In Vivo Efficacy of a Cocktail of Human Monoclonal Antibodies (CL184) Against Diverse North American Bat Rabies Virus Variants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Viruses
2.2. Biologics
2.3. Experimental Design
2.4. Model Validation/Post-Exposure Prophylaxis (PEP) Initiation Determination
2.5. Evaluation of the Efficacy of HRIG/Vaccine versus CL184/Vaccine during PEP
2.6. Laboratory Methods
2.6.1. Direct Fluorescent Antibody (DFA) Test
2.6.2. Rapid Fluorescent Focus Inhibition Test (RFFIT)
2.6.3. Reverse Transcription Polymerase Chain Reaction (RT-PCR), Hemi-Nested RT-PCR (hnRT-PCR) and Sequencing
2.7. Statistical Analysis
3. Results
3.1. Neutralization of Selected RABV Isolates In Vitro
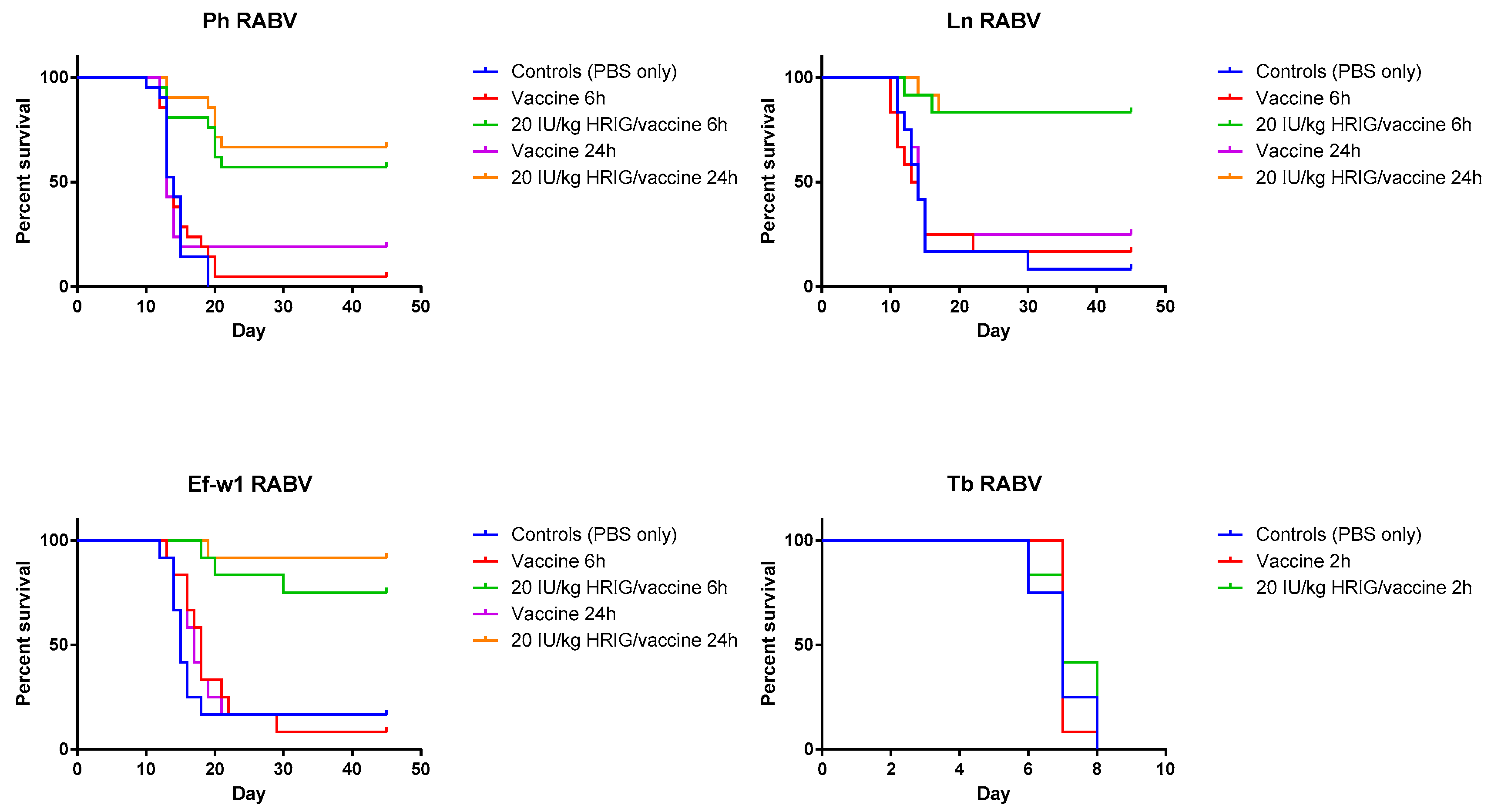
3.2. Model Validation/PEP Initiation Window
3.3. Evaluation of the Efficacy of HRIG/Vaccine versus CL184/Vaccine during PEP
3.4. Sequence Analyses of the Original Inoculum and Virus Detected in CNS of Experimental Animals
4. Discussion and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Hampson, K.; Coudeville, L.; Lembo, T.; Sambo, M.; Kieffer, A.; Attlan, M.; Barrat, J.; Blanton, J.D.; Briggs, D.J.; Cleaveland, S.; et al. Estimating the global burden of endemic canine rabies. PLoS Negl. Trop. Dis. 2015, 16, e0003709. [Google Scholar]
- World Health Organization. WHO Expert Consultation on Rabies, Second Report. 2013. Available online: http://apps.who.int/iris/bitstream/10665/85346/1/9789240690943_eng.pdf (accessed on 29 August 2016).
- Franka, R.; Wu, X.; Jackson, F.R.; Velasco-Villa, A.; Palmer, D.P.; Henderson, H.; Hayat, W.; Green, D.B.; Blanton, J.D.; Greenberg, L.; et al. Rabies virus pathogenesis in relationship to intervention with inactivated and attenuated rabies vaccines. Vaccine 2009, 27, 7149–7155. [Google Scholar] [CrossRef] [PubMed]
- Habel, K.; Koprowski, H. Laboratory data supporting the clinical trial of antirabies serum in persons bitten by a rabid wolf. Bull. World Health Organ. 1955, 13, 773–779. [Google Scholar] [PubMed]
- Nagarajan, T.; Marrisen, W.E.; Rupprecht, C.E. Monoclonal antibodies for the prevention of rabies: Theory and clinical practice. Antib. Technol. J. 2014, 4, 1–12. [Google Scholar] [CrossRef]
- Prosniak, M.; Faber, M.; Hanlon, C.A.; Rupprecht, C.E.; Hooper, D.C.; Dietzschold, B. Development of a cocktail of recombinant-expressed human rabies virus-neutralizing monoclonal antibodies for postexposure prophylaxis of rabies. J. Infect. Dis. 2003, 188, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Bakker, A.B.; Marissen, W.E.; Kramer, R.A.; Rice, A.B.; Weldon, W.C.; Niezgoda, M.; Hanlon, C.A.; Thijsse, S.; Backus, H.H.; De Kruif, J.; et al. Novel human monoclonal antibody combination effectively neutralizing natural rabies virus variants and individual in vitro escape mutants. J. Virol. 2005, 79, 9062–9068. [Google Scholar] [CrossRef] [PubMed]
- De Kruif, J.; Bakker, A.B.; Marissen, W.E.; Kramer, R.A.; Throsby, M.; Rupprecht, C.E.; Goudsmit, J. A human monoclonal antibody cocktail as a novel component of rabies postexposure prophylaxis. Annu. Rev. Med. 2007, 58, 359–368. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Consultation on a Monoclonal Antibody Cocktail for Rabies Post-Exposure Treatment. Available online: http://www.who.int/rabies/resources/WHO_consultation_on_RMAC_for_rabies_PEP/en/ (accessed on 15 August 2017).
- Marissen, W.E.; Kramer, R.A.; Rice, A.; Weldon, W.C.; Niezgoda, M.; Faber, M.; Slootstra, J.W.; Meloen, R.H.; Clijsters-Van der Horst, M.; Visser, T.J.; et al. Novel rabies virus-neutralizing epitope recognized by human monoclonal antibody: Fine mapping and escape mutant analysis. J. Virol. 2005, 79, 4672–4678. [Google Scholar] [CrossRef] [PubMed]
- Goudsmit, J.; Marissen, W.E.; Weldon, W.C.; Niezgoda, M.; Hanlon, C.A.; Rice, A.B.; De Kruif, J.; Dietzschold, B.; Bakker, A.B.; Rupprecht, C.E. Comparison of an anti-rabies human monoclonal antibody combination with human polyclonal anti-rabies immune globulin. J. Infect. Dis. 2006, 193, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Kuzmina, N.A.; Kuzmin, I.V.; Ellison, J.A.; Rupprecht, C.E. Conservation of binding epitopes for monoclonal antibodies on the rabies virus glycoprotein. J. Antivir. Antiretrovir. 2013, 5, 37–43. [Google Scholar] [CrossRef]
- Dean, D.J.; Abelseth, M.K.; Athanasiu, P. The fluorescent antibody test. In Laboratory Techniques in Rabies, 4th ed.; Meslin, F.X., Kaplan, M.M., Koprowski, H., Eds.; World Health Organization: Geneva, Switzerland, 1996; pp. 88–95. [Google Scholar]
- Smith, J.S.; Yager, P.A.; Baer, M. A rapid fluorescent focus inhibition test (RFFIT) for determining rabies virus-neutralizing antibody. In Laboratory Techniques in Rabies, 4th ed.; Meslin, F.X., Kaplan, M.M., Koprowski, H., Eds.; World Health Organization: Geneva, Switzerland, 1996; pp. 181–191. [Google Scholar]
- Trimarchi, C.V.; Smith, J.S. Diagnostic evaluation. In Rabies, 1st ed.; Jackson, A.C., Wunner, W.H., Eds.; Academic Press: New York, NY, USA, 2002; pp. 307–349. [Google Scholar]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Sloan, S.E.; Hanlon, C.; Weldon, W.; Niezgoda, M.; Blanton, J.; Self, J.; Rowley, K.J.; Mandell, R.B.; Babcock, G.J.; Thomas, W.D., Jr.; et al. Identification and characterization of a human monoclonal antibody that potently neutralizes a broad panel of rabies virus isolates. Vaccine 2007, 25, 2800–2810. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Dietzschold, B.; Ertl, H.; Fooks, A.R.; Freuling, C.; Fehlner-Gardiner, C.; Kliemt, J.; Meslin, F.X.; Franka, R.; Rupprecht, C.E.; et al. Development of a mouse monoclonal antibody cocktail for post-exposure rabies prophylaxis in humans. PLoS Negl. Trop. Dis. 2009, 3, e542. [Google Scholar] [CrossRef]
- De Benedictis, P.; Minola, A.; Rota Nodari, E.; Aiello, R.; Zecchin, B.; Salomoni, A.; Foglierini, M.; Agatic, G.; Vanzetta, F.; Lavenir, R.; et al. Development of broad-spectrum human monoclonal antibodies for rabies post-exposure prophylaxis. EMBO Mol. Med. 2016, 8, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Seif, I.; Coulon, P.; Rollin, P.E.; Flamand, A. Rabies virulence: Effect on pathogenicity and sequence characterization of rabies virus mutations affecting antigenic site III of the glycoprotein. J. Virol. 1985, 53, 926–934. [Google Scholar] [PubMed]


| Lyssaviruses | HRIG * | CR57 | CR4098 | CL184 |
|---|---|---|---|---|
| Cow/dog, Sri Lanka | + | + | + | + |
| Dog, China 2005 | + | + | + | + |
| Dog, China (RV342) | + | + | + | + |
| Dog, India (I 148) | NT | NT | NT | + |
| Dog, India (I 151) | + | + | + | + |
| Dog, India (I 155) | + | + | + | NT |
| Dog, Philippines | + | + | + | + |
| Dog, Philippines (231/002) | + | + | + | NT |
| Dog, Tunisia | + | + | + | + |
| Human/dog, UK ex India | NT | NT | NT | + |
| Human/wolf, Russia Siberia (RVHN) | + | + | + | + |
| Mongoose, South Africa | + | + | - | + |
| Raccoon dog, Russia/Far East | + | + | + | + |
| Skunk, south central (SK4384) | + | + | + | + |
| Bat, Lasiurus borealis, TN (tn132) | NT | NT | NT | + |
| Bat, Lasiurus borealis, TN (tn269) | NT | NT | NT | + |
| Bat, Lasiurus borealis, VA (VA399) | NT | NT | NT | + |
| Bat, Lasiurus cinereus, TN | NT | NT | NT | + |
| Survival after 45 days observation | ||||||||
|---|---|---|---|---|---|---|---|---|
| Group | Ln (%) | p-Value * | Ph (%) | p-Value | Ef-w1 (%) | p-Value | Tb (%) ‡ | p-Value |
| Control (PBS only) | 8.3 | - | 0 | - | 16.7 | - | 0 | - |
| Vaccine only, 6 h p.i. | 16.7 | 0.9984 | 4.8 | 0.5224 | 8.3 | 0.0023 | 0 | 0.8619 |
| Vaccine only, 24 h p.i. | 25 | 0.5174 | 19 | 0.6200 | 16.7 | 0.3391 | NA | |
| HRIG/vaccine, 6 h p.i. | 83.3 | 0.0002 0.0007 | 57 | <0.0001 <0.0001 | 75 | 0.0005 0.0002 | 0 | 0.3901 0.3161 |
| HRIG/vaccine, 24 h p.i. | 83.3 | <0.0001 0.0023 | 66.7 | <0.0001 0.0002 | 91.7 | <0.0001 0.0001 | NA | - |
| Survival after 45 Days Observation | ||||||||
|---|---|---|---|---|---|---|---|---|
| Groups | Ln (%) | p-Value * | Ph (%) | p-Value | Ef-w1 (%) | p-Value | Tb ‡ (%) | p-Value |
| Control (PBS only) | 11.1 | - | 16.7 | - | 33.3 | - | 0 | - |
| Vaccine only | 25 | 0.3630 | 0 | 0.0034 | 38.1 | 0.8464 | 8.3 | 0.6668 |
| 20 IU/kg HRIG/vaccine | 58.3 | 0.0430 0.1477 | 19 | 0.8705 0.0003 | 95.2 | <0.0001 <0.0001 | 66.7 | 0.0007 0.0006 |
| 24 μg/kg CL184/vaccine | NA | - | NA | - | NA | - | 100 | 0.0319 |
| 18 μg/kg CL184/vaccine | NA | - | NA | - | NA | - | 83.3 | 0.3959 |
| 16 μg/kg CL184/ vaccine | 66.7 | 0.5951 | 57.1 | 0.0177 | 100 | 0.3173 | NA | - |
| 12 μg/kg CL184/ vaccine | 50 | 0.8931 | 47.6 | 0.0699 | 95.2 | 0.9862 | 66.7 | 0.9483 |
| 6 μg/kg CL184/ vaccine | 41.7 | 0.5257 | 57.1 | 0.0062 | 85.7 | 0.2847 | NA | - |
| RABV Isolate | CR57 Epitope (226–231) | CR4098 Epitope (330–338) | CR57 Neutralization | CR4098 Neutralization |
|---|---|---|---|---|
| Bat, Lasionycteris noctivagans | KLCGVP | KSVRTWNEV | Yes | Yes |
| Bat, Parastrellus hesperus | KLCGVP | KSVRTWNET * | Yes | No |
| Bat, Eptesicus fuscus w1 lineage § | KLCGVP | KSIRTWNEI ‡ | Yes | Yes |
| Bat, Tadarida brasiliensis | KLCGVS | KSVRTWNEI | Yes | Yes |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franka, R.; Carson, W.C.; Ellison, J.A.; Taylor, S.T.; Smith, T.G.; Kuzmina, N.A.; Kuzmin, I.V.; Marissen, W.E.; Rupprecht, C.E. In Vivo Efficacy of a Cocktail of Human Monoclonal Antibodies (CL184) Against Diverse North American Bat Rabies Virus Variants. Trop. Med. Infect. Dis. 2017, 2, 48. https://doi.org/10.3390/tropicalmed2030048
Franka R, Carson WC, Ellison JA, Taylor ST, Smith TG, Kuzmina NA, Kuzmin IV, Marissen WE, Rupprecht CE. In Vivo Efficacy of a Cocktail of Human Monoclonal Antibodies (CL184) Against Diverse North American Bat Rabies Virus Variants. Tropical Medicine and Infectious Disease. 2017; 2(3):48. https://doi.org/10.3390/tropicalmed2030048
Chicago/Turabian StyleFranka, Richard, William C. Carson, James A. Ellison, Steven T. Taylor, Todd G. Smith, Natalia A. Kuzmina, Ivan V. Kuzmin, Wilfred E. Marissen, and Charles E. Rupprecht. 2017. "In Vivo Efficacy of a Cocktail of Human Monoclonal Antibodies (CL184) Against Diverse North American Bat Rabies Virus Variants" Tropical Medicine and Infectious Disease 2, no. 3: 48. https://doi.org/10.3390/tropicalmed2030048




