Microcentrifuge Tubes as Disposable Immunoelectrochemical Cells for the On-Site Determination of GFAP, Biomarker of Hemorrhagic Stroke †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Materials and Apparatus
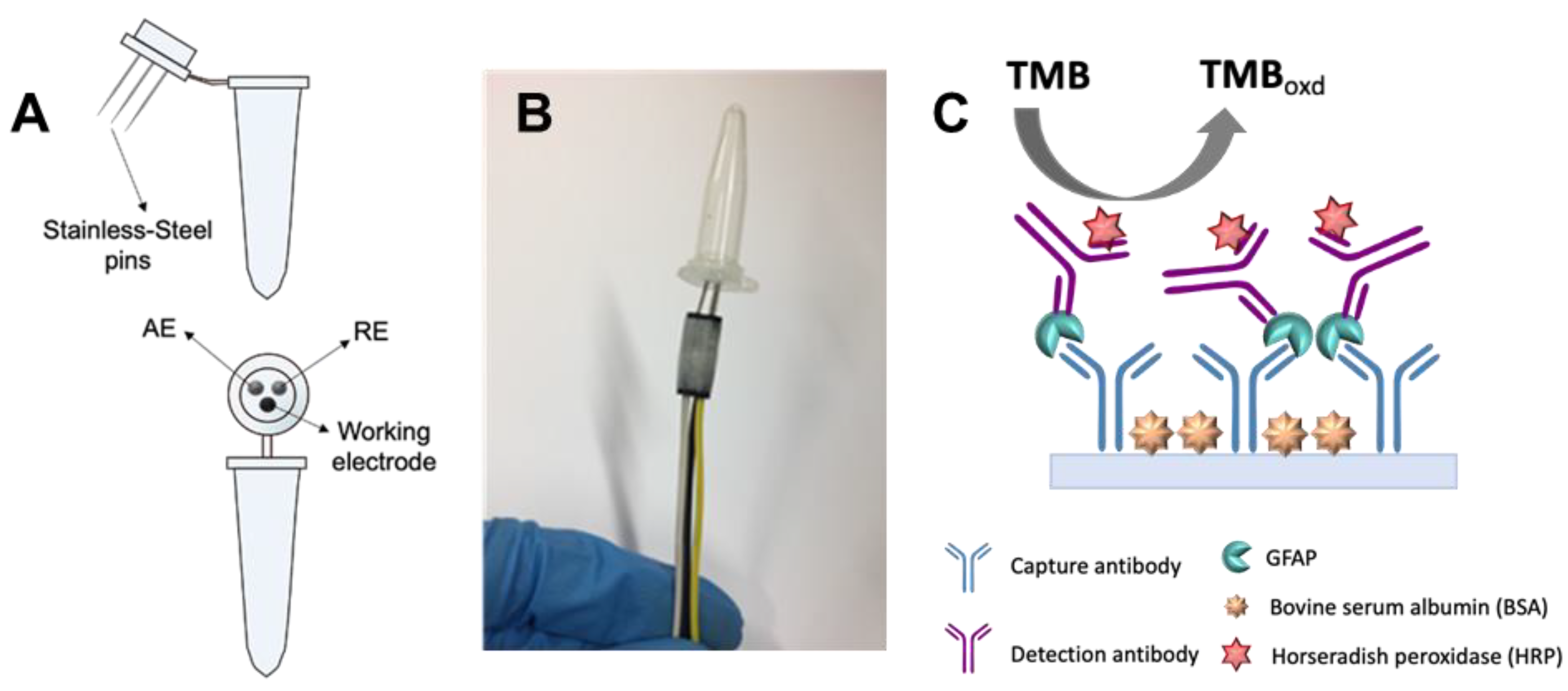
2.2. Electrochemical Cell Design
2.3. Immunoassay Procedure
2.4. Electrochemical Measurements
3. Results and Discussion
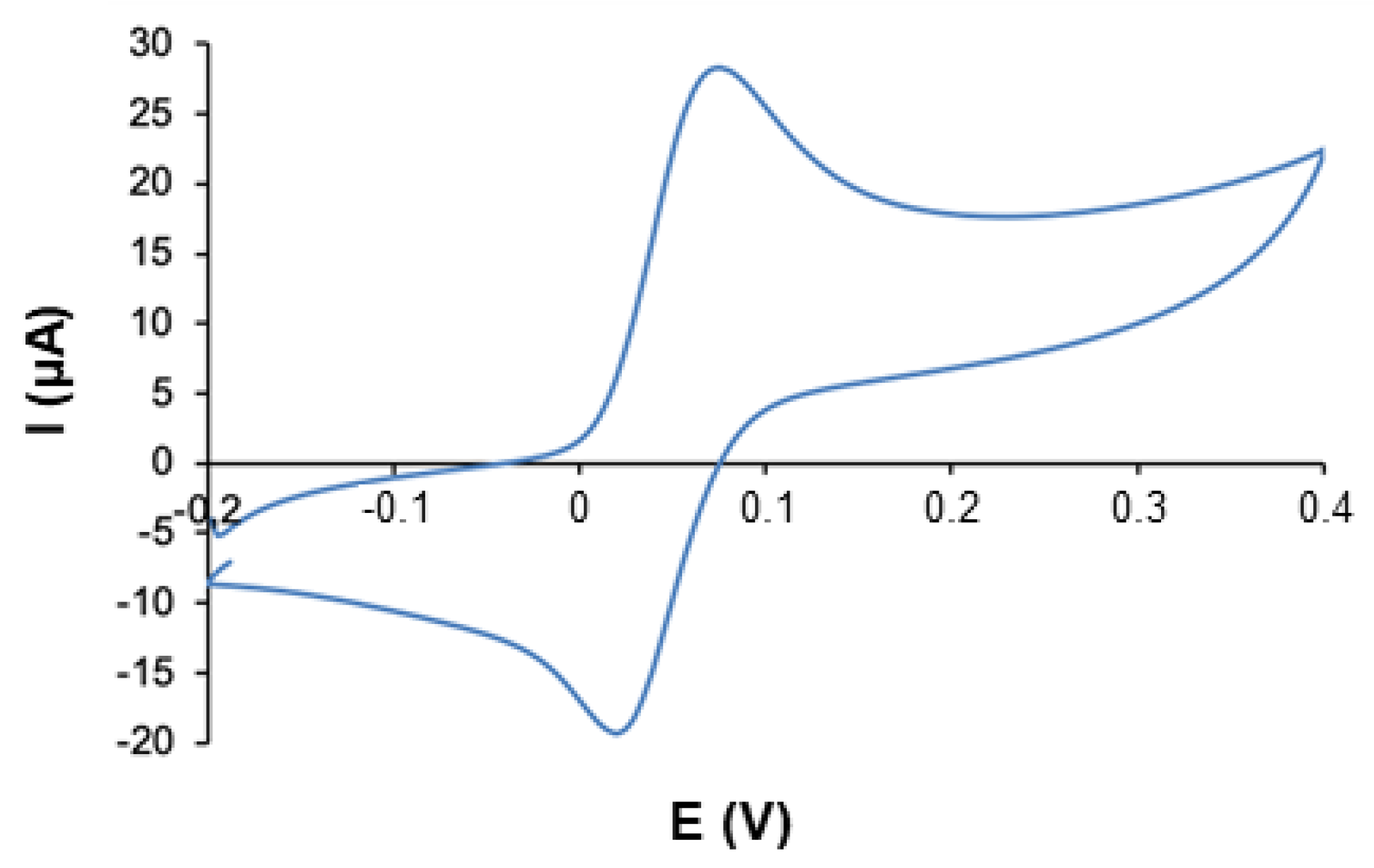
3.1. Evaluation of the Tube-Based Electrochemical Cell
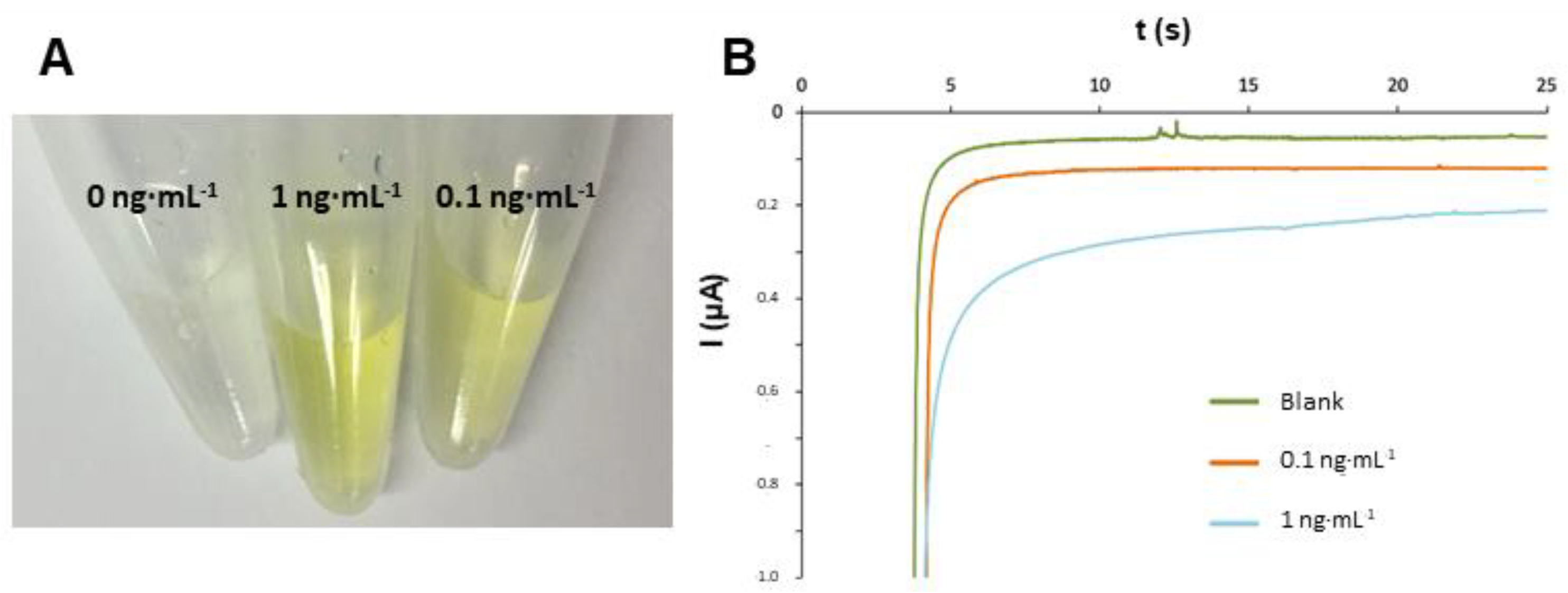
3.2. Analytical Signals of the Immunoassay
4. Conclusions
Acknowledgments
References
- García-Cabo, C.; Llano-Suárez, P.; Benavente-Fernández, L.; Calleja-Puerta, S.; Costa-Fernández, J.M.; Fernández-Abedul, M.T. Obtaining information from the brain in a non-invasive way: Determination of iron in nasal exudate to differentiate hemorrhagic and ischemic strokes. Clin. Chem. Lab. Med. 2020, 58, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Foerch, C.; Niessner, M.; Back, T.; Bauerle, M.; De Marchis, G.M.; Ferbert, A.; Grehl, H.; Hamann, G.F.; Jacobs, A.; Kastrup, A.; et al. Diagnostic accuracy of plasma glial fibrillary acidic protein for differentiating intracerebral hemorrhage and cerebral ischemia in patients with symptoms of acute stroke. Clin. Chem. 2012, 58, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Luppa, P.B.; Müller, C.; Schlichtiger, A.; Schlebusch, H. Point-of-care testing (POCT): Current techniques and future perspectives. TrAC - Trends Anal. Chem. 2011, 30, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Rama, E.C.; Costa-García, A.; Fernández-Abedul, M.T. Pin-based electrochemical glucose sensor with multiplexing possibilities. Biosens. Bioelectron. 2017, 88, 34–40. [Google Scholar] [CrossRef] [PubMed]
- García-Miranda Ferrari, A.; Amor-Gutiérrez, O.; Costa-Rama, E.; Fernández-Abedul, M.T. Batch injection electroanalysis with stainless-steel pins as electrodes in single and multiplexed configurations. Sens. Actuators B Chem. 2017, 253, 1207–1213. [Google Scholar] [CrossRef]
- Berg, K.E.; Adkins, J.A.; Boyle, S.E.; Henry, C.S. Manganese Detection Using Stencil-printed Carbon Ink Electrodes on Transparency Film. Electroanalysis 2016, 28, 679–684. [Google Scholar] [CrossRef]
- Glavan, A.C.; Ainla, A.; Hamedi, M.M.; Fernández-Abedul, M.T.; Whitesides, G.M. Electroanalytical devices with pins and thread. Lab. Chip. 2016, 16, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Nanni, P.I.; González-López, A.; Nunez-Bajo, E.; Madrid, R.E.; Fernández-Abedul, M.T. Staple-Based Paper Electrochemical Platform for Celiac Disease Diagnosis. ChemElectroChem 2018, 5, 4036–4045. [Google Scholar] [CrossRef]
- González-López, A.; Blanco-López, M.C.; Fernández-Abedul, M.T. Micropipette Tip-Based Immunoassay with Electrochemical Detection of Antitissue Transglutaminase to Diagnose Celiac Disease Using Staples and a Paper-Based Platform. ACS Sens. 2019, 4, 2679–2687. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-López, A.; Costa-Rama, E.; Fernández, C.G.-C.; Benavente-Fernández, L.; Calleja-Puerta, S.; Fernández-García, B.; Pereiro, R.; Fernández-Abedul, M.T. Microcentrifuge Tubes as Disposable Immunoelectrochemical Cells for the On-Site Determination of GFAP, Biomarker of Hemorrhagic Stroke. Proceedings 2020, 60, 10. https://doi.org/10.3390/IECB2020-07060
González-López A, Costa-Rama E, Fernández CG-C, Benavente-Fernández L, Calleja-Puerta S, Fernández-García B, Pereiro R, Fernández-Abedul MT. Microcentrifuge Tubes as Disposable Immunoelectrochemical Cells for the On-Site Determination of GFAP, Biomarker of Hemorrhagic Stroke. Proceedings. 2020; 60(1):10. https://doi.org/10.3390/IECB2020-07060
Chicago/Turabian StyleGonzález-López, Andrea, Estefanía Costa-Rama, Carmen García-Cabo Fernández, Lorena Benavente-Fernández, Sergio Calleja-Puerta, Beatriz Fernández-García, Rosario Pereiro, and M. Teresa Fernández-Abedul. 2020. "Microcentrifuge Tubes as Disposable Immunoelectrochemical Cells for the On-Site Determination of GFAP, Biomarker of Hemorrhagic Stroke" Proceedings 60, no. 1: 10. https://doi.org/10.3390/IECB2020-07060




