A Newborn with Arterial Tortuosity Syndrome: The Importance of Timely Diagnostic Work-Up in Patients Presenting with Cutis Laxa
Abstract
:1. Introduction
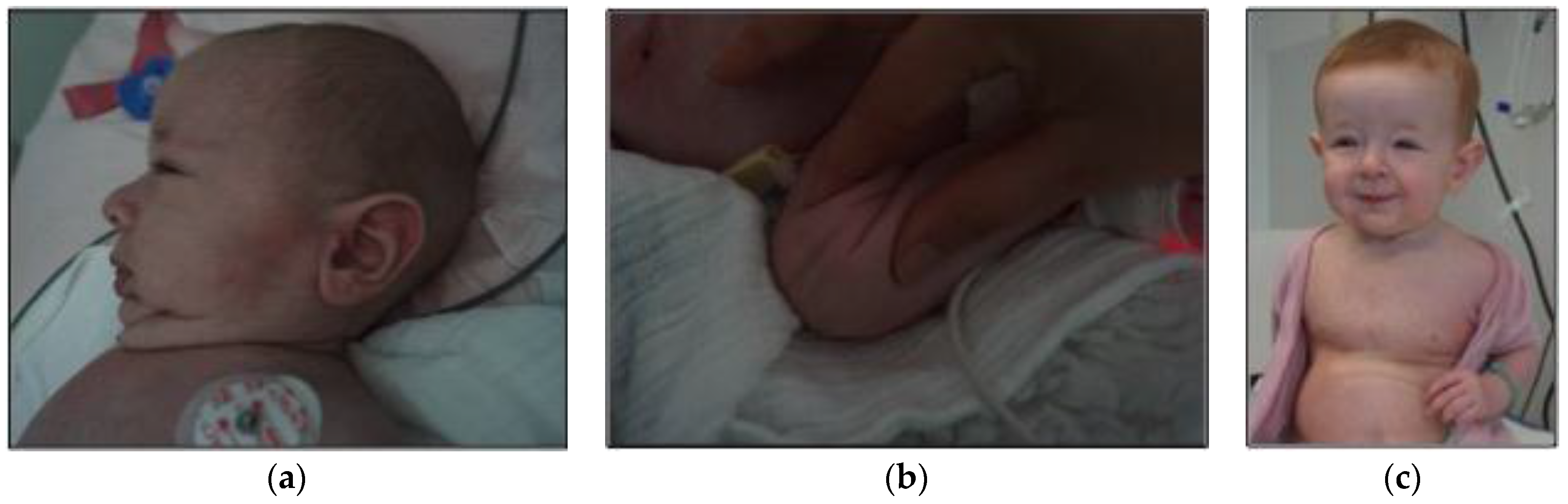
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| ATS | arterial tortuosity syndrome |
| GLUT10 | glucose transporter 10 |
| DHA | l-dehydroascorbic acid |
| PAS | pulmonary artery stenosis |
| CT | computer tomography |
| EEG | electroencephalography |
References
- Callewaert, B.; de Paepe, A.; Coucke, P. Arterial tortuosity syndrome. In GeneReviews®; Pagon, R.A., Adam, M.P., Ardinger, H.H., Wallace, S.E., Amemiya, A., Bean, L.J.H., Bird, T.D., Fong, C.T., Mefford, H.C., Smith, R.J.H., et al., Eds.; University of Washington: Seattle, WA, USA, 2014. [Google Scholar]
- Callewaert, B.L.; Willaert, A.; Kerstjens-Frederikse, W.S.; de Backer, J.; Devriendt, K.; Albrecht, B.; Ramos-Arroyo, M.A.; Doco-Fenzy, M.; Hennekam, R.C.; Pyeritz, R.E.; et al. Arterial tortuosity syndrome: Clinical and molecular findings in 12 newly identified families. Hum. Mutat. 2008, 29, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Huang, H.Y.; Chang, C.J.; Cheng, C.H.; Chen, Y.T. Mitochondrial GLUT10 facilitates dehydroascorbic acid import and protects cells against oxidative stress: Mechanistic insight into arterial tortuosity syndrome. Hum. Mol. Genet. 2010, 19, 3721–3733. [Google Scholar] [CrossRef] [PubMed]
- Castori, M.; Ritelli, M.; Zoppi, N.; Molisso, L.; Chiarelli, N.; Zaccagna, F.; Grammatico, P.; Colombi, M. Adult presentation of arterial tortuosity syndrome in a 51-year-old woman with a novel homozygous c.1411+1G>A mutation in the SLC2A10 gene. Am. J. Med. Genet. A 2012, 158A, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- Al-Khaldi, A.; Mohammed, Y.; Tamimi, O.; Alharbi, A. Early outcomes of total pulmonary arterial reconstruction in patients with arterial tortuosity syndrome. Ann. Thorac. Surg. 2011, 92, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Fujii, K.; Yoshida, A.; Morisaki, H.; Kohno, Y.; Morisaki, T. Artery tortuosity syndrome exhibiting early-onset emphysema with novel compound heterozygous SLC2A10 mutations. Am. J. Med. Genet. A 2013, 161A, 856–859. [Google Scholar] [CrossRef] [PubMed]
- Moceri, P.; Albuisson, J.; Saint-Faust, M.; Casagrande, F.; Giuliano, F.; Devos, C.; Benoit, P.; Hugues, N.; Ducreux, D.; Cerboni, P.; et al. Arterial tortuosity syndrome: Early diagnosis and association with venous tortuosity. J. Am. Coll. Cardiol. 2013, 61. [Google Scholar] [CrossRef] [PubMed]
- Ritelli, M.; Chiarelli, N.; Dordoni, C.; Reffo, E.; Venturini, M.; Quinzani, S.; Monica, M.D.; Scarano, G.; Santoro, G.; Russo, M.G.; et al. Arterial tortuosity syndrome: Homozygosity for two novel and one recurrent SLC2A10 missense mutations in three families with severe cardiopulmonary complications in infancy and a literature review. BMC Med. Genet. 2014, 15. [Google Scholar] [CrossRef] [PubMed]
- Ekici, F.; Uçar, T.; Fitöz, S.; Atalay, S.; Tutar, E. Cardiovascular findings in a boy with arterial tortuosity syndrome: Case report and review of the literature. Turk. J. Pediatr. 2011, 53, 104–107. [Google Scholar] [PubMed]
- Wessels, M.W.; Catsman-Berrevoets, C.E.; Mancini, G.M.; Breuning, M.H.; Hoogeboom, J.J.; Stroink, H.; Frohn-Mulder, I.; Coucke, P.J.; Paepe, A.D.; Niermeijer, M.F.; et al. Three new families with arterial tortuosity syndrome. Am. J. Med. Genet. A 2004, 131, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Allen, V.M.; Horne, S.G.; Penney, L.S.; Rapchuk, I.L.; Brock, J.A.; Thompson, D.L.; Stinson, D.A. Successful outcome in pregnancy with arterial tortuosity syndrome. Obstet. Gynecol. 2009, 114, 494–498. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pichler, K.; Ralser, E.; Resch, M.; Steichen-Gersdorf, E.; Maurer, K.; Trawöger, R.; Kiechl-Kohlendorfer, U. A Newborn with Arterial Tortuosity Syndrome: The Importance of Timely Diagnostic Work-Up in Patients Presenting with Cutis Laxa. J. Funct. Morphol. Kinesiol. 2016, 1, 249-253. https://doi.org/10.3390/jfmk1020249
Pichler K, Ralser E, Resch M, Steichen-Gersdorf E, Maurer K, Trawöger R, Kiechl-Kohlendorfer U. A Newborn with Arterial Tortuosity Syndrome: The Importance of Timely Diagnostic Work-Up in Patients Presenting with Cutis Laxa. Journal of Functional Morphology and Kinesiology. 2016; 1(2):249-253. https://doi.org/10.3390/jfmk1020249
Chicago/Turabian StylePichler, Karin, Elisabeth Ralser, Maria Resch, Elisabeth Steichen-Gersdorf, Kathrin Maurer, Rudolf Trawöger, and Ursula Kiechl-Kohlendorfer. 2016. "A Newborn with Arterial Tortuosity Syndrome: The Importance of Timely Diagnostic Work-Up in Patients Presenting with Cutis Laxa" Journal of Functional Morphology and Kinesiology 1, no. 2: 249-253. https://doi.org/10.3390/jfmk1020249




