Mapping Soil-Transmitted Helminth Parasite Infection in Rwanda: Estimating Endemicity and Identifying At-Risk Populations
Abstract
:1. Introduction
2. Results
2.1. Summary Statistics of STH Prevalence in Rwandan Schoolchildren
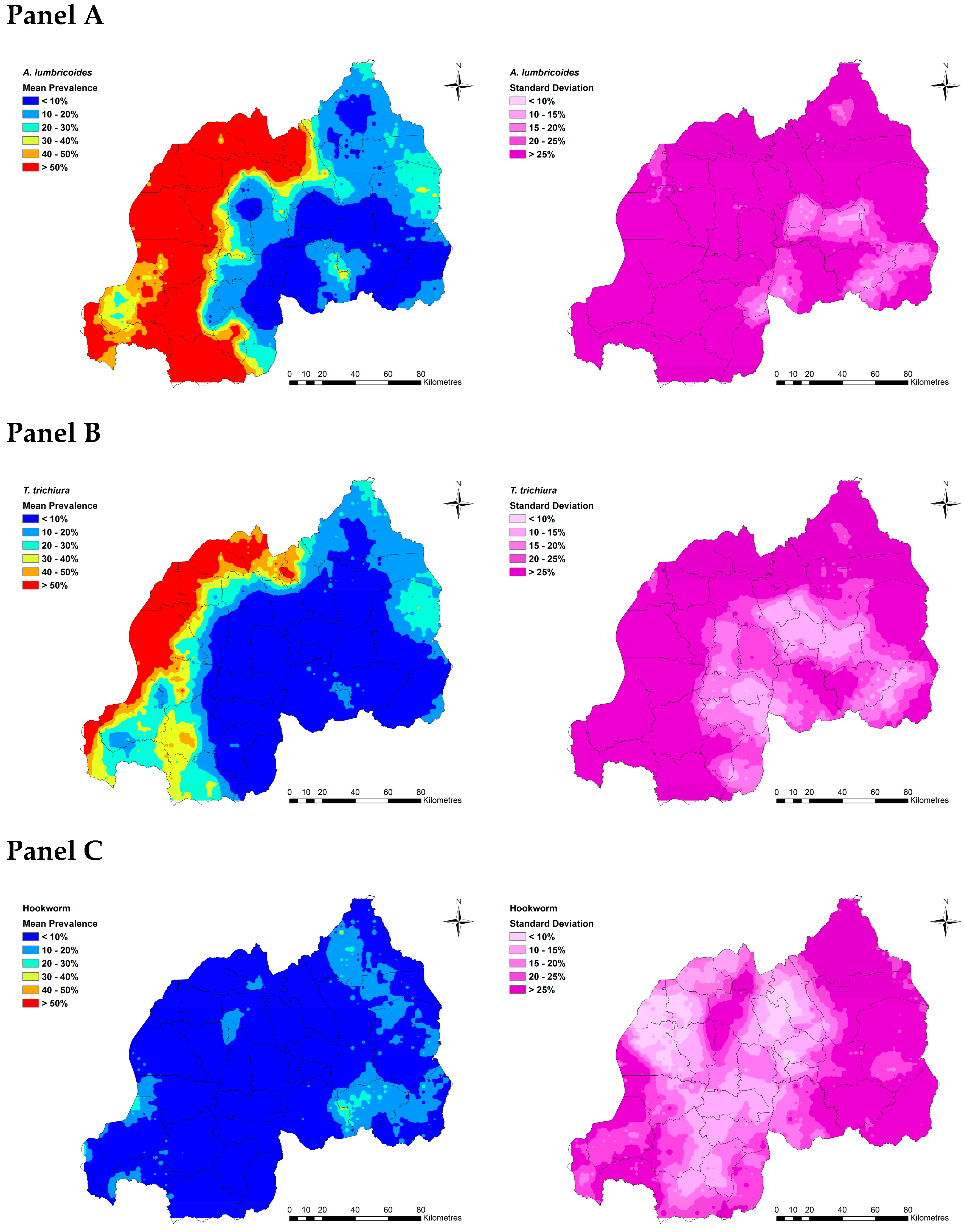
2.2. Spatial Clustering in STH Prevalence and Risk Prediction
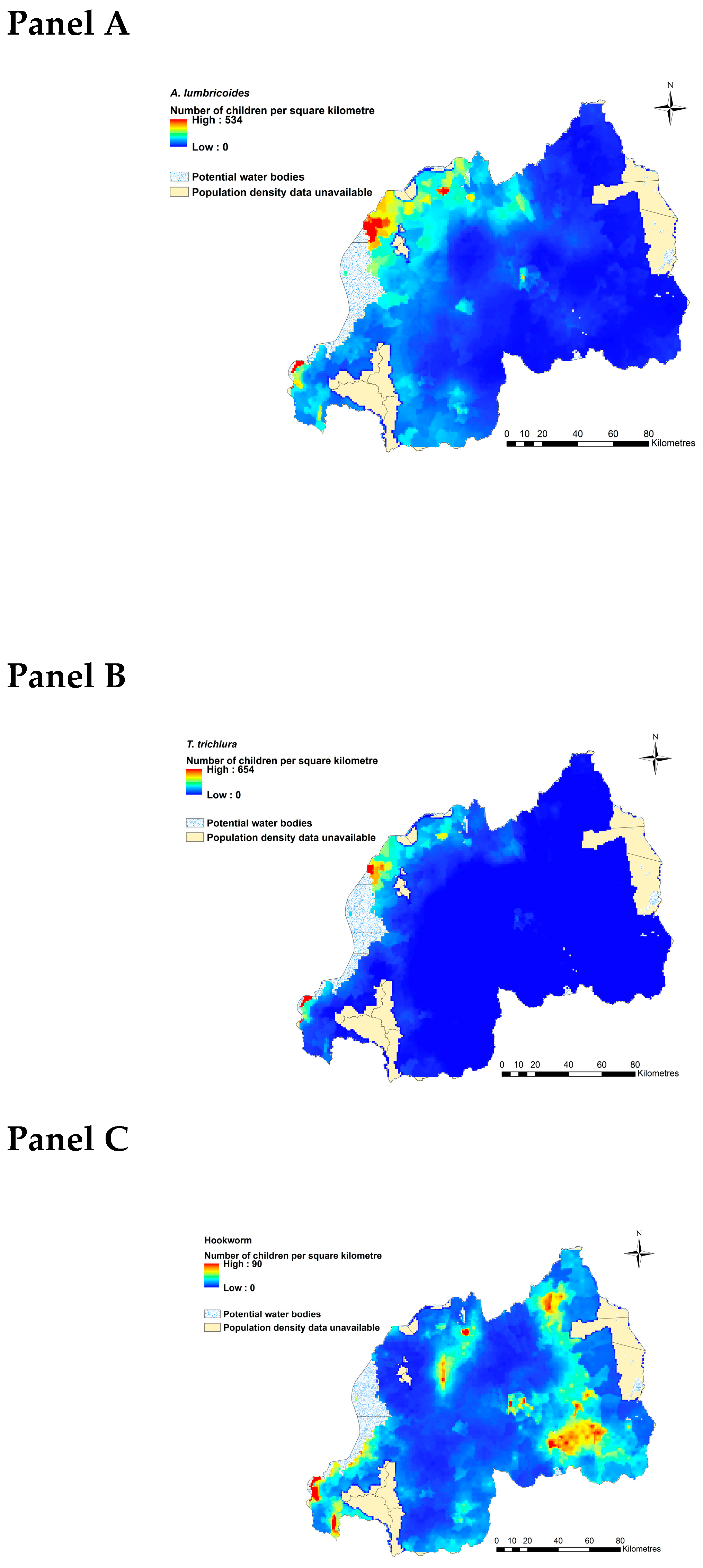
2.3. Estimated Current Burdens of A. lumbricoides, T. trichiura and Hookworms
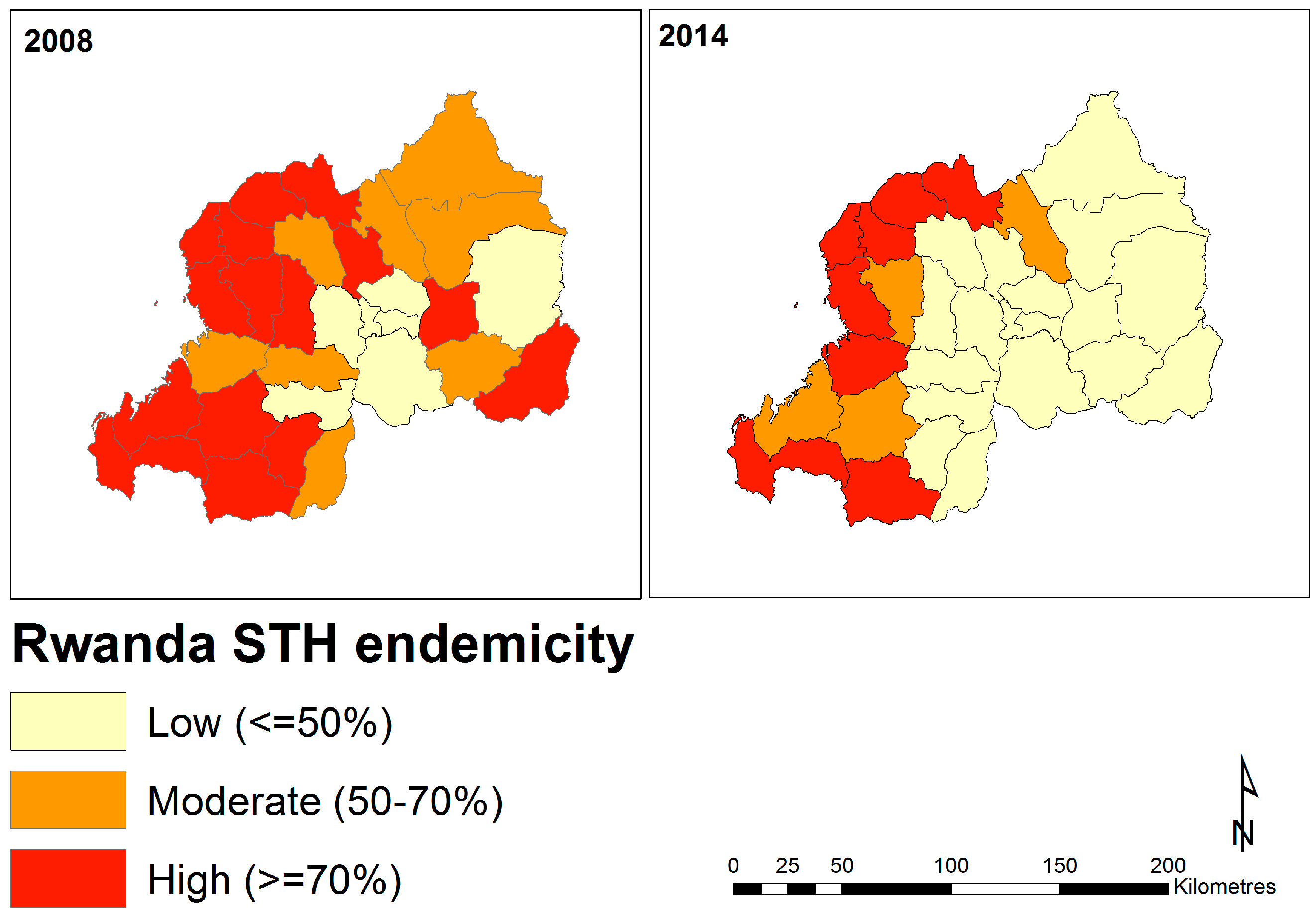
2.4. District-Level Temporal Trends in Soil-Transmitted Helminth Endemicity
3. Discussion
3.1. Delineating Regions in Rwanda at Greatest Need of STH Mitigation
3.2. Influence of Ecological Variables on STH Infection Probability
3.3. Study Limitations
3.4. Conclusions and Future Directions
4. Materials and Methods
4.1. Ethics Statement
4.2. STH Infection Data
4.3. Extraction of Environmental Covariate Information
4.4. Statistical Analysis
4.4.1. Frequentist Models for Variable Selection and Examination of Spatial Autocorrelation
4.4.2. Geostatistical Models of Soil-Transmitted Helminth Infection Risk
4.4.3. Estimating Current Burdens of A. lumbricoides, T. trichiura, and Hookworm Infections in an At-Risk Population in Rwanda
4.4.4. Visualising Temporal Trends in STH Endemicity
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ortu, G.; Assoum, M.; Wittmann, U.; Knowles, S.; Clements, M.; Ndayishimiye, O.; Basáñez, M.-G.; Lau, C.; Clements, A.; Fenwick, A.; et al. The Impact of an 8-Year Mass Drug Administration Programme on Prevalence, Intensity and Co-Infections of Soil-Transmitted Helminthiases in Burundi. Parasites Vectors 2016, 9, 513. [Google Scholar] [CrossRef] [PubMed]
- De Silva, N.R.; Brooker, S.; Hotez, P.J.; Montresor, A.; Engels, D.; Savioli, L. Soil-Transmitted Helminth Infections: Updating the Global Picture. Trends Parasitol. 2003, 19, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Stoll, N.R. This Wormy World. J. Parasitol. 1947, 33, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Soares Magalhães, R.J.; Biritwum, N.-K.; Gyapong, J.O.; Brooker, S.; Zhang, Y.; Blair, L.; Fenwick, A.; Clements, A.C.A. Mapping Helminth Co-Infection and Co-Intensity: Geostatistical Prediction in Ghana. PLoS Negl. Trop. Dis. 2011, 5, e1200. [Google Scholar] [CrossRef] [PubMed]
- Kightlinger, L.K.; Seed, J.R.; Kightlinger, M.B. The Epidemiology of Ascaris lumbricoides, Trichuris trichiura, and Hookworm in Children in the Ranomafana Rainforest, Madagascar. J. Parasitol. 1995, 81, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.; Kurscheid, J.; Jones, M.; Gray, D.; McManus, D. Soil-Transmitted Helminths in Tropical Australia and Asia. Trop. Med. Infect. Dis. 2017, 2, 56. [Google Scholar] [CrossRef] [PubMed]
- Pullan, R.L.; Smith, J.L.; Jasrasaria, R.; Brooker, S.J. Global Numbers of Infection and Disease Burden of Soil Transmitted Helminth Infections in 2010. Parasites Vectors 2014, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Drake, L.; Jukes, M.; Sternberg, R.; Bundy, D. Geohelminth Infections (Ascariasis, Trichuriasis, and Hookworm): Cognitive and Developmental Impacts. In Seminars in Pediatric Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2000; pp. 245–251. [Google Scholar]
- Hotez, P.J.; Bethony, J.; Bottazzi, M.E.; Brooker, S.; Buss, P. Hookworm: “The Great Infection of Mankind”. PLoS Med. 2005, 2, e67. [Google Scholar] [CrossRef]
- Brooker, S.; Kabatereine, N.B.; Smith, J.L.; Mupfasoni, D.; Mwanje, M.T.; Ndayishimiye, O.; Lwambo, N.J.; Mbotha, D.; Karanja, P.; Mwandawiro, C. An Updated Atlas of Human Helminth Infections: The Example of East Africa. Int. J. Health Geogr. 2009, 8, 42. [Google Scholar] [CrossRef]
- London Declaration on Neglected Tropical Diseases. Neglected Tropical Diseases, Uniting to Combat: London Declaration on Neglected Tropical Diseases. Available online: http://unitingtocombatntds.org/sites/default/files/document/london_declaration_on_ntds.pdf (accessed on 27 May 2018).
- Strunz, E.C.; Addiss, D.G.; Stocks, M.E.; Ogden, S.; Utzinger, J.; Freeman, M.C. Water, Sanitation, Hygiene, and Soil-Transmitted Helminth Infection: A Systematic Review and Meta-Analysis. PLoS Med. 2014, 11, e1001620. [Google Scholar] [CrossRef]
- Campbell, S.J.; Savage, G.B.; Gray, D.J.; Atkinson, J.-A.M.; Soares Magalhães, R.J.; Nery, S.V.; McCarthy, J.S.; Velleman, Y.; Wicken, J.H.; Traub, R.J.; et al. Water, Sanitation, and Hygiene (Wash): A Critical Component for Sustainable Soil-Transmitted Helminth and Schistosomiasis Control. PLoS Negl. Trop. Dis. 2014, 8, e2651. [Google Scholar] [CrossRef] [PubMed]
- Jamison, D.T.; Breman, J.G.; Measham, A.R.; Alleyne, G.; Claeson, M.; Evans, D.B.; Jha, P.; Mills, A.; Musgrove, P. Disease Control. Priorities in Developing Countries; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Campbell, S.; Osei-Atweneboana, M.; Stothard, R.; Koukounari, A.; Cunningham, L.; Armoo, S.; Biritwum, N.-K.; Gyapong, M.; MacPherson, E.; Theobald, S.; et al. The Countdown Study Protocol for Expansion of Mass Drug Administration Strategies against Schistosomiasis and Soil-Transmitted Helminthiasis in Ghana. Trop. Med. Infect. Dis. 2018, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Assoum, M.; Ortu, G.; Basáñez, M.-G.; Lau, C.; Clements, A.C.A.; Halton, K.; Fenwick, A.; Soares Magalhães, R.J. Spatiotemporal Distribution and Population at Risk of Soil-Transmitted Helminth Infections Following an Eight-Year School-Based Deworming Programme in Burundi, 2007–2014. Parasites Vectors 2017, 10, 583. [Google Scholar] [CrossRef] [PubMed]
- Brooker, S.; Clements, A.C.; Bundy, D.A. Global Epidemiology, Ecology and Control of Soil-Transmitted Helminth Infections. Adv. Parasitol. 2006, 62, 221–261. [Google Scholar] [PubMed] [Green Version]
- Wolstenholme, A.J.; Fairweather, I.; Prichard, R.; von Samson-Himmelstjerna, G.; Sangster, N.C. Drug Resistance in Veterinary Helminths. Trends Parasitol. 2004, 20, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Kopp, S.R.; Kotze, A.C.; McCarthy, J.S.; Coleman, G.T. High-Level Pyrantel Resistance in the Hookworm Ancylostoma caninum. Vet. Parasitol. 2007, 143, 299–304. [Google Scholar] [CrossRef]
- Kotze, A.C.; Kopp, S.R. The Potential Impact of Density Dependent Fecundity on the Use of the Faecal Egg Count Reduction Test for Detecting Drug Resistance in Human Hookworms. PLoS Negl. Trop. Dis. 2008, 2, e297. [Google Scholar] [CrossRef]
- Truscott, J.; Turner, H.; Farrell, S.; Anderson, R. Soil-Transmitted Helminths: Mathematical Models of Transmission, the Impact of Mass Drug Administration and Transmission Elimination Criteria. In Advances in Parasitology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 94, pp. 1331–1398. [Google Scholar]
- Staudacher, O.; Heimer, J.; Steiner, F.; Kayonga, Y.; Havugimana, J.M.; Ignatius, R.; Musemakweri, A.; Ngabo, F.; Harms, G.; Gahutu, J.B. Soil-Transmitted Helminths in Southern Highland Rwanda: Associated Factors and Effectiveness of School-Based Preventive Chemotherapy. Trop. Med. Int. Health 2014, 19, 812–824. [Google Scholar] [CrossRef]
- Soares Magalhães, R.J.; Clements, A.C.A.; Patil, A.P.; Gething, P.W.; Brooker, S. The Applications of Model-Based Geostatistics in Helminth Epidemiology and Control. Adv. Parasitol. 2011, 74, 267–296. [Google Scholar] [Green Version]
- Clark, N.; Soares Magalhães, R.J. Airborne Geographical Dispersal of Q Fever from Livestock Holdings to Human Communities: A Systematic Review and Critical Appraisal of Evidence. BMC Infect. Dis. 2018. [Google Scholar] [CrossRef]
- Brooker, S.; Michael, E. The Potential of Geographical Information Systems and Remote Sensing in the Epidemiology and Control of Human Helminth Infections. Adv. Parasitol. 2000, 47, 245–288. [Google Scholar] [PubMed]
- Traub, R.J. Ancylostoma ceylanicum, a Re-Emerging but Neglected Parasitic Zoonosis. Int. J. Parasitol. 2013, 43, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Pavlovsky, E.N. Natural Nidality of Transmissible Diseases with Special Reference to the Landscape Epidemiology of Zooanthroponoses; University of Illinois Press: Urbana, IL, USA, 1966. [Google Scholar]
- Pullan, R.L.; Gething, P.W.; Smith, J.L.; Mwandawiro, C.S.; Sturrock, H.J.; Gitonga, C.W.; Hay, S.I.; Brooker, S. Spatial Modelling of Soil-Transmitted Helminth Infections in Kenya: A Disease Control Planning Tool. PLoS Negl. Trop. Dis. 2011, 5, e958. [Google Scholar] [CrossRef] [PubMed]
- Hay, S.; Tatem, A.; Graham, A.; Goetz, S.; Rogers, D. Global Environmental Data for Mapping Infectious Disease Distribution. Adv. Parasitol. 2006, 62, 37–77. [Google Scholar] [Green Version]
- World Bank. The World Bank in Rwanda. Available online: http://www.worldbank.org/en/country/rwanda/overview (accessed on 27 May 2018).
- Rujeni, N.; Morona, D.; Ruberanziza, E.; Mazigo, H.D. Schistosomiasis and Soil-Transmitted Helminthiasis in Rwanda: An Update on Their Epidemiology and Control. Infect. Dis Poverty 2017, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- World Health Report. Working Together for Health; World Health Organization: Geneva, Switzerland, 2006; Available online: http://www.who.int/whr/2006/en (accessed on 27 May 2018).
- Binagwaho, A.; Kyamanywa, P.; Farmer, P.E.; Nuthulaganti, T.; Umubyeyi, B.; Nyemazi, J.P.; Mugeni, S.D.; Asiimwe, A.; Ndagijimana, U.; Lamphere McPherson, H. The Human Resources for Health Program in Rwanda—A New Partnership. N. Engl. J. Med. 2013, 369, 2054–2059. [Google Scholar] [CrossRef]
- Rwanda Human Resources for Health Program. Strategic Plan 2011–2016; Ministry of Health of the Republic of Rwanda: Kigali, Rwanda, 2011. Available online: http://www.brown.edu/academics/medical/bright/sites/brown.edu.academics.medical.bright/files/uploads/MOH%20Rwanda%20HRH%20Strategic%20Plan%202011%20-%202016.pdf (accessed on 27 May 2018).
- Ignatius, R.; Gahutu, J.B.; Klotz, C.; Steininger, C.; Shyirambere, C.; Lyng, M.; Musemakweri, A.; Aebischer, T.; Martus, P.; Harms, G.; et al. High Prevalence of Giardia Duodenalis Assemblage B Infection and Association with Underweight in Rwandan Children. PLoS Negl. Trop. Dis. 2012, 6, e1677. [Google Scholar] [CrossRef]
- Soares Magalhães, R.J.; Salamat, M.S.; Leonardo, L.; Gray, D.J.; Carabin, H.; Halton, K.; McManus, D.P.; Williams, G.M.; Rivera, P.; Saniel, O.; et al. Mapping the Risk of Soil-Transmitted Helminthic Infections in the Philippines. PLoS Negl. Trop. Dis. 2015, 9, e0003915. [Google Scholar] [CrossRef]
- Basáñez, M.-G.; McCarthy, J.S.; French, M.D.; Yang, G.-J.; Walker, M.; Gambhir, M.; Prichard, R.K.; Churcher, T.S. A Research Agenda for Helminth Diseases of Humans: Modelling for Control and Elimination. PLoS Negl. Trop. Dis. 2012, 6, e1548. [Google Scholar] [CrossRef]
- ICF International. Rwanda Demographic and Health Survey 2014–2015; National Institute of Statistics of Rwanda: Rockville, MA, USA, 2016.
- Colón-González, F.J.; Tompkins, A.M.; Biondi, R.; Bizimana, J.P.; Namanya, D.B. Assessing the Effects of Air Temperature and Rainfall on Malaria Incidence: An Epidemiological Study across Rwanda and Uganda. Geospat. Health 2016, 11, 18–37. [Google Scholar] [CrossRef]
- García-Leiva, J.; Barreto-Zuñiga, R.; Estradas, J.; Torre, A. Ascaris lumbricoides and Iron Deficiency Anemia. Am. J. Gastroenterol. 2008, 103, 1051. [Google Scholar] [CrossRef] [PubMed]
- Amuga, G.; Onwuliri, C.; Oniye, S. Relative Contribution of Hookworm and Ascaris lumbricoides to Iron Deficiency Anemia among School Pupils in Nasarawa Area, Nigeria. Int. J. Nat. Appl. Sci. 2006, 2, 205–209. [Google Scholar] [CrossRef]
- Brooker, S.; Bethony, J.; Hotez, P.J. Human Hookworm Infection in the 21st Century. Adv. Parasitol. 2004, 58, 197–288. [Google Scholar] [PubMed] [Green Version]
- World Health Organization. Bench Aids for the Diagnosis of Intestinal Parasites; WHO: Geneva, Switzterland, 2012; p. 23. [Google Scholar]
- Center for International Earth Science Information Network (CIESIN). Documentation for the Gridded Population of the World, Version 4 (Gpwv4). Available online: http://dx.doi.org/10.7927/H4D50JX4 (accessed on 21 January 2018).
- Crompton, D.W.; Savioli, L. Intestinal Parasitic Infections and Urbanization. Bull. World Health Organ. 1993, 71, 1–7. [Google Scholar] [PubMed]
- Bradley, C.A.; Altizer, S. Urbanization and the Ecology of Wildlife Diseases. Trends Ecol. Evol. 2007, 22, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Clark, N.J.; Seddon, J.M.; Šlapeta, J.; Wells, K. Parasite Spread at the Domestic Animal-Wildlife Interface: Anthropogenic Habitat Use, Phylogeny and Body Mass Drive Risk of Cat and Dog Flea (Ctenocephalides spp.) Infestation in Wild Mammals. Parasites Vectors 2018, 11, 8. [Google Scholar] [CrossRef]
- Wells, K.; O’Hara, R.B.; Morand, S.; Lessard, J.-P.; Ribas, A. The Importance of Parasite Geography and Spillover Effects for Global Patterns of Host–Parasite Associations in Two Invasive Species. Divers. Distrib. 2015, 21, 477–486. [Google Scholar] [CrossRef]
- Thompson, R.A. Parasite Zoonoses and Wildlife: One Health, Spillover and Human Activity. Int. J. Parasitol. 2013, 43, 1079–1088. [Google Scholar] [CrossRef]
- Lau, C.L.; Smythe, L.D.; Craig, S.B.; Weinstein, P. Climate Change, Flooding, Urbanisation and Leptospirosis: Fuelling the Fire? Trans. Roy. Soc. Trop. Med. Hyg. 2010, 104, 631–638. [Google Scholar] [CrossRef]
- Brooker, S. Spatial Epidemiology of Human Schistosomiasis in Africa: Risk Models, Transmission Dynamics and Control. Trans. Roy. Soc. Trop. Med. Hyg. 2007, 101, 1–8. [Google Scholar] [CrossRef]
- Haque, U.; Soares Magalhães, R.J.; Reid, H.L.; Clements, A.C.; Ahmed, S.M.; Islam, A.; Yamamoto, T.; Haque, R.; Glass, G.E. Spatial Prediction of Malaria Prevalence in an Endemic Area of Bangladesh. Malar. J. 2010, 9, 120. [Google Scholar] [CrossRef] [PubMed]
- Clark, N.J.; Wells, K.; Dimitrov, D.; Clegg, S.M. Co-Infections and Environmental Conditions Drive the Distributions of Blood Parasites in Wild Birds. J. Anim. Ecol. 2016, 85, 1461–1470. [Google Scholar] [CrossRef] [PubMed]
- Wells, K.; Gibson, D.I.; Clark, N.J.; Ribas, A.; Morand, S.; McCallum, H.I. Global Spread of Helminth Parasites at the Human–Domestic Animal–Wildlife Interface. Glob. Chang. Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Clark, N.J.; Seddon, J.M.; Kyaw-Tanner, M.; Al-Alawneh, J.; Harper, G.; McDonagh, P.; Meers, J. Emergence of Canine Parvovirus Subtype 2b (Cpv-2b) Infections in Australian Dogs. Infect. Gen. Evol. 2018, 58, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Raso, G.; Vounatsou, P.; Gosoniu, L.; Tanner, M.; N’Goran, E.K.; Utzinger, J. Risk Factors and Spatial Patterns of Hookworm Infection among Schoolchildren in a Rural Area of Western Côte D’ivoire. Int. J. Parasitol. 2006, 36, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Bethony, J.; Brooker, S.; Albonico, M.; Geiger, S.M.; Loukas, A.; Diemert, D.; Hotez, P.J. Soil-Transmitted Helminth Infections: Ascariasis, Trichuriasis, and Hookworm. Lancet 2006, 367, 1521–1532. [Google Scholar] [CrossRef]
- Pullan, R.L.; Kabatereine, N.B.; Quinnell, R.J.; Brooker, S. Spatial and Genetic Epidemiology of Hookworm in a Rural Community in Uganda. PLoS Negl. Trop. Dis. 2010, 4, e713. [Google Scholar] [CrossRef]
- Anderson, R.M.; May, R.M. Helminth Infections of Humans: Mathematical Models, Population Dynamics, and Control. In Advances in Parasitology; Baker, J.R., Muller, R., Eds.; Academic Press: New York, NY, USA, 1985; Volume 24, pp. 1–101. [Google Scholar]
- Booth, M.; Vounatsou, P.; N’goran, E.; Tanner, M.; Utzinger, J. The influence of sampling effort and the performance of the Kato-Katz technique in diagnosing Schistosoma mansoni and hookworm co-infections in rural Côte d’Ivoire. Parasitology 2003, 127, 525–531. [Google Scholar] [CrossRef]
- Mationg, M.L.S.; Gordon, C.A.; Tallo, V.L.; Olveda, R.M.; Alday, P.P.; Renosa, M.D.C.; Bieri, F.A.; Williams, G.M.; Clements, A.C.A.; Steinmann, P.; et al. Status of Soil-Transmitted Helminth Infections in Schoolchildren in Laguna Province, the Philippines: Determined by Parasitological and Molecular Diagnostic Techniques. PLoS Negl. Trop. Dis. 2017, 11, e0006022. [Google Scholar] [CrossRef]
- Clark, N.J.; Wells, K.; Lindberg, O. Unravelling Changing Interspecific Interactions across Environmental Gradients Using Markov Random Fields. Ecology 2018, 99, 1277–1283. [Google Scholar] [CrossRef]
- Muturi, E.J.; Jacob, B.G.; Kim, C.-H.; Mbogo, C.M.; Novak, R.J. Are Coinfections of Malaria and Filariasis of Any Epidemiological Significance? Parasitol. Res. 2008, 102, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Owada, K.; Nielsen, M.; Lau, C.L.; Clements, A.C.; Yakob, L.; Soares Magalhães, R.J. Measuring the Effect of Soil-Transmitted Helminth Infections on Cognitive Function in Children: Systematic Review and Critical Appraisal of Evidence. Adv. Parasitol. 2017, 98, 1–37. [Google Scholar] [PubMed]
- Asaolu, S.; Ofoezie, I. The Role of Health Education and Sanitation in the Control of Helminth Infections. Acta Trop. 2003, 86, 283–294. [Google Scholar] [CrossRef]
- Karema, C.; Fenwick, A.; Colley, G.D. Mapping of Schistosomiasis and Soil-Transmitted Helminthiasis in Rwanda 2014: Mapping Survey Report; Rwanda Biomedical Center: Kigali, Rwanda, 2015; p. 47.
- World Health Organization. Basic Laboratory Methods in Medical Parasitology; World Health Organization: Geneva, Switzerland, 1991. [Google Scholar]
- Brooker, S.; Alexander, N.; Geiger, S.; Moyeed, R.A.; Stander, J.; Fleming, F.; Hotez, P.J.; Correa-Oliveira, R.; Bethony, J. Contrasting Patterns in the Small-Scale Heterogeneity of Human Helminth Infections in Urban and Rural Environments in Brazil. Int J. Parasitol. 2006, 36, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Environmental Systems Research Institute. Arcgis Desktop: Release 10.4; Environmental Systems Research Institute: Redlands, CA, USA, 2015. [Google Scholar]
- QGIS Development Team. QGIS Geographic Information System; Open Source Geospatial Foundation Project. Available online: http://qgis.osgeo.org (accessed on 17 May 2018).
- Ribeiro, P.J.; Diggle, P.J. Geor: Analysis of Geostatistical Data R Package Version 1.7–5.2. Available online: https://cran.r-project.org/web/packages/geoR/geoR.pdf (accessed on 17 May 2018).
- Lunn, D.; Jackson, C.; Best, N.; Thomas, A.; Spiegelhalter, D. The Bugs Book: A Practical Introduction to Bayesian Analysis; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]


| Variable | Observation | Mean | 95%CI | Missing Data |
|---|---|---|---|---|
| Number of schools | 186 | - | - | - |
| Units (district) | 30 | - | - | - |
| Number of individuals surveyed | 9251 | |||
| Demographic | ||||
| Age | 9226 | 13.31 (Range 9 to 18) | 13.29, 13.33 | Location 232 R1 to R25 (N = 25) |
| Age category | 9226 | - | - | - |
| 9 to 11 years old | 151 (1.64%) | - | - | - |
| 12 to 14 years old | 8931 (96.80%) | - | - | - |
| 15 to 18 years old | 144 (1.56%) | - | - | - |
| Sex | 9251 | - | - | - |
| Female | 4617 (49.9%) | - | - | - |
| Male | 4634 (50.1%) | - | - | - |
| Prevalence of infections | ||||
| A. lumbricoides | 9251 | 0.37 | 0.36, 0.38 | |
| T. trichiura | 9250 | 0.23 | 0.22, 0.24 | Location 104/R46 |
| Hookworm | 9250 | 0.05 | 0.04, 0.05 | Location 104/R46 |
| Environmental variables | ||||
| LST 2014 | 9251 | −0.001 | −0.021, 0.020 | - |
| NDVI 2014 | 9251 | 0.002 | −0.018, 0.023 | - |
| NDWI 2014 | 9251 | 0.002 | −0.019, 0.022 | - |
| Observed | Residual | |
|---|---|---|
| A. lumbricoides | ||
| Partial sill | 0.167 | 0.013 |
| Nugget | 0.001 | 0.000 |
| Practical range * | 1.686 | 0.421 |
| % of the variance due to clustering | 99.84 | 99.41 |
| T. trichiura | ||
| Partial sill | 0.083 | 0.01 |
| Nugget | 0.007 | 0.005 |
| Practical range * | 1.526 | 0.94 |
| % of the variance that is due to cluster ** | 92.67 | 64.76 |
| Hookworm | ||
| Partial sill | 0.003 | 0.001 |
| Nugget | 0.000 | 0.006 |
| Practical range * | 0.463 | 0.149 |
| % of the variance that is due to cluster ** | 88.23 | 11.07 |
| Coefficient | A. lumbricoides Mean (95% CI) b | T. trichiura Mean (95% CI) b | Hookworm Mean (95% CI) b |
|---|---|---|---|
| Intercept | −1.651(−3.981, 0.214) | −2.891 (−4.417, −1.121) | −4.585 (−7.184, −2.656) |
| Male (versus female) | −0.032 (−0.150, 0.086) | 0.036 (−0.098, 0.167) | −0.043 (−0.257, 0.159) |
| Age 12 to 14 years old (versus 9 to 11 years old) | 0.357 (−0.502, 1.248) | 0.998 (0.015, 2.024) | 0.996 (−0.890, 3.573) |
| 15 to 18 years old (versus 9 to 11 years old) | 0.316 (−0.651, 1.323) | 0.791(−0.308, 1.931) | 0.542 (−1.703, 3.192) |
| Normalized difference vegetation index a | 0.166 (0.015, 0.322) | 0.083 (−0.088, 0.249) | −0.117 (−0.337, 0.089) |
| Land surface temperature a | −0.080 (−0.251, 0.100) | 0.045 (−0.141, 0.230) | −0.065 (−0.288, 0.178) |
| Normalized difference water index a | 0.296 (0.107, 0.491) | 0.263 (0.074, 0.450) | 0.308 (0.054, 0.583) |
| φ Phi (rate of decay) c | 3.154 (0.988, 5.696) | 2.912 (0.541, 5.520) | 7.911 (3.343, 13.610) |
| σ2Sigma (variance) | 5.888 (2.788, 13.550) | 6.639 (2.818, 23.750) | 2.268 (1.336, 3.910) |
| Tau (precision) | 0.210 (0.074, 0.359) | 0.197 (0.042, 0.355) | 0.475 (0.256, 0.748) |
| Total Population for 2015 (in Thousands) a | Annual Population Growth Rate for 2015–2020 (Percentage) a | Percentage of Individuals Aged 12–14y a | Predicted Number of Individuals with Infection in 2018 | ||
|---|---|---|---|---|---|
| Infections | |||||
| A. lumbricoides | T. trichiua | Hookworm | |||
| 11,629.6 | 2.53 | 12.20% | 589,673 | 332,144 | 83,749 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruberanziza, E.; Owada, K.; Clark, N.J.; Umulisa, I.; Ortu, G.; Lancaster, W.; Munyaneza, T.; Mbituyumuremyi, A.; Bayisenge, U.; Fenwick, A.; et al. Mapping Soil-Transmitted Helminth Parasite Infection in Rwanda: Estimating Endemicity and Identifying At-Risk Populations. Trop. Med. Infect. Dis. 2019, 4, 93. https://doi.org/10.3390/tropicalmed4020093
Ruberanziza E, Owada K, Clark NJ, Umulisa I, Ortu G, Lancaster W, Munyaneza T, Mbituyumuremyi A, Bayisenge U, Fenwick A, et al. Mapping Soil-Transmitted Helminth Parasite Infection in Rwanda: Estimating Endemicity and Identifying At-Risk Populations. Tropical Medicine and Infectious Disease. 2019; 4(2):93. https://doi.org/10.3390/tropicalmed4020093
Chicago/Turabian StyleRuberanziza, Eugene, Kei Owada, Nicholas J. Clark, Irenee Umulisa, Giuseppina Ortu, Warren Lancaster, Tharcisse Munyaneza, Aimable Mbituyumuremyi, Ursin Bayisenge, Alan Fenwick, and et al. 2019. "Mapping Soil-Transmitted Helminth Parasite Infection in Rwanda: Estimating Endemicity and Identifying At-Risk Populations" Tropical Medicine and Infectious Disease 4, no. 2: 93. https://doi.org/10.3390/tropicalmed4020093





