Effect of Fatigue on Electromyographic Activity Patterns of the Knee Joint Muscles in Anterior Cruciate Ligament Reconstructed and Deficient Patients during Landing Task
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Experimental Design
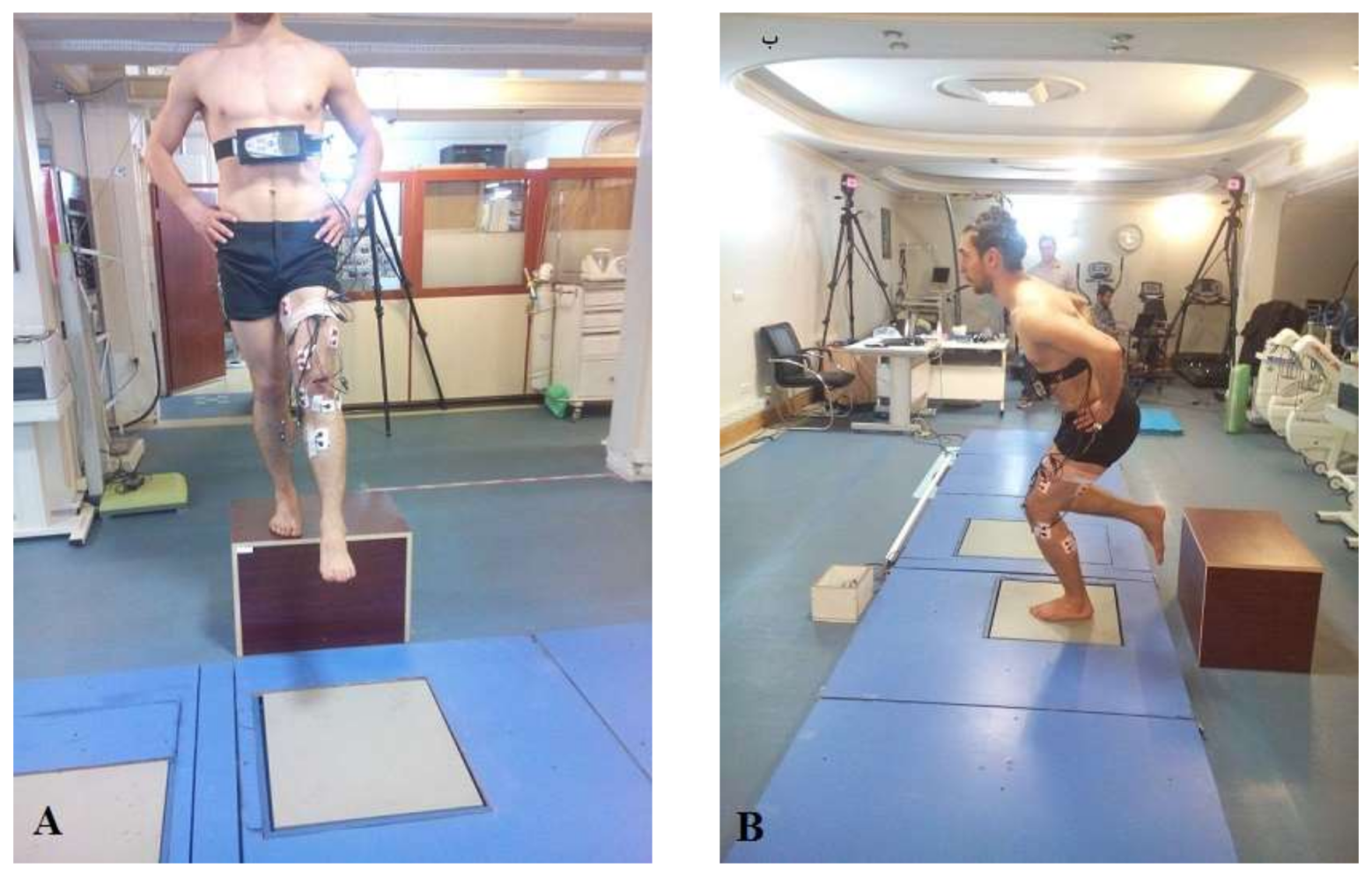
2.3. Testing Procedures
2.4. Data Analysis
2.5. Statistical Analysis
3. Results
3.1. Muscle Activity Onset Time
3.2. Reactive Muscle Activity
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ACLR | Anterior cruciate ligament reconstructed |
| ACLD | Anterior cruciate ligament deficit |
| NA | Not applicable |
| PT | Patellar tendon |
| STG | Semitendinosus/gracilis |
| VM | Vastus medialis: |
| VL | Vastus lateralis |
| MH | Medial hamstring |
| LH | Lateral hamstring |
| MG | Medial gastrocnemius |
| LG | Lateral gastrocnemius. |
References
- Ardern, C.L.; Webster, K.E.; Taylor, N.F.; Feller, J.A. Return to sport following anterior cruciate ligament reconstruction surgery: A systematic review and meta-analysis of the state of play. Br. J. Sports Med. 2011, 45, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, A.; Francisco, R. Factors affecting return to sports after anterior cruciate ligament reconstruction with patellar tendon and hamstring graft: A prospective clinical investigation. Knee Surg. Sports Traumatol. Arthrosc. 2006, 14, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Myklebust, G.; Holm, I.; Mæhlum, S.; Engebretsen, L.; Bahr, R. Clinical, functional, and radiologic outcome in team handball players 6 to 11 years after anterior cruciate ligament injury: A follow-up study. Am. J. Sports Med. 2003, 31, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Strehl, A.; Eggli, S. The value of conservative treatment in ruptures of the anterior cruciate ligament (ACL). J. Trauma Acute Care Surg. 2007, 62, 1159–1162. [Google Scholar] [CrossRef] [PubMed]
- Blagojevic, M.; Jinks, C.; Jeffery, A.; Jordan, K. Risk factors for onset of osteoarthritis of the knee in older adults: A systematic review and meta-analysis. Osteoarthr. Cartil. 2010, 18, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Luc, B.; Gribble, P.A.; Pietrosimone, B.G. Osteoarthritis prevalence following anterior cruciate ligament reconstruction: A systematic review and numbers-needed-to-treat analysis. J. Athl. Train. 2014, 49, 806–819. [Google Scholar] [CrossRef] [PubMed]
- Di Stasi, S.; Myer, G.D.; Hewett, T.E. Neuromuscular training to target deficits associated with second anterior cruciate ligament injury. J. Orthop. Sports Phys. Ther. 2013, 43, 777–792. [Google Scholar] [CrossRef] [PubMed]
- Lass, P.; Kaalund, S.; Iefevre, S.; Arendt-Nielsen, L.; Sinkjæ, T.; Simonsen, O. Muscle coordination following rupture of the anterior cruciate ligament: Electromyographic studies of 14 patients. Acta Orthop. Scand. 1991, 62, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Gokeler, A.; Hof, A.; Arnold, M.; Dijkstra, P.; Postema, K.; Otten, E. Abnormal landing strategies after ACL reconstruction. Scand. J. Med. Sci. Sports 2010, 20, e12–e19. [Google Scholar] [CrossRef] [PubMed]
- Klyne, D.M.; Keays, S.L.; Bullock-Saxton, J.E.; Newcombe, P.A. The effect of anterior cruciate ligament rupture on the timing and amplitude of gastrocnemius muscle activation: A study of alterations in EMG measures and their relationship to knee joint stability. J. Electromyogr. Kinesiol. 2012, 22, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Lindström, M.; Felländer-Tsai, L.; Wredmark, T.; Henriksson, M. Adaptations of gait and muscle activation in chronic ACL deficiency. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Solomonow, M.; Krogsgaard, M. Sensorimotor control of knee stability: A review. Scand. J. Med. Sci. Sports 2001, 11, 64–80. [Google Scholar] [CrossRef] [PubMed]
- Rozzi, S.L.; Lephart, S.M.; Fu, F.H. Effects of muscular fatigue on knee joint laxity and neuromuscular characteristics of male and female athletes. J. Athl. Train. 1999, 34, 106. [Google Scholar] [PubMed]
- Wojtys, E.M.; Wylie, B.B.; Huston, L.J. The effects of muscle fatigue on neuromuscular function and anterior tibial translation in healthy knees. Am. J. Sports Med. 1996, 24, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Lepley, L.K.; Thomas, A.C.; McLean, S.G.; Palmieri-Smith, R.M. Fatigue’s Lack of Effect on Thigh-Muscle Activity in Anterior Cruciate Ligament–Reconstructed Patients During a Dynamic-Landing Task. J. Sport Rehabil. 2013, 22, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Lessi, G.C.; Serrão, F.V. Effects of fatigue on lower limb, pelvis and trunk kinematics and lower limb muscle activity during single-leg landing after anterior cruciate ligament reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 2550–2558. [Google Scholar] [CrossRef] [PubMed]
- Berchuck, M.; Andriacchi, T.; Bach, B.; Reider, B. Gait adaptations by patients who have a deficient anterior cruciate ligament. J. Bone Jt. Surg. 1990, 72, 871–877. [Google Scholar] [CrossRef]
- Swanik, C.B.; Lephart, S.M.; Giraldo, J.L.; DeMont, R.G.; Fu, F.H. Reactive muscle firing of anterior cruciate ligament-injured females during functional activities. J. Athl. Train. 1999, 34, 121. [Google Scholar] [PubMed]
- Van Lent, M.; Drost, M.; van den Wildenberg, F. EMG profiles of ACL-deficient patients during walking: The influence of mild fatigue. Int. J. Sports Med. 1994, 15, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, T.L.; Hurd, W.J.; Rudolph, K.S.; Axe, M.J.; Snyder-Mackler, L. Perturbation training improves knee kinematics and reduces muscle co-contraction after complete unilateral anterior cruciate ligament rupture. Phys. Ther. 2005, 85, 740–749. [Google Scholar] [PubMed]
- Webster, K.E.; Santamaria, L.J.; Mcclelland, J.A.; Feller, J.A. Effect of fatigue on landing biomechanics after anterior cruciate ligament reconstruction surgery. Med. Sci. Sports Exerc. 2012, 44, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Hefti, E.; Müller, W.; Jakob, R.; Stäubli, H.-U. Evaluation of knee ligament injuries with the IKDC form. Knee Surg. Sports Traumatol. Arthrosc. 1993, 1, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Borotikar, B.S.; Newcomer, R.; Koppes, R.; McLean, S.G. Combined effects of fatigue and decision making on female lower limb landing postures: Central and peripheral contributions to ACL injury risk. Clin. Biomech. 2008, 23, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Gokeler, A.; Eppinga, P.; Dijkstra, P.; Welling, W.; Padua, D.; Otten, E.; Benjaminse, A. Effect of fatigue on landing performance assessed with the landing error scoring system (less) in patients after ACL reconstruction: A pilot study. Int. J. Sports Phys. Ther. 2014, 9, 302. [Google Scholar] [PubMed]
- Mclean, S.G.; Samorezov, J.E. Fatigue-induced ACL injury risk stems from a degradation in central control. Med. Sci. Sports Exerc. 2009, 41, 1661–1672. [Google Scholar] [CrossRef] [PubMed]
- Horita, T.; Komi, P.; Nicol, C.; Kyröläinen, H. Interaction between pre-landing activities and stiffness regulation of the knee joint musculoskeletal system in the drop jump: Implications to performance. Eur. J. Appl. Physiol. 2002, 88, 76–84. [Google Scholar] [PubMed]
- Pfeifer, K.; Banzer, W. Motor performance in different dynamic tests in knee rehabilitation. Scand. J. Med. Sci. Sports 1999, 9, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Dyhre-Poulsen, P.; Simonsen, E.B.; Voigt, M. Dynamic control of muscle stiffness and H reflex modulation during hopping and jumping in man. J. Physiol. 1991, 437, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Bryant, A.L.; Newton, R.U.; Steele, J. Successful feed-forward strategies following ACL injury and reconstruction. J. Electromyogr. Kinesiol. 2009, 19, 988–997. [Google Scholar] [CrossRef] [PubMed]
- Di Fabio, R.; Graf, B.; Badke, M.; Breunig, A.; Jensen, K. Effect of knee joint laxity on long-loop postural reflexes: Evidence for a human capsular-hamstring reflex. Exp. Brain Res. 1992, 90, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Webster, K.E.; Wittwer, J.E.; O’brien, J.; Feller, J.A. Gait patterns after anterior cruciate ligament reconstruction are related to graft type. Am. J. Sports Med. 2005, 33, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, K.; Axe, M.; Snyder-Mackler, L. Dynamic stability after ACL injury: Who can hop? Knee Surg. Sports Traumatol. Arthrosc. 2000, 8, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Sinkjær, T.; Arendt-Nielsen, L. Knee stability and muscle coordination in patients with anterior cruciate ligament injuries: An electromyographic approach. J. Electromyogr. Kinesiol. 1991, 1, 209–217. [Google Scholar] [CrossRef]
- Ciccotti, M.G.; Kerlan, R.K.; Perry, J.; Pink, M. An electromyographic analysis of the knee during functional activities: II. The anterior cruciate ligament-deficient and-reconstructed profiles. Am. J. Sports Med. 1994, 22, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Woods, J.; Furbush, F.; Bigland-Ritchie, B. Evidence for a fatigue-induced reflex inhibition of motoneuron firing rates. J. Neurophysiol. 1987, 58, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Padua, D.A.; Arnold, B.L.; Perrin, D.H.; Gansneder, B.M.; Carcia, C.R.; Granata, K.P. Fatigue, vertical leg stiffness, and stiffness control strategies in males and females. J. Athl. Train. 2006, 41, 294. [Google Scholar] [PubMed]
- Fleming, B.C.; Renstrom, P.A.; Ohlen, G.; Johnson, R.J.; Peura, G.D.; Beynnon, B.D.; Badger, G.J. The gastrocnemius muscle is an antagonist of the anterior cruciate ligament. J. Orthop. Res. 2001, 19, 1178–1184. [Google Scholar] [CrossRef]

| ACLD | ACLR | Control | p | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| Age (year) | 24.5 ± 2.32 | 23.83 ± 5.49 | 24.92 ± 2.81 | 0.78 |
| Height (cm) | 174.5 ± 4.62 | 175.25 ± 4.78 | 175.00 ± 5.23 | 0.77 |
| Mass (kg) | 75.25 ± 7.13 | 76.45 ± 5.93 | 74.75 ± 7.50 | 0.65 |
| Months since surgery or initial injury | 23.25 ± 6.95 | 23.75 ± 6.30 | NA | |
| Graft type | NA | PT = 4 | NA | |
| STG = 6 | ||||
| Allograft = 2 | ||||
| Injury grade | Grade 2 = 7 | NA | NA | |
| Grade 3 = 5 |
| Level | Sports Activity | Occupation Activity |
|---|---|---|
| I | Jumping, cutting, pivoting (soccer, team handball, basketball) | Activity comparable to Level I sports |
| II | Lateral movements, less pivoting than Level I (racket sports, martial arts, wrestling, gymnastics, aerobics) | Heavy manual labor, working on uneven surfaces |
| III | Straight ahead activities, no jumping or pivoting (running, mountaineering, weightlifting) | Light manual work |
| IV | Sedentary | Activities of daily living |
| Muscles | Pre-Fatigue | Post-Fatigue |
|---|---|---|
| Mean ± SD | Mean ± SD | |
| VM | ||
| ACLD | 168.21 ± 85.23 | 121.53 ± 33.47 |
| ACLR | 119.77 ± 97.39 | 167.58 ± 81.98 |
| Control | 101.22 ± 57.17 | 100.03 ± 38.06 |
| VL a,b | ||
| ACLD | 122.03 ± 55.81 | 107.62 ± 47.81 |
| ACLR | 111.65 ± 83.06 | 194.79 ± 119.35 |
| Control | 89.16 ± 55.41 | 95.04 ± 44.69 |
| MH b | ||
| ACLD | 108.93 ± 56.87 | 134.41 ± 38.65 |
| ACLR | 148.65 ± 81.18 | 163.79 ± 85.59 |
| Control | 99.46 ± 36.21 | 100.77 ± 35.19 |
| LH a,b | ||
| ACLD | 186.00 ± 99.75 | 158.13 ± 61.82 |
| ACLR | 142.10 ± 92.79 | 227.75 ± 104.99 |
| Control | 117.42 ± 42.36 | 125.60 ± 50.57 |
| MG | ||
| ACLD | 214.95 ± 83.03 | 242.09 ± 93.05 |
| ACLR | 286.83 ± 99.33 | 335.06 ± 123.59 |
| Control | 239.36 ± 70.95 | 270.65 ± 128.80 |
| LG | ||
| ACLD | 167.81 ± 71.61 | 203.89 ± 89.36 |
| ACLR | 186.91 ± 101.06 | 193.90 ± 79.94 |
| Control | 208.02 ± 69.33 | 179.54 ± 74.57 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dashti Rostami, K.; Alizadeh, M.H.; Minoonejad, H.; Yazdi, H.; Thomas, A. Effect of Fatigue on Electromyographic Activity Patterns of the Knee Joint Muscles in Anterior Cruciate Ligament Reconstructed and Deficient Patients during Landing Task. J. Funct. Morphol. Kinesiol. 2018, 3, 22. https://doi.org/10.3390/jfmk3020022
Dashti Rostami K, Alizadeh MH, Minoonejad H, Yazdi H, Thomas A. Effect of Fatigue on Electromyographic Activity Patterns of the Knee Joint Muscles in Anterior Cruciate Ligament Reconstructed and Deficient Patients during Landing Task. Journal of Functional Morphology and Kinesiology. 2018; 3(2):22. https://doi.org/10.3390/jfmk3020022
Chicago/Turabian StyleDashti Rostami, Komeil, Mohammad Hossein Alizadeh, Hooman Minoonejad, Hamidreza Yazdi, and Abbey Thomas. 2018. "Effect of Fatigue on Electromyographic Activity Patterns of the Knee Joint Muscles in Anterior Cruciate Ligament Reconstructed and Deficient Patients during Landing Task" Journal of Functional Morphology and Kinesiology 3, no. 2: 22. https://doi.org/10.3390/jfmk3020022




