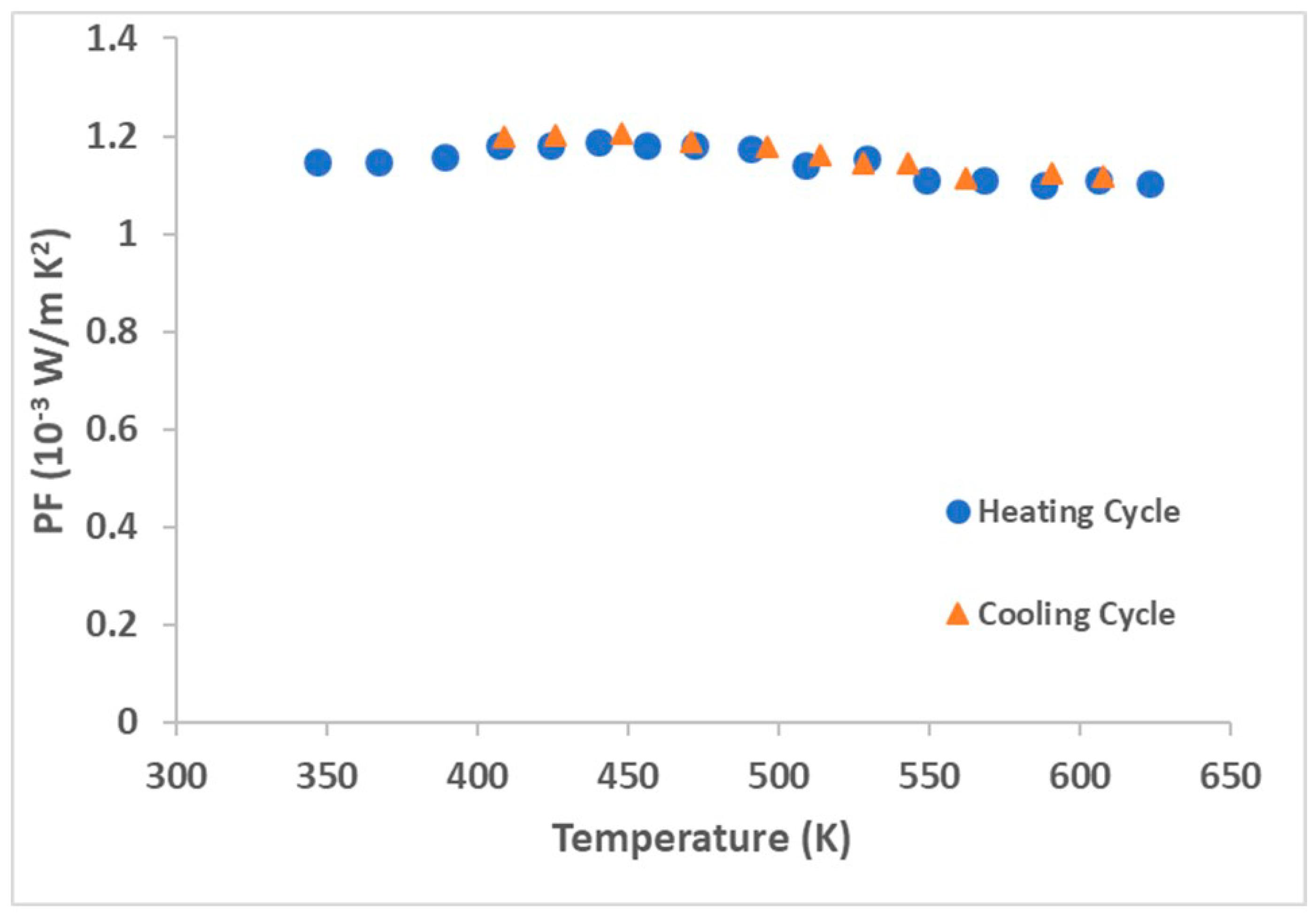
3.4. Thermoelectric Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Goldsmid, H.J. Introduction to Thermoelectricity; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2010; p. XVI, 242. [Google Scholar]
- Mehdizadeh Dehkordi, A.; Zebarjadi, M.; He, J.; Tritt, T.M. Thermoelectric power factor: Enhancement mechanisms and strategies for higher performance thermoelectric materials. Mater. Sci. Eng. R Rep. 2015, 97, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Iversen, B.B. Fulfilling thermoelectric promises: β-Zn4Sb3 from materials research to power generation. J. Mater. Chem. 2010, 20, 10778–10787. [Google Scholar] [CrossRef]
- Shaver, P.; Blair, J. Thermal and electronic transport properties of p-type ZnSb. Phys. Rev. 1966, 141, 649. [Google Scholar] [CrossRef]
- Caillat, T.; Fleurial, J.-P.; Borshchevsky, A. Preparation and thermoelectric properties of semiconducting Zn4Sb3. J. Phys. Chem. Solids 1997, 58, 1119–1125. [Google Scholar] [CrossRef]
- Song, X.; Finstad, T. Review of research on the thermoelectric material ZnSb. In Thermoelectrics for Power Generation: A Look at Trends in the Technology; IntechOpen: London, UK, 2016. [Google Scholar]
- Ur, S.-C.; Nash, P.; Kim, I.-H. Solid-state syntheses and properties of Zn4Sb3 thermoelectric materials. J. Alloy. Compd. 2003, 361, 84–91. [Google Scholar] [CrossRef]
- Saadat, S.; Tay, Y.Y.; Zhu, J.; Teh, P.F.; Maleksaeedi, S.; Shahjamali, M.M.; Shakerzadeh, M.; Srinivasan, M.; Tay, B.Y.; Hng, H.H. Template-free electrochemical deposition of interconnected ZnSb nanoflakes for li-ion battery anodes. Chem. Mater. 2011, 23, 1032–1038. [Google Scholar] [CrossRef]
- Wang, G.; Shen, X.; Lu, Y.; Dai, S.; Nie, Q.; Xu, T. Investigation on pseudo-binary ZnSb–Sb2Te3 material for phase change memory application. J. Alloys Compd. 2015, 622, 341–346. [Google Scholar] [CrossRef]
- Song, X.; Valset, K.; Graff, J.; Thøgersen, A.; Gunnæs, A.; Luxsacumar, S.; Løvvik, O.; Snyder, G.; Finstad, T. Nanostructuring of undoped ZnSb by cryo-milling. J. Electron. Mater. 2015, 44, 2578–2584. [Google Scholar] [CrossRef]
- Okamura, C.; Ueda, T.; Hasezaki, K. Preparation of Single-Phase ZnSb Thermoelectric Materials Using a Mechanical Grinding Process. Mater. Trans. 2010, 51, 860–862. [Google Scholar] [CrossRef] [Green Version]
- Eklöf, D.; Fischer, A.; Wu, Y.; Scheidt, E.W.; Scherer, W.; Häussermann, U. Transport properties of the II–V semiconductor ZnSb. J. Mater. Chem. A 2013, 1, 1407–1414. [Google Scholar] [CrossRef]
- Kostur, N.; Psarev, V. Electrical properties of alloyed ZNSB single crystals. Izvestiya Vysshikh Uchebnykh Zavedenii Fizika 1967, 2, 39. [Google Scholar]
- Komiya, H.; Masumoto, K.; Fan, H. Optical and electrical properties and energy band structure of ZnSb. Phys. Rev. 1964, 133, A1679. [Google Scholar] [CrossRef]
- Keshavarz, M.K.; Vasilevskiy, D.; Masut, R.A.; Turenne, S. Mechanical properties of bismuth telluride based alloys with embedded MoS2 nano-particles. Mater. Des. 2016, 103, 114–121. [Google Scholar] [CrossRef]
- Vasilevskiy, D.; Masut, R.; Turenne, S. Thermoelectric and mechanical properties of novel hot-extruded PbTe n-type material. J. Electron. Mater. 2012, 41, 1057–1061. [Google Scholar] [CrossRef]
- Vasilevskiy, D.; Keshavarz, M.K.; Dufourcq, J.; Ihou-Mouko, H.; Navonne, C.; Masut, R.A.; Turenne, S. Bulk Mg2Si based n-type thermoelectric material produced by gas atomization and hot extrusion. Mater. Today Proc. 2015, 2, 523–531. [Google Scholar] [CrossRef]
- Fedorov, M.I.; Prokof’eva, L.V.; Pshenay-Severin, D.A.; Shabaldin, A.A.; Konstantinov, P.P. New Interest in Intermetallic Compound ZnSb. J. Electron. Mater. 2014, 43, 2314–2319. [Google Scholar] [CrossRef]
- Shai, X.; Deng, S.; Meng, D.; Shen, L.; Li, D. Thermal stability and electrical transport properties of β-Zn4Sb3 single crystal prepared by Sn-flux method. Phys. B Condens. Matter 2014, 452, 148–151. [Google Scholar] [CrossRef]
- Snyder, G.J.; Christensen, M.; Nishibori, E.; Caillat, T.; Iversen, B.B. Disordered zinc in Zn4Sb3 with phonon-glass and electron-crystal thermoelectric properties. Nat. Mater. 2004, 3, 458–463. [Google Scholar] [CrossRef] [PubMed]


| Sample | Zn13Sb10 (wt.%) | ZnSb (wt.%) | Zn (wt.%) | Sb (wt.%) | Unit Cell Volume (Å3) |
|---|---|---|---|---|---|
| Zn13Sb10 (SM) | 100% | --- | --- | --- | 1608.07 (7) |
| Zn13Sb10 (Extruded) | 58.53 (3) | 40.33 (3) | 1.21 (3) | --- | 1609.92 (6) |
| ZnSb (SM) | 4.65 (1) | 95.25 (5) | --- | 0.14 (3) | 388.31 (1) |
| ZnSb (Extruded) | 0.83 (1) | 98.24 (4) | --- | 0.26 (5) | 388.10 (2) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keshavarz, M.K.; Lo, C.-W.T.; Turenne, S.; Mozharivskyj, Y.; Quitoriano, N.J. Hot Extrusion of ZnSb-Based Thermoelectric Materials; A Novel Approach for Scale-Up Production. J. Manuf. Mater. Process. 2019, 3, 58. https://doi.org/10.3390/jmmp3030058
Keshavarz MK, Lo C-WT, Turenne S, Mozharivskyj Y, Quitoriano NJ. Hot Extrusion of ZnSb-Based Thermoelectric Materials; A Novel Approach for Scale-Up Production. Journal of Manufacturing and Materials Processing. 2019; 3(3):58. https://doi.org/10.3390/jmmp3030058
Chicago/Turabian StyleKeshavarz, Mohsen K., Chun-Wan Timothy Lo, Sylvain Turenne, Yurij Mozharivskyj, and Nathaniel J. Quitoriano. 2019. "Hot Extrusion of ZnSb-Based Thermoelectric Materials; A Novel Approach for Scale-Up Production" Journal of Manufacturing and Materials Processing 3, no. 3: 58. https://doi.org/10.3390/jmmp3030058





