Effects of Synthetic Anti-Inflammatory Sterol in CB3V-Induced Myocarditis: A Morphological Study on Heart Muscle Tissue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs
2.3. Experimental Design
- (1)
- HE3286-treated mice (n = 12);
- (2)
- Dex-treated mice (n = 12);
- (3)
- HERF405-treated mice (n = 12);
- (4)
- Control (healthy) mice (n = 5).
2.4. Histology and Histochemistry
2.5. Immunohistochemistry (IHC)
2.6. Evaluation of Immunohistochemistry
2.7. Statistical Analysis
3. Results
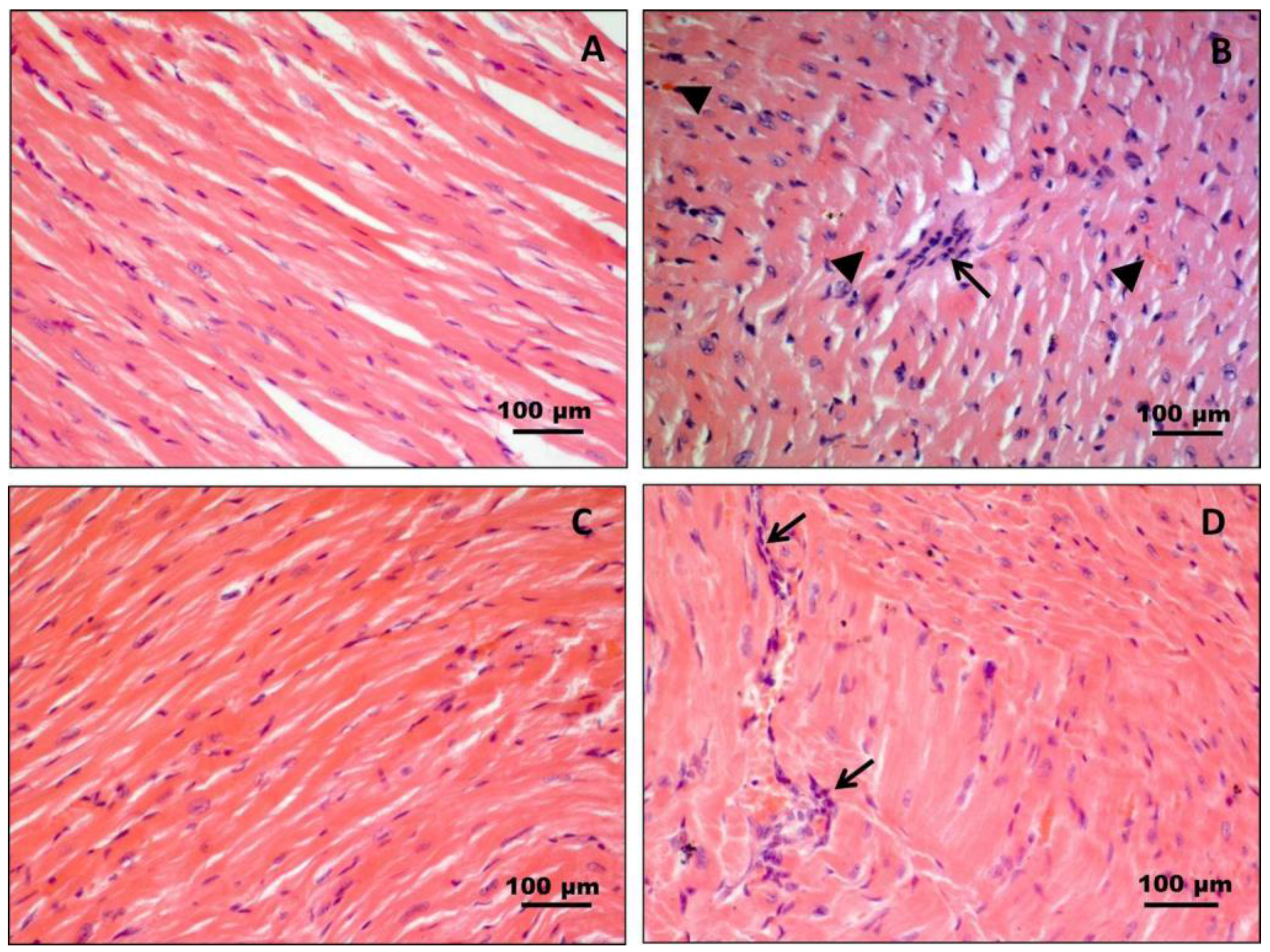
3.1. Histology and Histochemistry
3.2. Immunohistochemistry (IHC)
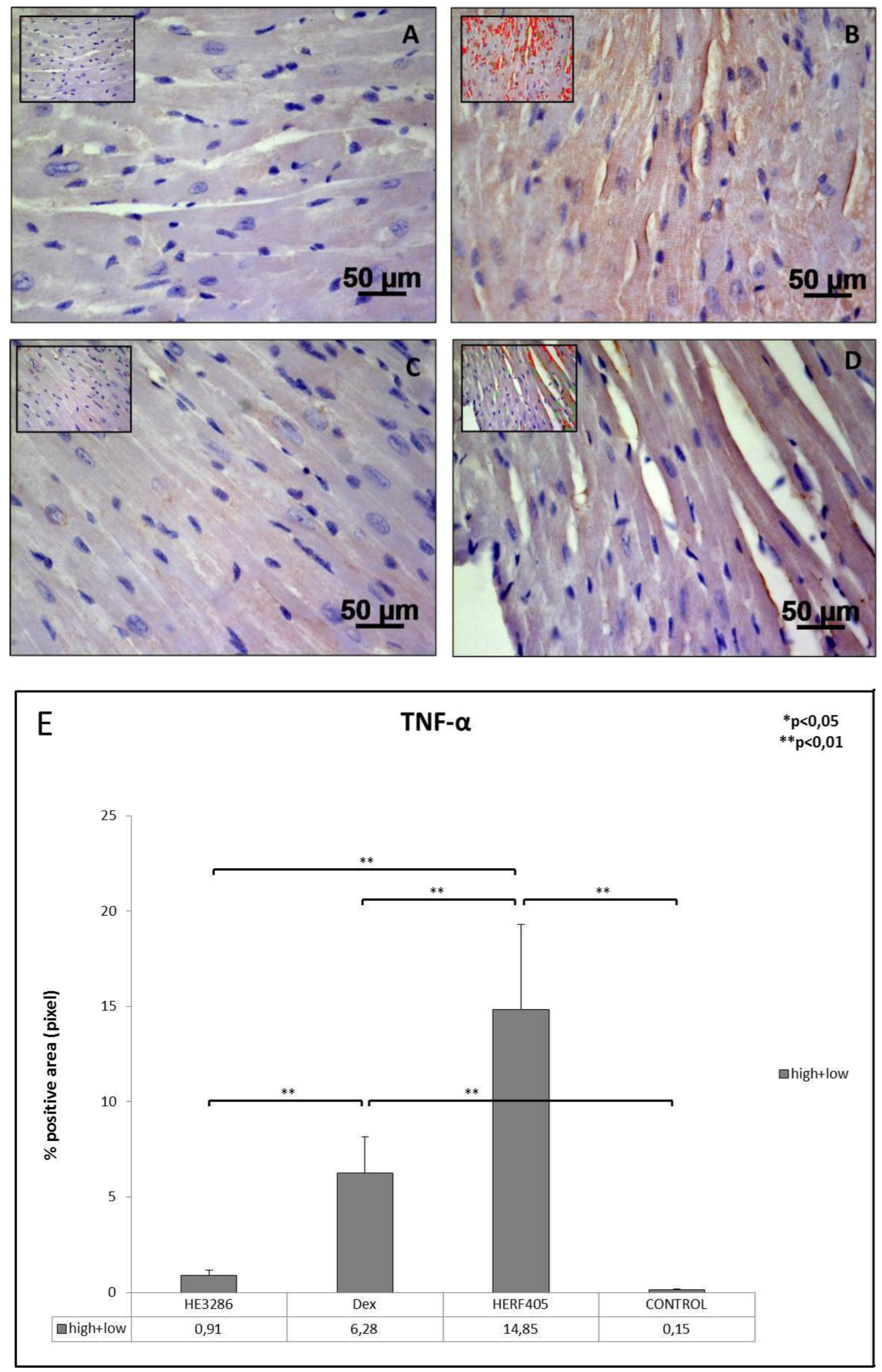
3.2.1. TNF-α
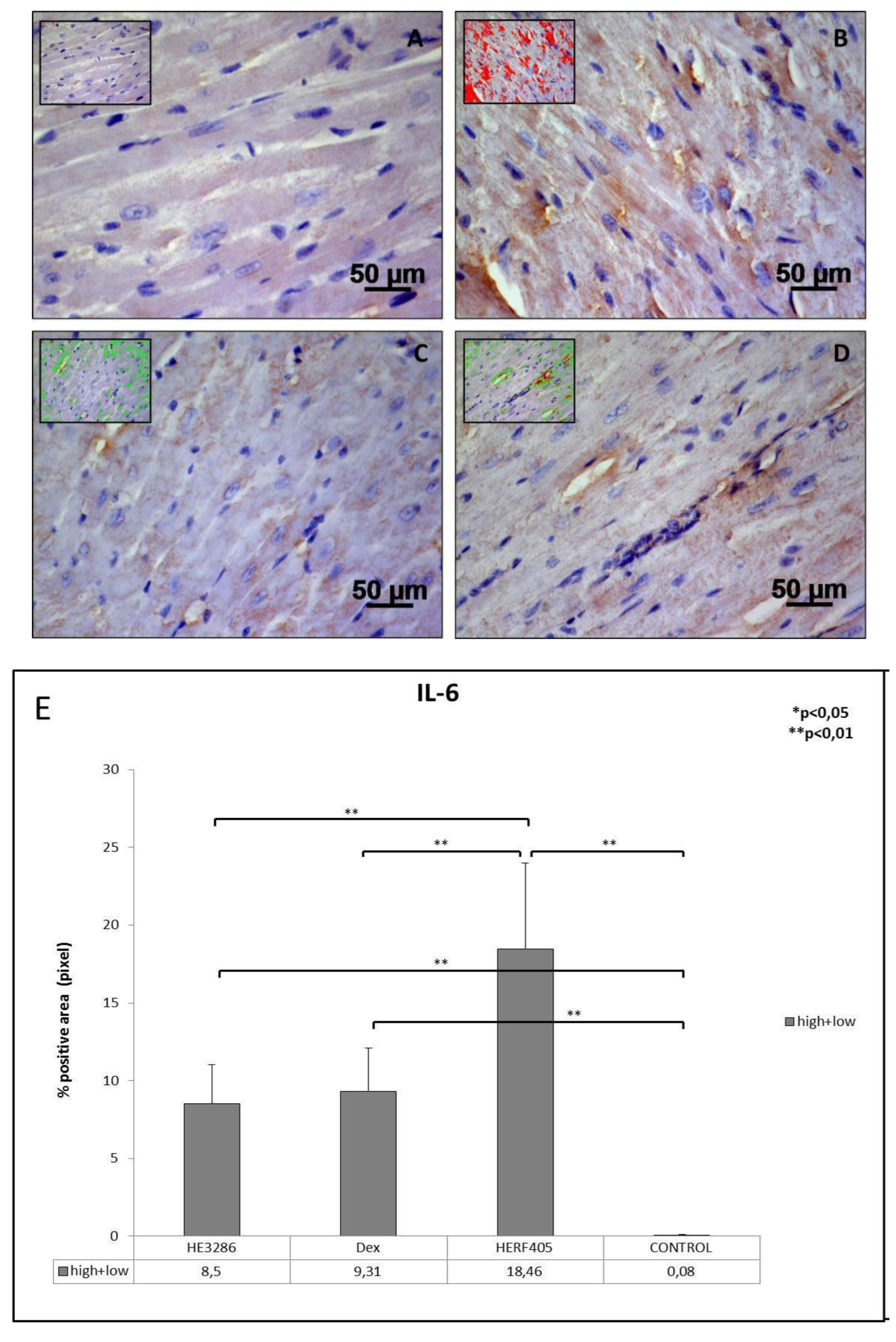
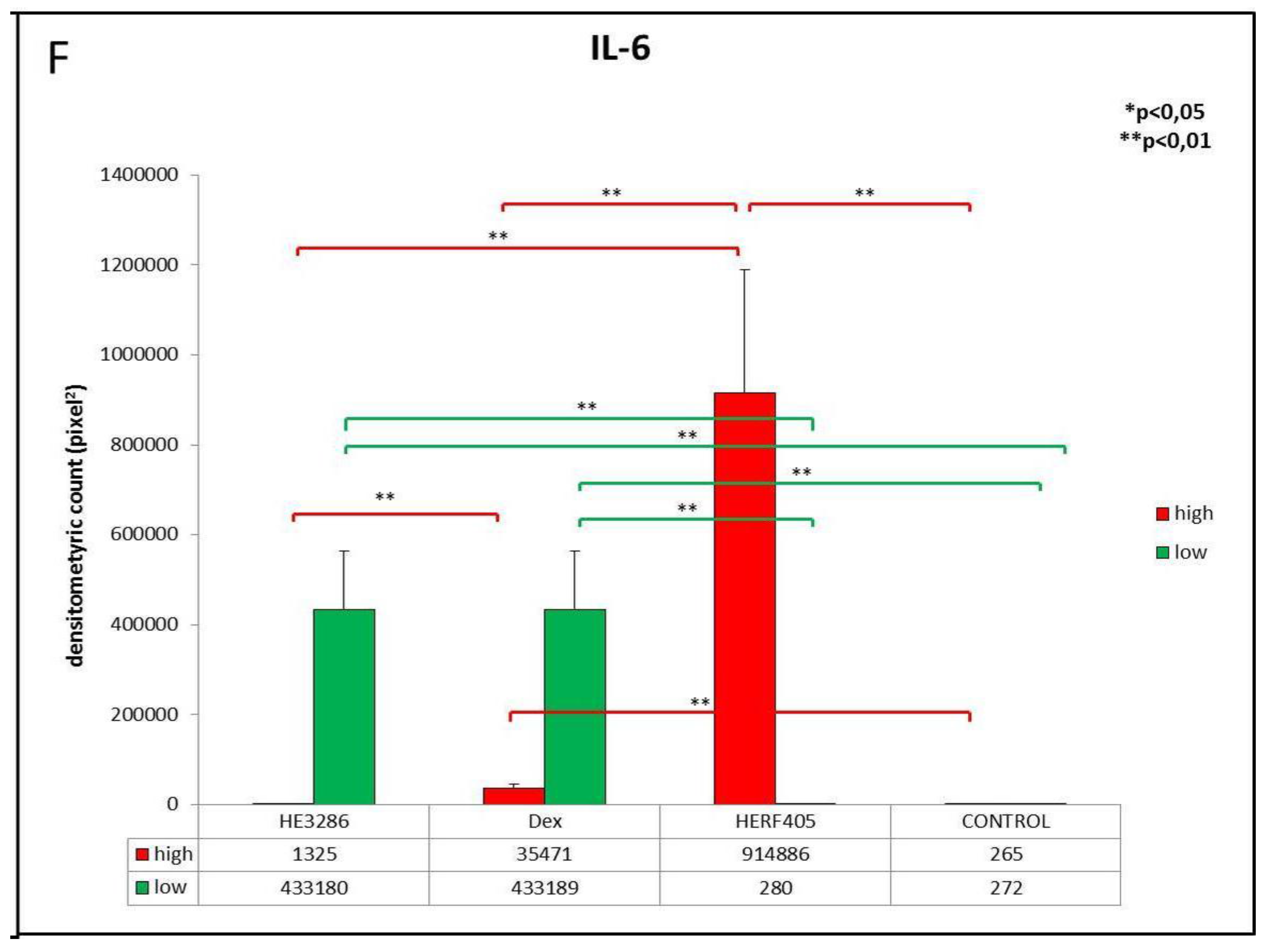
3.2.2. IL-6
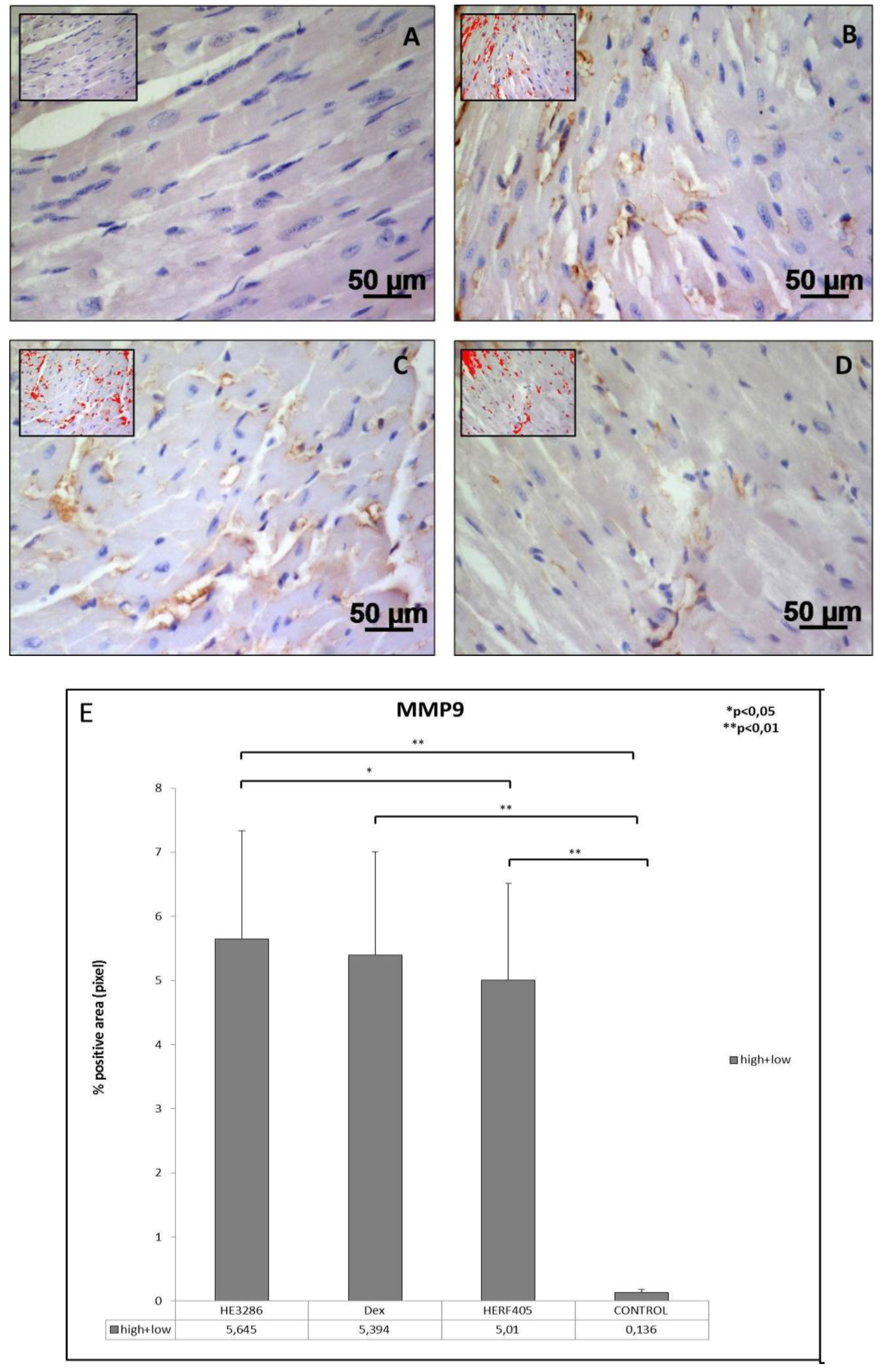
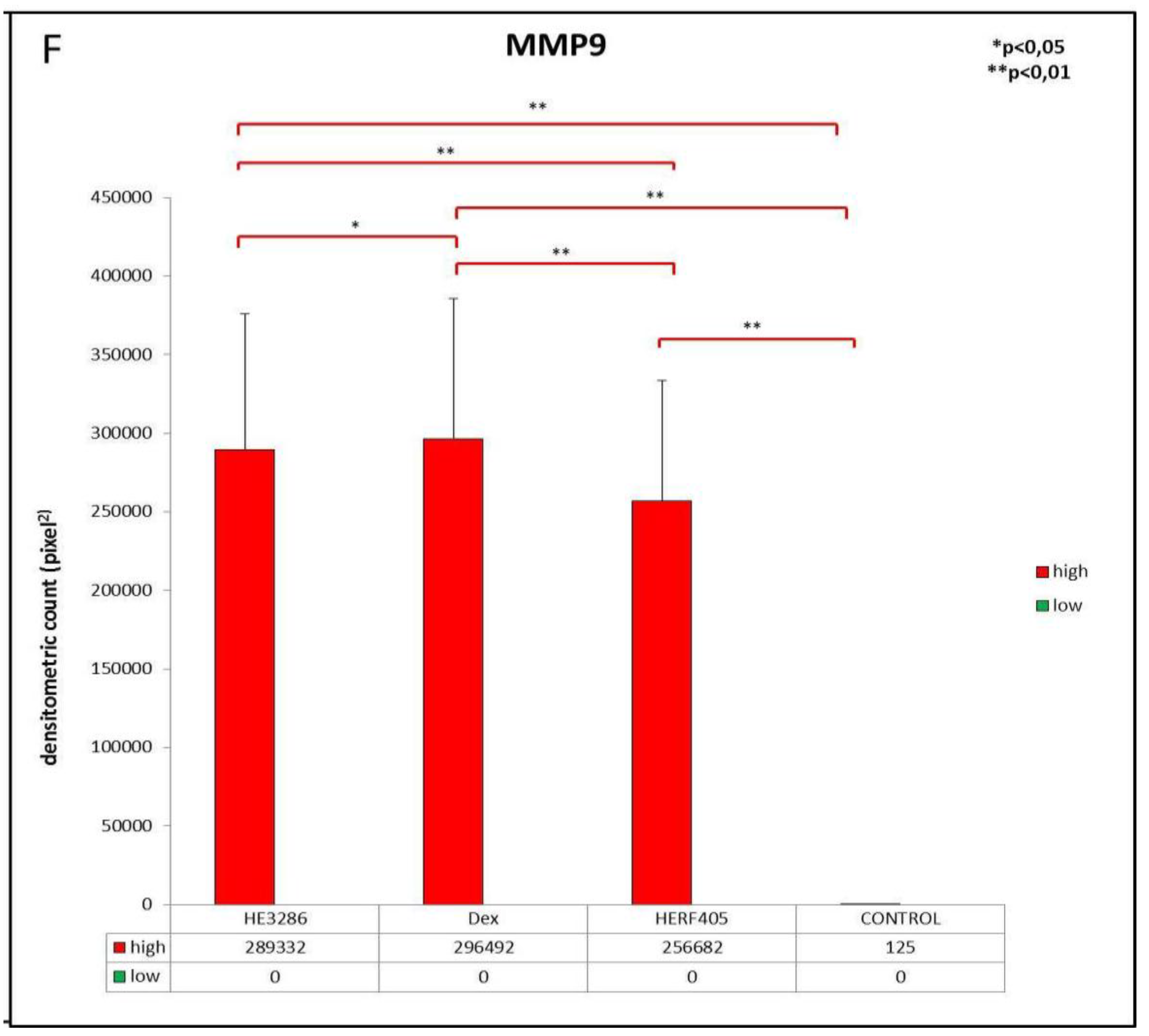
3.2.3. MMP9
3.2.4. ADAM10
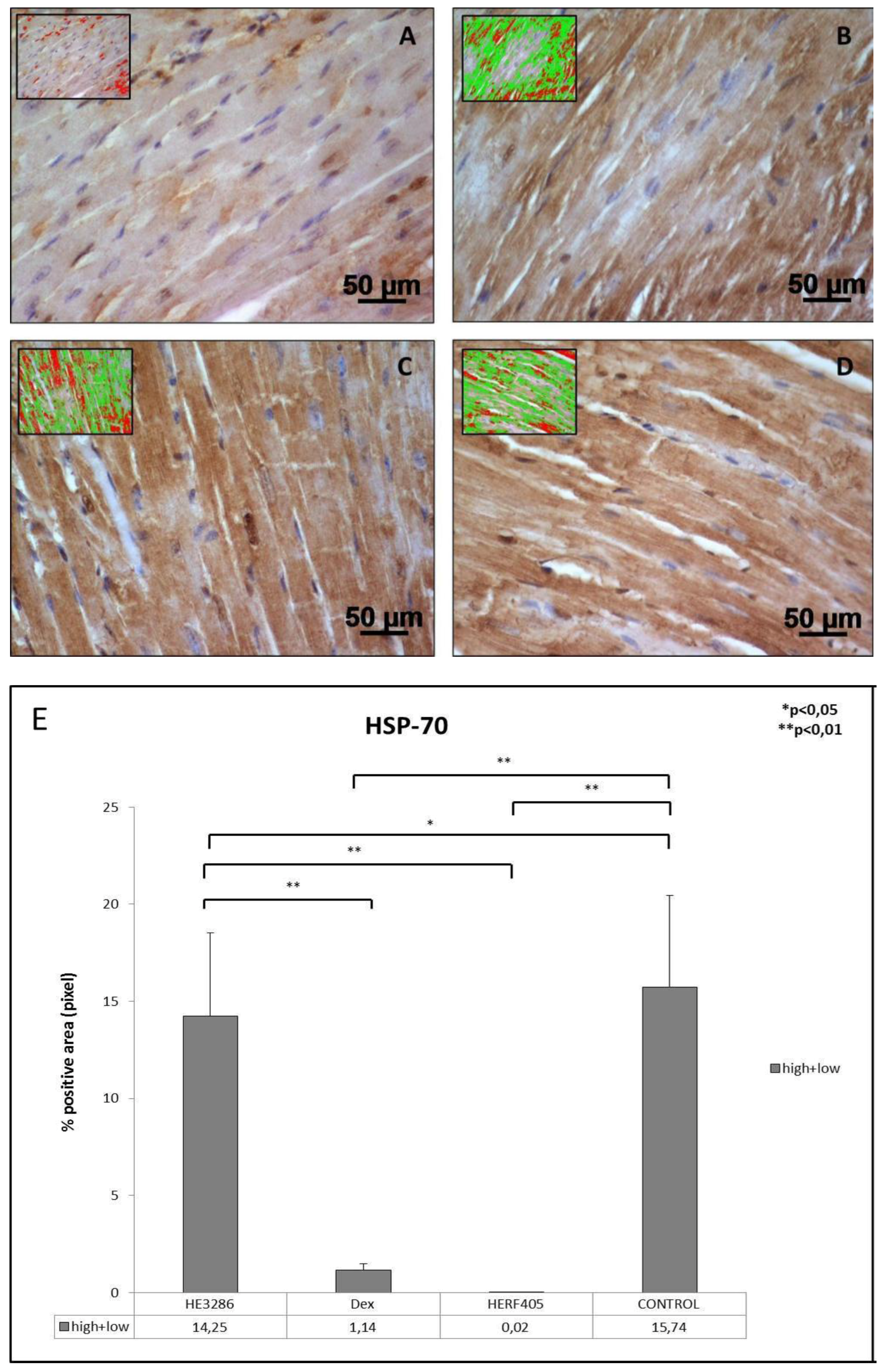
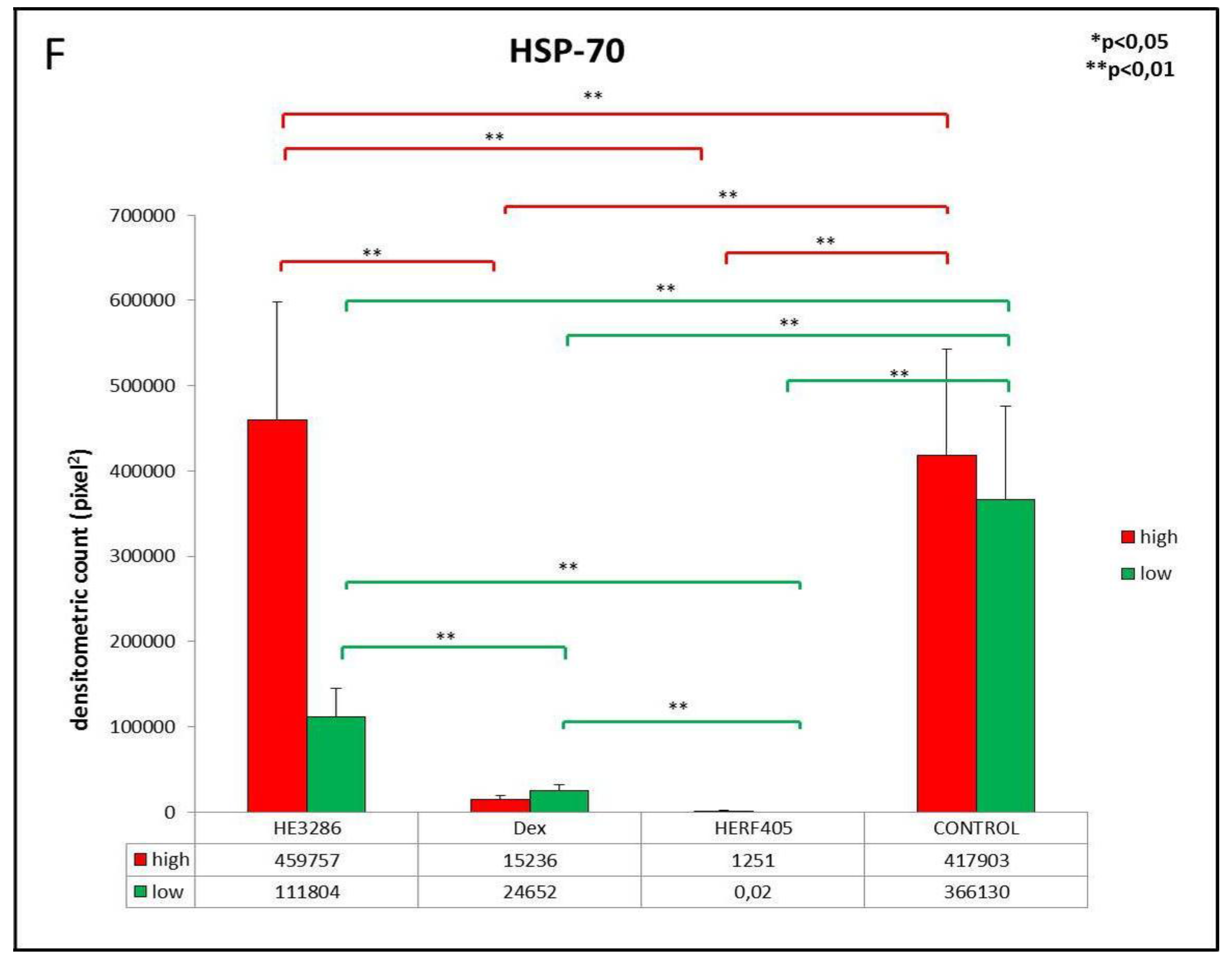
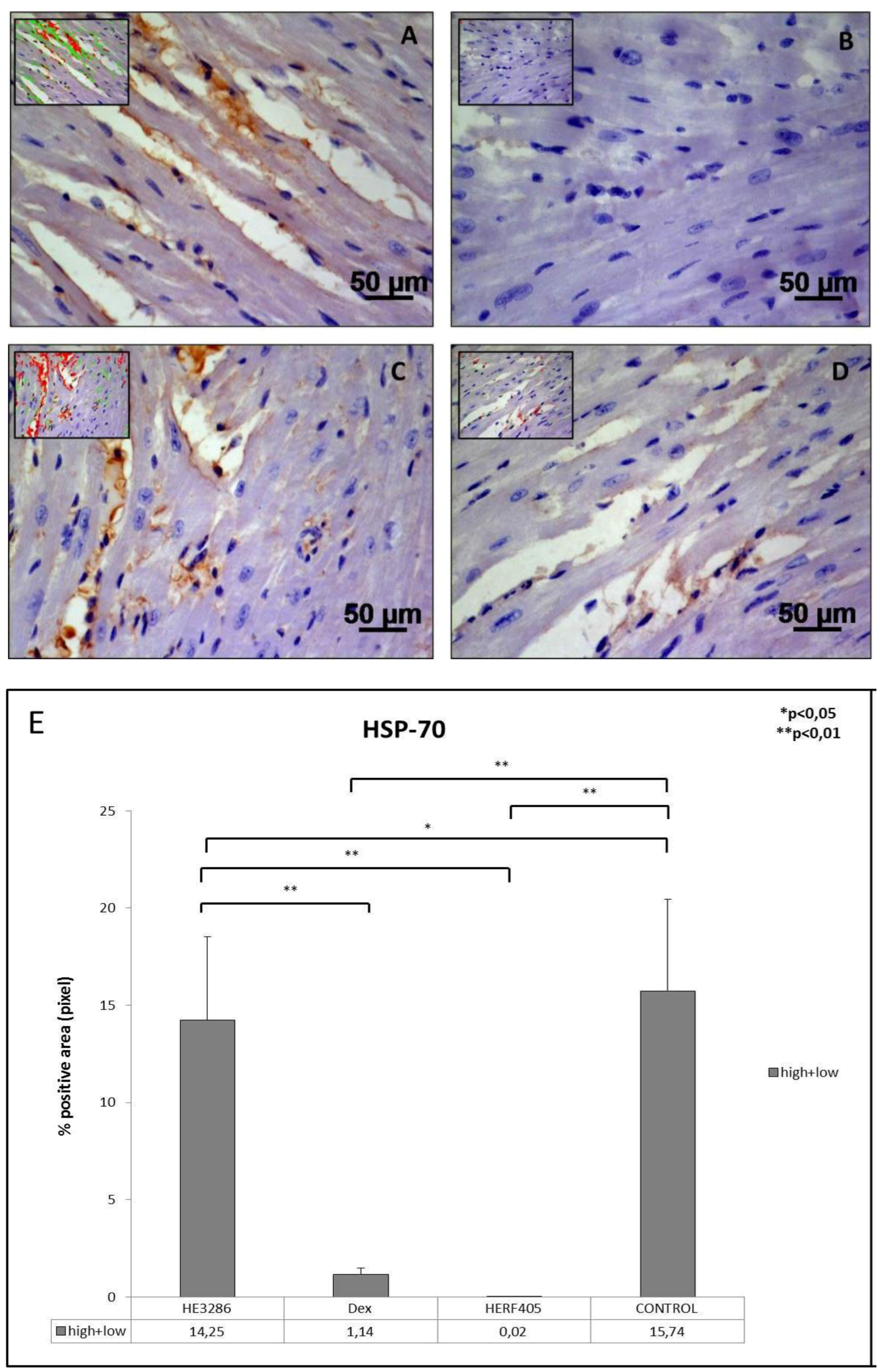
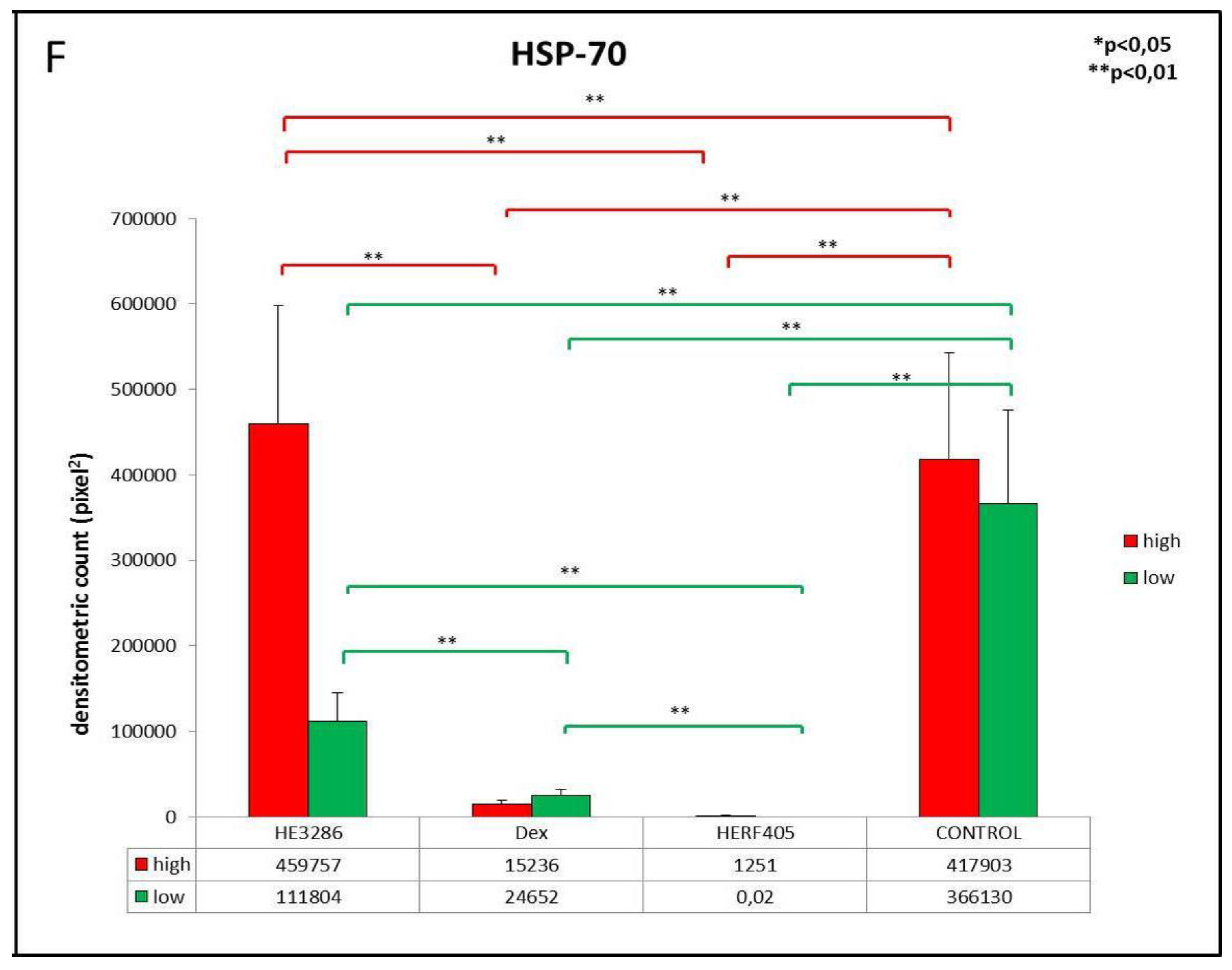
3.2.5. HSP-70
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gouda, Z.A.; Elewa, Y.H.A.; Selim, A.O. Histological architecture of cardiac myofibers composing the left ventricle of murine heart. J. Histol. Histopathol. 2015, 2. [Google Scholar] [CrossRef]
- Kühl, U.; Schultheiss, H.-P.P. Viral myocarditis. Swiss Med. Wkly. 2014, 144. [Google Scholar] [CrossRef] [PubMed]
- Schultz, J.C.; Hilliard, A.A.; Cooper, L.T.; Rihal, C.S. Diagnosis and treatment of viral myocarditis. Mayo Clin. Proc. 2009, 84, 1001–1009. [Google Scholar] [CrossRef]
- Nakamura, H.; Kunitsugu, I.; Fukuda, K.; Matsuzaki, M.; Sano, M. Diverse stage-dependent effects of glucocorticoids in a murine model of viral myocarditis. J. Cardiol. 2013, 61, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Aziz, K.U.; Patel, N.; Sadullah, T.; Tasneem, H.; Thawerani, H.; Talpur, S. Acute viral myocarditis: Role of immunosuppression: A prospective randomised study. Cardiol. Young 2010, 20, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Moreels, M.; Delforge, M.-L.L.; Renard, M. Fulminant myocarditis with dramatic response to corticoids. Acta Cardiol. 2010, 65, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.S.; Dine, K.; Luna, E.; Ahlem, C.; Shindler, K.S. HE3286 reduces axonal loss and preserves retinal ganglion cell function in experimental optic neuritis. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5744–5751. [Google Scholar] [CrossRef] [PubMed]
- Auci, D.; Kaler, L.; Subramanian, S.; Huang, Y.; Frincke, J.; Reading, C.; Offner, H. A new orally bioavailable synthetic androstene inhibits collagen-induced arthritis in the mouse: Androstene hormones as regulators of regulatory T cells. Ann. N. Y. Acad. Sci. 2007, 1110, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Auci, D.L.; Reading, C.L.; Frincke, J.M. 7-hydroxy androstene steroids and a novel synthetic analogue with reduced side effects as a potential agent to treat autoimmune diseases. Autoimmunity Rev. 2009, 8, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Hegen, M.; Keith, J.C.; Collins, M.; Nickerson-Nutter, C.L. Utility of animal models for identification of potential therapeutics for rheumatoid arthritis. Ann. Rheumatic Dis. 2008, 67, 1505–1515. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, F.; Philippens, I.; Fagone, P.; Ahlem, C.N.; Reading, C.L.; Frincke, J.M.; Auci, D.L. 17α-ethynyl-androst-5-ene-3β,7β,17β-triol (HE3286) is neuroprotective and reduces motor impairment and neuroinflammation in a murine mptp model of parkinson’s disease. Parkinson’s Dis. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Offner, H.; Firestein, G.S.; Boyle, D.L.; Pieters, R.; Frincke, J.M.; Garsd, A.; White, S.K.; Reading, C.L.; Auci, D.L. An orally bioavailable synthetic analog of an active dehydroepiandrosterone metabolite reduces established disease in rodent models of rheumatoid arthritis. J. Pharmacol. Exp. Ther. 2009, 329, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Reading, C.L.; Flores-Riveros, J.; Stickney, D.R.; Frincke, J.M. An anti-inflammatory sterol decreases obesity-related inflammation-induced insulin resistance and metabolic dysregulation. Med. Inflamm. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Reading, C.L.; Stickney, D.R.; Flores-Riveros, J.; Destiche, D.A.; Ahlem, C.N.; Cefalu, W.T.; Frincke, J.M. A synthetic anti-inflammatory sterol improves insulin sensitivity in insulin-resistant obese impaired glucose tolerance subjects. Obesity 2013, 21. [Google Scholar] [CrossRef] [PubMed]
- Corsten, M.F.; Schroen, B.; Heymans, S. Inflammation in viral myocarditis: Friend or foe? Trends Mol. Med. 2012, 18, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Fairweather, D.; Frisancho-Kiss, S.; Gatewood, S.; Njoku, D.; Steele, R.; Barrett, M.; Rose, N.R. Mast cells and innate cytokines are associated with susceptibility to autoimmune heart disease following coxsackievirus B3 infection. Autoimmunity 2004, 37, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.R.; Neumann, D.A.; Lafond-Walker, A.; Herskowitz, A.; Rose, N.R. Role of IL-1 and tumor necrosis factor in coxsackie virus-induced autoimmune myocarditis. J. Immunol. 1993, 151, 1682–1690. [Google Scholar] [PubMed]
- Rose, N.R. Critical cytokine pathways to cardiac inflammation. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 2011, 31, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Rehren, F.; Ritter, B.; Dittrich-Breiholz, O.; Henke, A.; Lam, E.; Kati, S.; Kracht, M.; Heim, A. Induction of a broad spectrum of inflammation-related genes by coxsackievirus B3 requires interleukin-1 signaling. Med. Microbiol. Immunol. 2013, 202, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Rutschow, S.; Leschka, S.; Westermann, D.; Puhl, K.; Weitz, A.; Ladyszenskij, L.; Jaeger, S.; Zeichhardt, H.; Noutsias, M.; Schultheiss, H.-P.P.; et al. Left ventricular enlargement in coxsackievirus-B3 induced chronic myocarditis—Ongoing inflammation and an imbalance of the matrix degrading system. Eur. J. Pharmacol. 2010, 630, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Jibiki, T.; Terai, M.; Tateno, S.; Toyozaki, T.; Horie, H.; Nakajima, H.; Niwa, K.; Niimi, H. Expression of tumor necrosis factor-α protein in the myocardium in fatal myocarditis. Pediatr. Int. Off. J. Jpn. Pediatr. Soc. 2000, 42, 43–47. [Google Scholar] [CrossRef]
- Torre-Amione, G.; Kapadia, S.; Lee, J.; Durand, J.B.; Bies, R.D.; Young, J.B.; Mann, D.L. Tumor necrosis factor-α and tumor necrosis factor receptors in the failing human heart. Circulation 1996, 93, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.C. Interrelation between expression of ADAM 10 and MMP 9 and synthesis of peroxynitrite in doxorubicin induced cardiomyopathy. Biomol. Ther. 2013, 21, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Castrogiovanni, P.; Imbesi, R. Oxidative stress and skeletal muscle in exercise. Ital. J. Anat. Embryol. 2012, 117, 107–117. [Google Scholar] [PubMed]
- Musumeci, G.; Castrogiovanni, P.; Trovato, F.M.; Parenti, R.; Szychlinska, M.A.; Imbesi, R. Pregnancy, embryo-fetal development and nutrition: Physiology around fetal programming. J. Histol. Histopathol. 2015, 2. [Google Scholar] [CrossRef]
- Musumeci, G.; Maria Trovato, F.; Imbesi, R.; Castrogiovanni, P. Effects of dietary extra-virgin olive oil on oxidative stress resulting from exhaustive exercise in rat skeletal muscle: A morphological study. Acta Histochem. 2014, 116, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, A.-P.P.; Heymans, S. Interactions between the extracellular matrix and inflammation during viral myocarditis. Immunobiology 2012, 217, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Senf, S.M.; Howard, T.M.; Ahn, B.; Ferreira, L.F.; Judge, A.R. Loss of the inducible Hsp70 delays the inflammatory response to skeletal muscle injury and severely impairs muscle regeneration. PLoS ONE 2013, 8, e62687. [Google Scholar]
- Musumeci, G.; Coleman, R.; Imbesi, R.; Magro, G.; Parenti, R.; Szychlinska, M.A.; Scuderi, R.; Cinà, C.S.; Castorina, S.; Castrogiovanni, P. ADAM-10 could mediate cleavage of n-cadherin promoting apoptosis in human atherosclerotic lesions leading to vulnerable plaque: A morphological and immunohistochemical study. Acta Histochem. 2014, 116, 1148–1158. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Loreto, C.; Imbesi, R.; Szychlinska, M.A.; Castrogiovanni, P. N-cadherin, ADAM-10 and aquaporin1 expression in lung tissue exposed to fluoro-edenite fibres: An immunohistochemical study. J. Histol. Histopathol. 2015, 30, 987–999. [Google Scholar]
- Cheung, P.Y.; Sawicki, G.; Wozniak, M.; Wang, W.; Radomski, M.W.; Schulz, R. Matrix metalloproteinase-2 contributes to ischemia-reperfusion injury in the heart. Circulation 2000, 101, 1833–1839. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.L.; Spinale, F.G. Activation of matrix metalloproteinases in the failing human heart: Breaking the tie that binds. Circulation 1998, 98, 1699–1702. [Google Scholar] [CrossRef] [PubMed]
- Spinale, F.G. Matrix metalloproteinases: Regulation and dysregulation in the failing heart. Circ. Res. 2002, 90, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.V.; Coker, M.L.; Zellner, J.L.; Handy, J.R.; Crumbley, A.J.; Spinale, F.G. Increased matrix metalloproteinase activity and selective upregulation in LV myocardium from patients with end-stage dilated cardiomyopathy. Circulation 1998, 97, 1708–1715. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, A.; Postma, D.S.; Noordhoek, J.A.; Lodewijk, M.E.; Kauffman, H.F.; ten Hacken, N.H.; Timens, W. Expression of ADAMs (“a disintegrin and metalloprotease”) in the human lung. Virch. Arch. Int. J. Pathol. 2009, 454, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Reiss, K.; Saftig, P. The “a disintegrin and metalloprotease” (ADAM) family of sheddases: Physiological and cellular functions. Semin. Cell Dev. Biol. 2009, 20, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Tousseyn, T.; Jorissen, E.; Reiss, K.; Hartmann, D. (Make) stick and cut loose—Disintegrin metalloproteases in development and disease. Birth Defects Res. Part C Embryo Today Rev. 2006, 78, 24–46. [Google Scholar] [CrossRef] [PubMed]
- Kohutek, Z.A.; di Pierro, C.G.; Redpath, G.T.; Hussaini, I.M. ADAM-10-mediated n-cadherin cleavage is protein kinase C-α dependent and promotes glioblastoma cell migration. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 4605–4615. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Imbesi, R.; Magro, G.; Parenti, R.; Szychlinska, M.A.; Scuderi, R.; Castorina, S.; Castrogiovanni, P. N-cadherin has a protective role in stable human atherosclerotic plaques: A morphological and immunohistochemical study. J. Histol. Histopathol. 2014, 1. [Google Scholar] [CrossRef]
- Hikita, A.; Tanaka, N.; Yamane, S.; Ikeda, Y.; Furukawa, H.; Tohma, S.; Suzuki, R.; Tanaka, S.; Mitomi, H.; Fukui, N. Involvement of a disintegrin and metalloproteinase 10 and 17 in shedding of tumor necrosis factor-α. Biochem. Cell Biol. 2009, 87, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Hundhausen, C.; Schulte, A.; Schulz, B.; Andrzejewski, M.G.; Schwarz, N.; von Hundelshausen, P.; Winter, U.; Paliga, K.; Reiss, K.; Saftig, P.; et al. Regulated shedding of transmembrane chemokines by the disintegrin and metalloproteinase 10 facilitates detachment of adherent leukocytes. J. Immunol. 2007, 178, 8064–8072. [Google Scholar] [CrossRef] [PubMed]
- Schulte, M.; Reiss, K.; Lettau, M.; Maretzky, T.; Ludwig, A.; Hartmann, D.; de Strooper, B.; Janssen, O.; Saftig, P. ADAM10 regulates fasl cell surface expression and modulates fasl-induced cytotoxicity and activation-induced cell death. Cell Death Differ. 2007, 14, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Schulz, B.; Pruessmeyer, J.; Maretzky, T.; Ludwig, A.; Blobel, C.P.; Saftig, P.; Reiss, K. ADAM10 regulates endothelial permeability and T-cell transmigration by proteolysis of vascular endothelial cadherin. Circ. Res. 2008, 102, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- Arndt, M.; Lendeckel, U.; Röcken, C.; Nepple, K.; Wolke, C.; Spiess, A.; Huth, C.; Ansorge, S.; Klein, H.U.; Goette, A. Altered expression of ADAMs (a disintegrin and metalloproteinase) in fibrillating human atria. Circulation 2002, 105, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Hunt, M.J.; Aru, G.M.; Hayden, M.R.; Moore, C.K.; Hoit, B.D.; Tyagi, S.C. Induction of oxidative stress and disintegrin metalloproteinase in human heart end-stage failure. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 283. [Google Scholar] [CrossRef] [PubMed]
- Kayani, A.C.; Close, G.L.; Dillmann, W.H.; Mestril, R.; Jackson, M.J.; McArdle, A. Overexpression of HSP10 in skeletal muscle of transgenic mice prevents the age-related fall in maximum tetanic force generation and muscle cross-sectional area. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299. [Google Scholar] [CrossRef] [PubMed]
- Staib, J.L.; Tümer, N.; Powers, S.K. Increased temperature and protein oxidation lead to HSP72 mRNA and protein accumulation in the in vivo exercised rat heart. Exp. Physiol. 2009, 94, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Gulbahar, M.Y.; Kabak, Y.B.; Karayigit, M.O.; Yarim, M.; Guvenc, T.; Parlak, U. The expressions of HSP70 and αb-crystallin in myocarditis associated with foot-and-mouth disease virus in lambs. J. Vet. Sci. 2011, 12, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Radons, J.; Multhoff, G. Immunostimulatory functions of membrane-bound and exported heat shock protein 70. Exerc. Immunol. Rev. 2005, 11, 17–33. [Google Scholar] [PubMed]
- Tsan, M.-F.F.; Gao, B. Heat shock protein and innate immunity. Cell. Mol. Immunol. 2004, 1, 274–279. [Google Scholar] [PubMed]
- Tsan, M.-F.F.; Gao, B. Cytokine function of heat shock proteins. Am. J. Physiol. Cell Physiol. 2004, 286. [Google Scholar] [CrossRef] [PubMed]
- Delogu, G.; Signore, M.; Mechelli, A.; Famularo, G. Heat shock proteins and their role in heart injury. Curr. Opin. Crit. Care 2002, 8, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, S.R.; Gupta, S.; Knowlton, A.A. Cytosolic heat shock protein 60, apoptosis, and myocardial injury. Circulation 2002, 105, 2899–2904. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.; Suryakumar, G.; Prasad, R.; Ganju, L. Upregulation of cytoprotective defense mechanisms and hypoxia-responsive proteins imparts tolerance to acute hypobaric hypoxia. High Alt. Med. Biol. 2013, 14, 65–77. [Google Scholar] [CrossRef] [PubMed]
- McArdle, A.; Vasilaki, A.; Jackson, M. Exercise and skeletal muscle ageing: Cellular and molecular mechanisms. Ageing Res. Rev. 2002, 1, 79–93. [Google Scholar] [CrossRef]
- Ahlem, C.N.; Kennedy, M.R.; Page, T.M.; Reading, C.L.; White, S.K.; McKenzie, J.J.; Cole, P.I.; Stickney, D.R.; Frincke, J.M. Studies of the pharmacology of 17α-ethynyl-androst-5-ene-3β,7β,17β-triol, a synthetic anti-inflammatory androstene. Int. J. Clin. Exp. Med. 2011, 4, 119–135. [Google Scholar] [PubMed]
- Auci, D.L.; Mangano, K.; Destiche, D.; White, S.K.; Huang, Y.; Boyle, D.; Frincke, J.; Reading, C.L.; Nicoletti, F. Oral treatment with HE3286 ameliorates disease in rodent models of rheumatoid arthritis. Int. J. Mol. Med. 2010, 25, 625–633. [Google Scholar] [PubMed]
- Ahlem, C.; Auci, D.; Mangano, K.; Reading, C.; Frincke, J.; Stickney, D.; Nicoletti, F. HE3286: A novel synthetic steroid as an oral treatment for autoimmune disease. Ann. N. Y. Acad. Sci. 2009, 1173, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Conrad, D.; Wang, A.; Pieters, R.; Nicoletti, F.; Mangano, K.; van Heeckeren, A.M.; White, S.K.; Frincke, J.M.; Reading, C.L.; Stickney, D.; et al. HE3286, an oral synthetic steroid, treats lung inflammation in mice without immune suppression. J. Inflamm. 2010, 7. [Google Scholar] [CrossRef] [PubMed]
- Lathe, R. Steroid and sterol 7-hydroxylation: Ancient pathways. Steroids 2002, 67, 967–977. [Google Scholar] [CrossRef]
- Wang, T.; Villegas, S.; Huang, Y.; White, S.K.; Ahlem, C.; Lu, M.; Olefsky, J.M.; Reading, C.; Frincke, J.M.; Alleva, D.; et al. Amelioration of glucose intolerance by the synthetic androstene HE3286: Link to inflammatory pathways. J. Pharmacol. Exp. Ther. 2010, 333, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Kosiewicz, M.M.; Auci, D.L.; Fagone, P.; Mangano, K.; Caponnetto, S.; Tucker, C.F.; Azeem, N.; White, S.K.; Frincke, J.M.; Reading, C.L.; et al. HE3286, an orally bioavailable synthetic analogue of an active DHEA metabolite suppresses spontaneous autoimmune diabetes in the non-obese diabetic (nod) mouse. Eur. J. Pharmacol. 2011, 658, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Zwar, T.D.; van Driel, I.R.; Gleeson, P.A. Guarding the immune system: Suppression of autoimmunity by CD4+CD25+ immunoregulatory T cells. Immunol. Cell Biol. 2006, 84, 487–501. [Google Scholar] [CrossRef] [PubMed]
- Lohr, J.; Knoechel, B.; Wang, J.J.; Villarino, A.V.; Abbas, A.K. Role of IL-17 and regulatory T lymphocytes in a systemic autoimmune disease. J. Exp. Med. 2006, 203, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Marin, V.; Montero-Julian, F.A.; Grès, S.; Boulay, V.; Bongrand, P.; Farnarier, C.; Kaplanski, G. The IL-6-soluble IL-6α autocrine loop of endothelial activation as an intermediate between acute and chronic inflammation: An experimental model involving thrombin. J. Immunol. 2001, 167, 3435–3442. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Nakanishi, Y. The pathogenesis of copd: Lessons learned from in vivo animal models. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2007, 13, RA19–RA24. [Google Scholar]
- Koehler, D.R.; Downey, G.P.; Sweezey, N.B.; Tanswell, A.K.; Hu, J. Lung inflammation as a therapeutic target in cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 2004, 31, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Moon, C.; Lee, H.W.; Park, E.-M.M.; Cho, M.-S.S.; Kang, J.L. Src tyrosine kinases mediate activations of NF-κB and integrin signal during lipopolysaccharide-induced acute lung injury. J. Immunol. 2007, 179, 7001–7011. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Patsouris, D.; Li, P.; Flores-Riveros, J.; Frincke, J.M.; Watkins, S.; Schenk, S.; Olefsky, J.M. A new antidiabetic compound attenuates inflammation and insulin resistance in zucker diabetic fatty rats. Am. J. Physiol. Endocrinol. Metabol. 2010, 298. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, K.A.; Chapman, N.M.; Carson, S.D. Caspase-3 activation and ERK phosphorylation during CVB3 infection of cells: Influence of the coxsackievirus and adenovirus receptor and engineered variants. Virus Res. 2003, 92, 179–186. [Google Scholar] [CrossRef]
- Huber, M.; Watson, K.A.; Selinka, H.C.; Carthy, C.M.; Klingel, K.; McManus, B.M.; Kandolf, R. Cleavage of rasgap and phosphorylation of mitogen-activated protein kinase in the course of coxsackievirus B3 replication. J. Virol. 1999, 73, 3587–3594. [Google Scholar] [PubMed]
- Luo, H.; Yanagawa, B.; Zhang, J.; Luo, Z.; Zhang, M.; Esfandiarei, M.; Carthy, C.; Wilson, J.E.; Yang, D.; McManus, B.M. Coxsackievirus B3 replication is reduced by inhibition of the extracellular signal-regulated kinase (ERK) signaling pathway. J. Virol. 2002, 76, 3365–3373. [Google Scholar] [CrossRef] [PubMed]
- Marchant, D.; Sall, A.; Si, X.; Abraham, T.; Wu, W.; Luo, Z.; Petersen, T.; Hegele, R.G.; McManus, B.M. ERK map kinase-activated ARF6 trafficking directs coxsackievirus type B3 into an unproductive compartment during virus host-cell entry. J. Gen. Virol. 2009, 90, 854–862. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castrogiovanni, P.; Trovato, F.M.; Szychlinska, M.A.; Loreto, C.; Giunta, S.; Scuderi, S.; Passanisi, R.; Fidone, F.; Fagone, P.; Imbesi, R.; et al. Effects of Synthetic Anti-Inflammatory Sterol in CB3V-Induced Myocarditis: A Morphological Study on Heart Muscle Tissue. J. Funct. Morphol. Kinesiol. 2016, 1, 69-89. https://doi.org/10.3390/jfmk1010069
Castrogiovanni P, Trovato FM, Szychlinska MA, Loreto C, Giunta S, Scuderi S, Passanisi R, Fidone F, Fagone P, Imbesi R, et al. Effects of Synthetic Anti-Inflammatory Sterol in CB3V-Induced Myocarditis: A Morphological Study on Heart Muscle Tissue. Journal of Functional Morphology and Kinesiology. 2016; 1(1):69-89. https://doi.org/10.3390/jfmk1010069
Chicago/Turabian StyleCastrogiovanni, Paola, Francesca Maria Trovato, Marta Anna Szychlinska, Carla Loreto, Salvatore Giunta, Soraya Scuderi, Roberta Passanisi, Federica Fidone, Paolo Fagone, Rosa Imbesi, and et al. 2016. "Effects of Synthetic Anti-Inflammatory Sterol in CB3V-Induced Myocarditis: A Morphological Study on Heart Muscle Tissue" Journal of Functional Morphology and Kinesiology 1, no. 1: 69-89. https://doi.org/10.3390/jfmk1010069
APA StyleCastrogiovanni, P., Trovato, F. M., Szychlinska, M. A., Loreto, C., Giunta, S., Scuderi, S., Passanisi, R., Fidone, F., Fagone, P., Imbesi, R., Nicoletti, F., & Castorina, S. (2016). Effects of Synthetic Anti-Inflammatory Sterol in CB3V-Induced Myocarditis: A Morphological Study on Heart Muscle Tissue. Journal of Functional Morphology and Kinesiology, 1(1), 69-89. https://doi.org/10.3390/jfmk1010069










