Numerical Simulation of the Dynamics of Listeria Monocytogenes Biofilms †
Abstract
1. Introduction
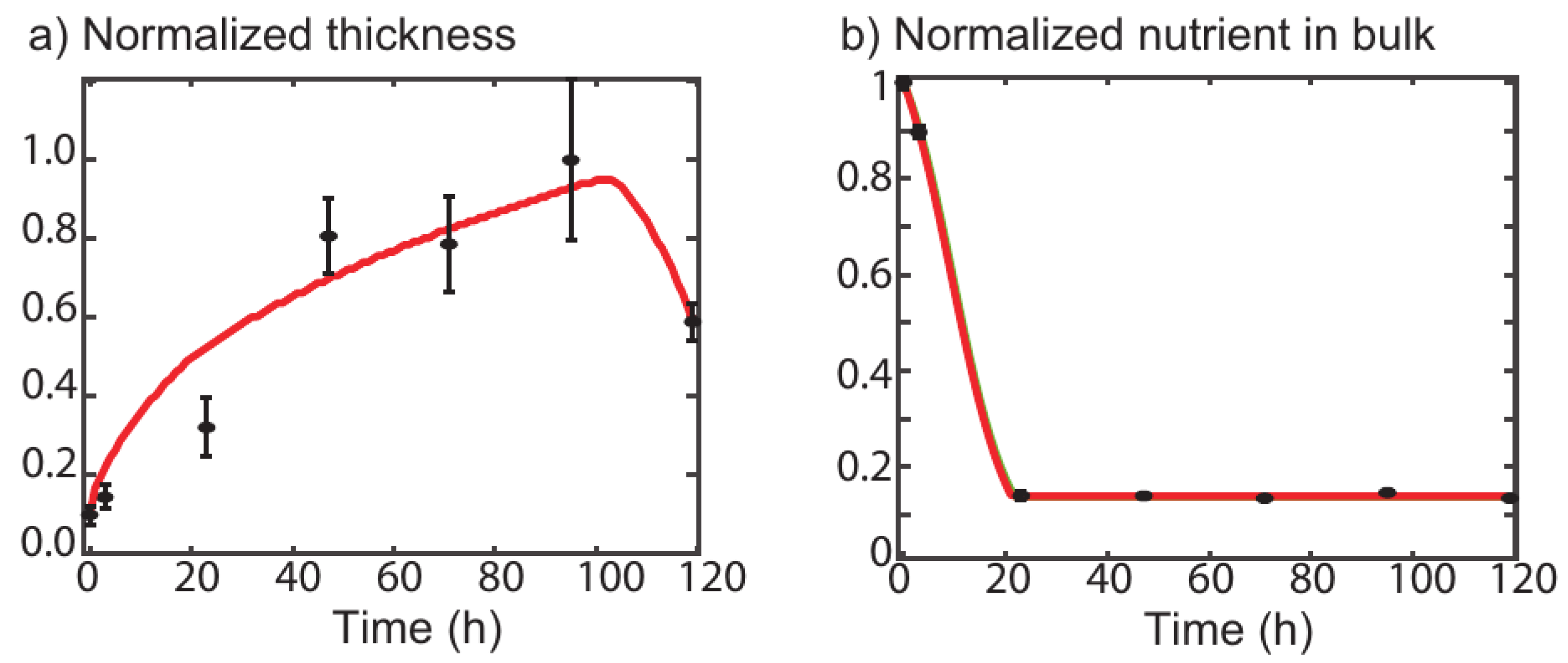
2. One-Dimensional Model
- There is a sharp front of biomass at the bulk/solid transition.
- Biomass density can not exceed a maximum bound which is a parameter of the model.
- Biomass production is due to nutrient consumption.
- Nutrients diffuse in the bulk and in the biofilm with different diffusion constants.
- The detachment is related to biofilm ageing.
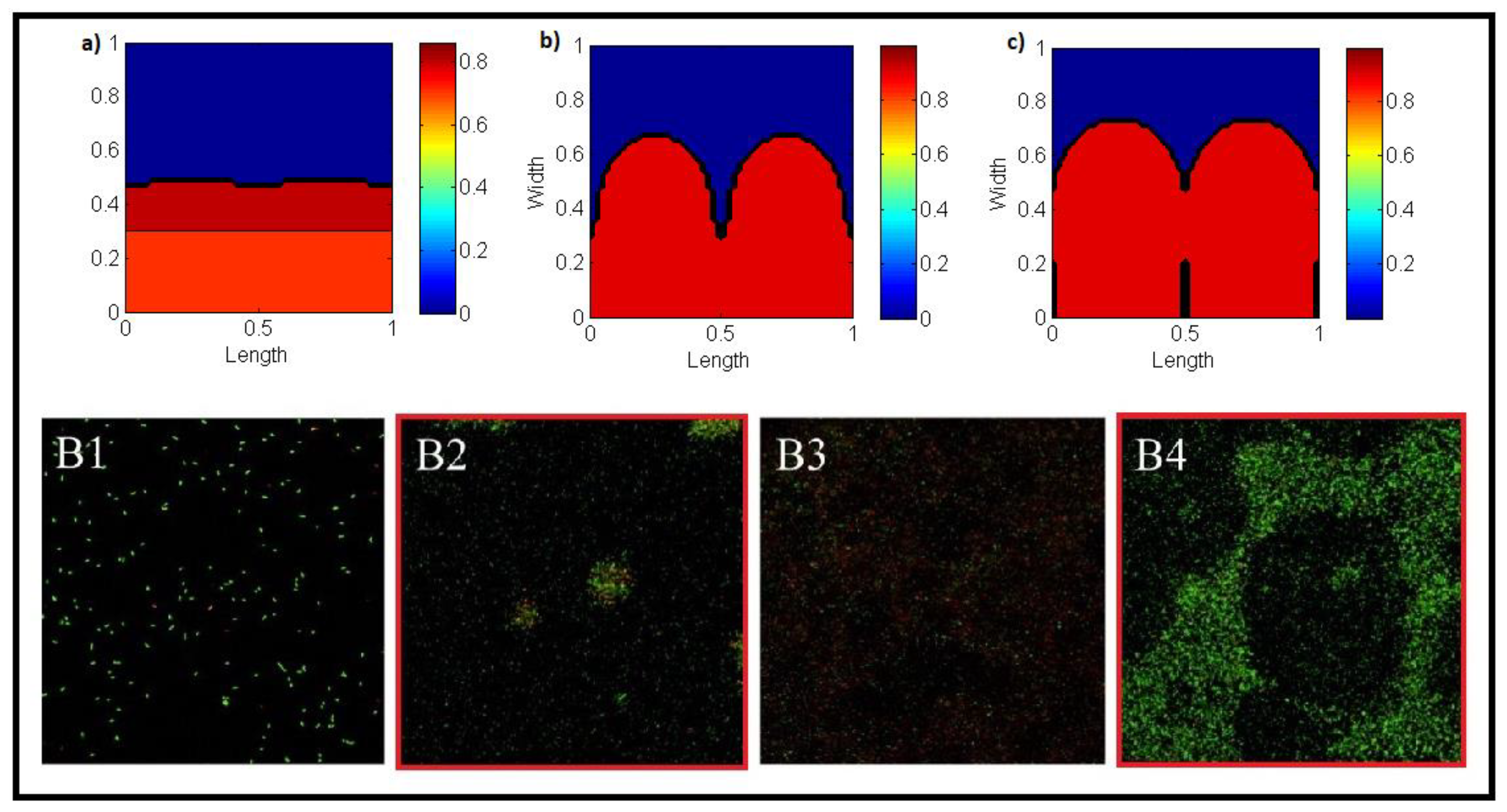
3. Two-Dimensional Model
- Biofilm described as a viscous fluid.
- Nutrients and biomasses concentrations governed by a mass conservation law.
- Active and inactive biomasses are of the same microbial species and incompressible.
- The time scale for the biomass-related processes is much slower that for the nutrients-related.
- Nutrients are diluted in the media. Biomass exists only inside the biofilm.
- The detachment is related to cells death and the degradation of the extracellular DNA, i.e., to biofilm ageing.
4. Results
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Swaminathan, B.; Gerner-Smidt, P. The epidemiology of human listeriosis. Microbes Infect. 2007, 9, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Balsa-Canto, E.; Vilas, C.; López-Núñez, A.; Mosquera-Fernández, M.; Briandet, R.; Cabo, M.L.; Vázquez, C. Modeling reveals the role of aging and glucose uptake impairment in L1A1 Listeria monocytogenes biofilm life cycle. Front. Microbiol. 2017, 8, 2118. [Google Scholar] [CrossRef] [PubMed]
- López-Núñez, A. Contributions to Mathematical Modelling and Numerical Simulation of Biofilms. Ph.D. Thesis, Universidade da Coruña, A Coruña, Spain, 2018. [Google Scholar]
- Balsa-Canto, E.; López-Núñez, A.; Vázquez, C. Numerical methods for a non-linear reaction-diffusion system modelling a batch culture of biofilm. Appl. Math. Model. 2017, 41, 164–179. [Google Scholar] [CrossRef][Green Version]
- Balsa-Canto, E.; Henriques, D.; Gabor, A.; Banga, J.R. AMIGO2, a toolbox for dynamic modeling optimization and control in systems biology. Bioinformatics 2016, 32, 3357–3359. [Google Scholar] [CrossRef] [PubMed]
- Mosquera-Fernández, M.; Sánchez-Vizuete, P.; Briandet, R.; Cabo, M.L.; Balsa-Canto, E. Quantitative image analysis to characterize the dynamics of Listeria monocytogenes biofilms. Int. J. Food Microbiol. 2016, 236, 130–137. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balsa-Canto, E.; López-Núñez, A.; Vázquez, C. Numerical Simulation of the Dynamics of Listeria Monocytogenes Biofilms. Proceedings 2018, 2, 1182. https://doi.org/10.3390/proceedings2181182
Balsa-Canto E, López-Núñez A, Vázquez C. Numerical Simulation of the Dynamics of Listeria Monocytogenes Biofilms. Proceedings. 2018; 2(18):1182. https://doi.org/10.3390/proceedings2181182
Chicago/Turabian StyleBalsa-Canto, Eva, Alejandro López-Núñez, and Carlos Vázquez. 2018. "Numerical Simulation of the Dynamics of Listeria Monocytogenes Biofilms" Proceedings 2, no. 18: 1182. https://doi.org/10.3390/proceedings2181182
APA StyleBalsa-Canto, E., López-Núñez, A., & Vázquez, C. (2018). Numerical Simulation of the Dynamics of Listeria Monocytogenes Biofilms. Proceedings, 2(18), 1182. https://doi.org/10.3390/proceedings2181182



