Study of NO2 Sensing Properties of UV-Activated Graft Comb Copolymer and ZnO Blends in ppm and Sub-ppm Range at Room Temperature †
Abstract
:1. Introduction
2. Materials and Methods
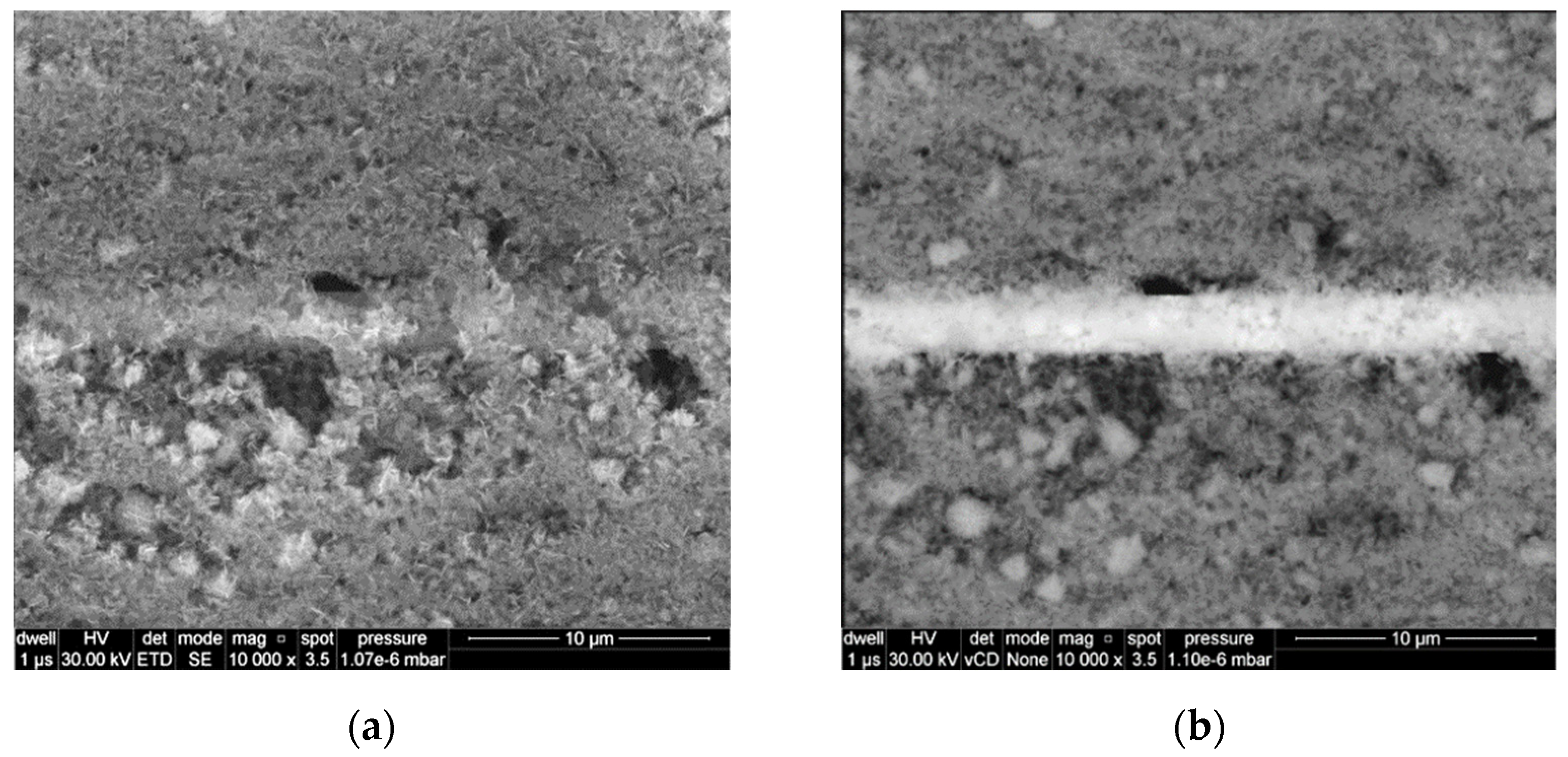
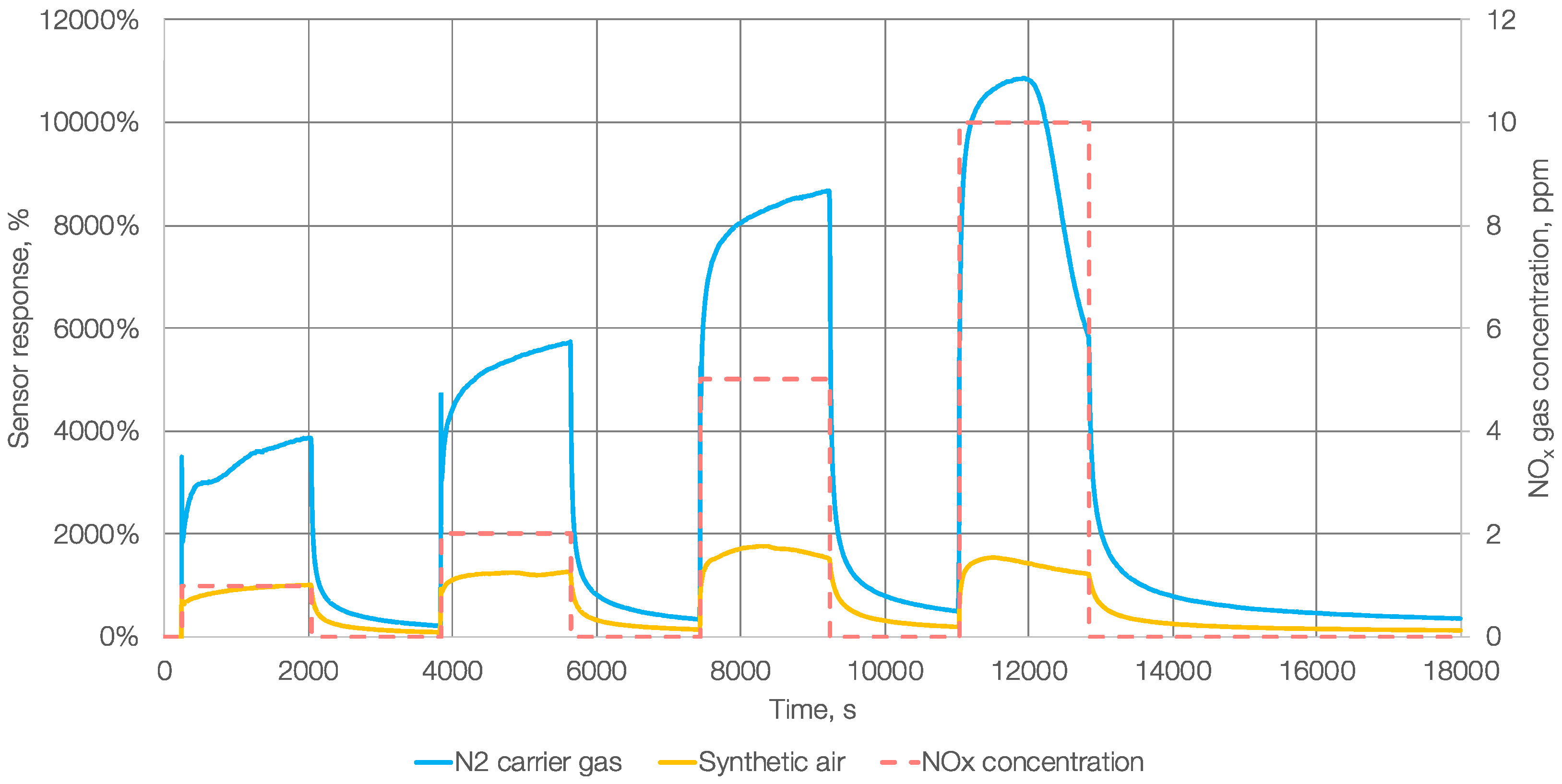
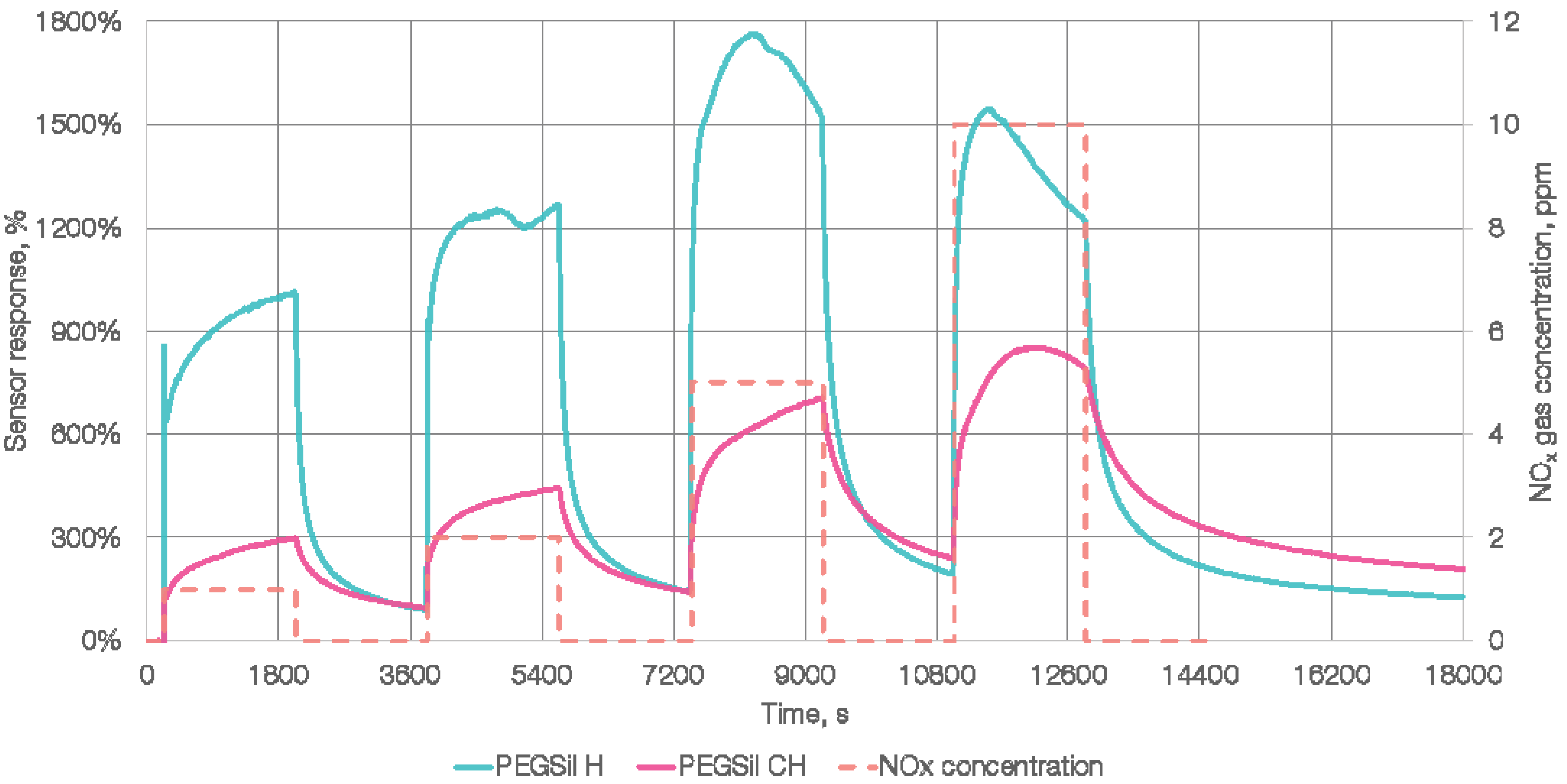
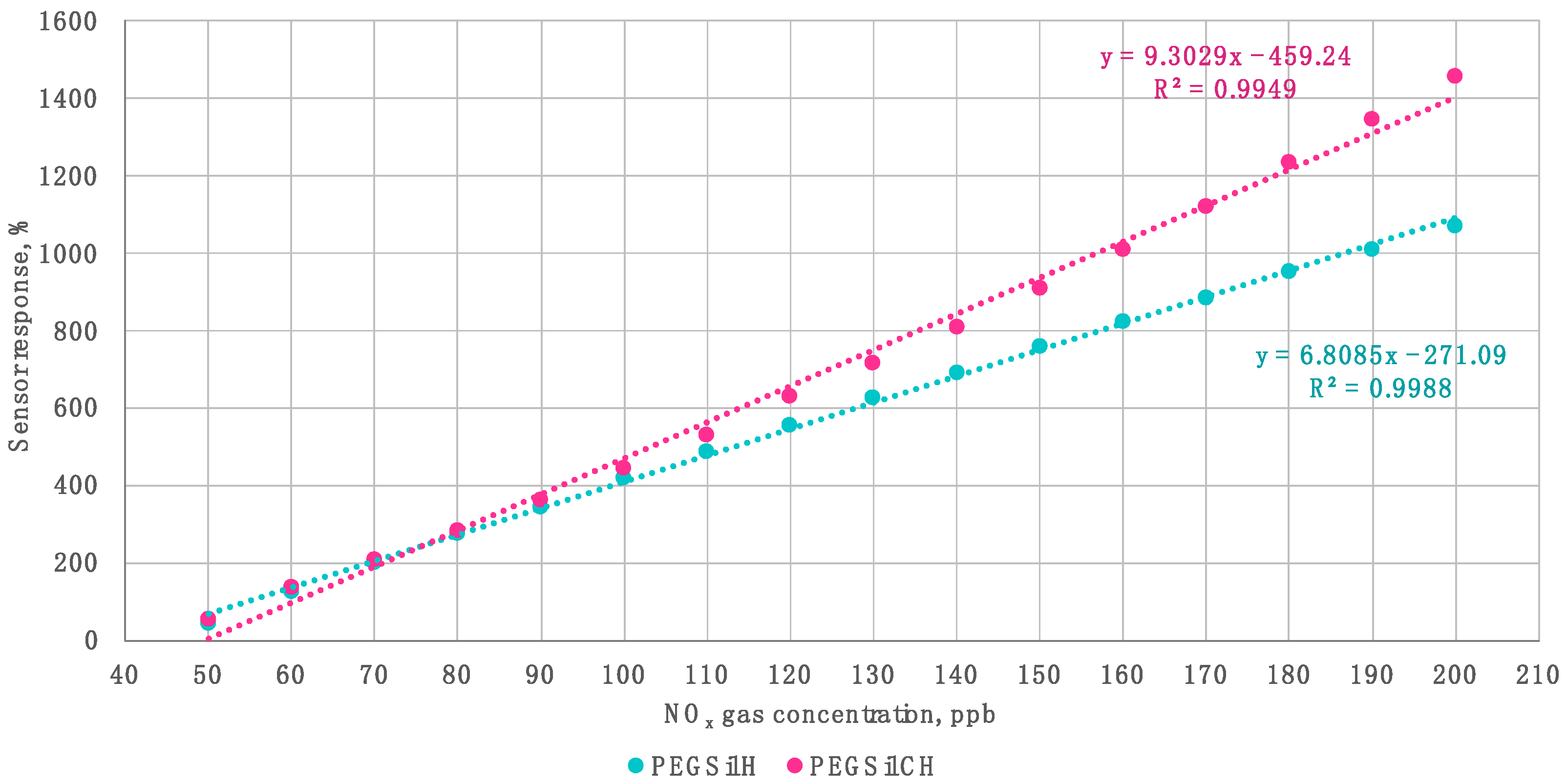
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xia, Y.; Wang, J.; Xu, J.L.; Li, X.; Xie, D.; Xiang, L.; Komarneni, S. Confined Formation of Ultrathin ZnO Nanorods/Reduced Graphene Oxide Mesoporous Nanocomposites for High-Performance Room-Temperature NO2 Sensors. ACS Appl. Mater. Interfaces 2016, 8, 35454–35463. [Google Scholar] [CrossRef] [PubMed]
- Yunusa, Z.; Hamidon, M.N.; Kaiser, A.; Awang, Z. Gas sensors: A review. Sens. Transducers 2014, 168, 61–75. [Google Scholar]
- Ponzoni, A.; Baratto, C.; Cattabiani, N.; Falasconi, M.; Galstyan, V.; Nunez-Carmona, E.; Rigoni, F.; Sberveglieri, V.; Zambotti, G.; Zappa, D. Metal Oxide Gas Sensors, a Survey of Selectivity Issues Addressed at the SENSOR Lab. Sensors 2017, 17, 714. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Shi, G. Gas Sensors Based on Conducting Polymers. Sensors 2007, 7, 267–307. [Google Scholar] [CrossRef]
- Jarosz, T.; Kępska, K.; Ledwon, P.; Procek, M.; Domagala, W.; Stolarczyk, A. Poly(3-hexylthiophene) Grafting and Molecular Dilution: Study of a Class of Conjugated Graft Copolymers. Polymers 2019, 11, 205. [Google Scholar] [CrossRef] [PubMed]
- Djurišić, A.B.; Ng, A.M.C.; Chen, X.Y. ZnO Nanostructures for Optoelectronics: Material Properties and Device Applications. Prog. Quantum Electron. 2010, 34, 191–259. [Google Scholar] [CrossRef]
- Xie, T.; Xie, G.Z.; Du, H.F.; Su, Y.J.; Ye, Z.B.; Chen, Y.Y.; Jiang, Y.D. Two novel methods for evaluating the performance of OTFT gas sensors. Sens. Actuators B 2016, 230, 176–183. [Google Scholar] [CrossRef]
- Stolarczyk, A.; Turczyn, R.; Januszkiewicz-Kaleniak, A.; Kepska, K. The macromolecule comb-graft polymer of polydimethylsiloxane graft-conjugated polyether and a method for its preparation. Pol. Pat. Appl. 2014, 407784, RR10. [Google Scholar]
- Procek, M.; Pustelny, T.; Stolarczyk, A.; Maciak, E. Studies of changes in electrical resistance of zinc oxide nanostructures under the influence of variable gaseous environments. Bull. Polish Acad. Sci. Tech. Sci. 2014, 62, 635–639. [Google Scholar] [CrossRef]
- Procek, M.; Pustelny, T.; Stolarczyk, A. Influence of External Gaseous Environments on the Electrical Properties of ZnO Nanostructures Obtained by a Hydrothermal Method. Nanomaterials 2016, 6, 227. [Google Scholar] [CrossRef]
- Procek, M.; Stolarczyk, A.; Pustelny, T. Impact of Temperature and UV Irradiation on Dynamics of NO2 Sensors Based on ZnO Nanostructures. Nanomaterials 2017, 7, 312. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kałużynski, P.; Procek, M.; Stolarczyk, A. Study of NO2 Sensing Properties of UV-Activated Graft Comb Copolymer and ZnO Blends in ppm and Sub-ppm Range at Room Temperature. Proceedings 2020, 42, 82. https://doi.org/10.3390/ecsa-6-06558
Kałużynski P, Procek M, Stolarczyk A. Study of NO2 Sensing Properties of UV-Activated Graft Comb Copolymer and ZnO Blends in ppm and Sub-ppm Range at Room Temperature. Proceedings. 2020; 42(1):82. https://doi.org/10.3390/ecsa-6-06558
Chicago/Turabian StyleKałużynski, Piotr, Marcin Procek, and Agnieszka Stolarczyk. 2020. "Study of NO2 Sensing Properties of UV-Activated Graft Comb Copolymer and ZnO Blends in ppm and Sub-ppm Range at Room Temperature" Proceedings 42, no. 1: 82. https://doi.org/10.3390/ecsa-6-06558




