Inventions and Innovations in Preclinical Platforms for Cancer Research
Abstract
:1. Introduction
2. Spheroid Formation Phases
3. Conventional Methods for Spheroid Formation
3.1. Bioreactor Flasks
3.2. Liquid Overlay Method
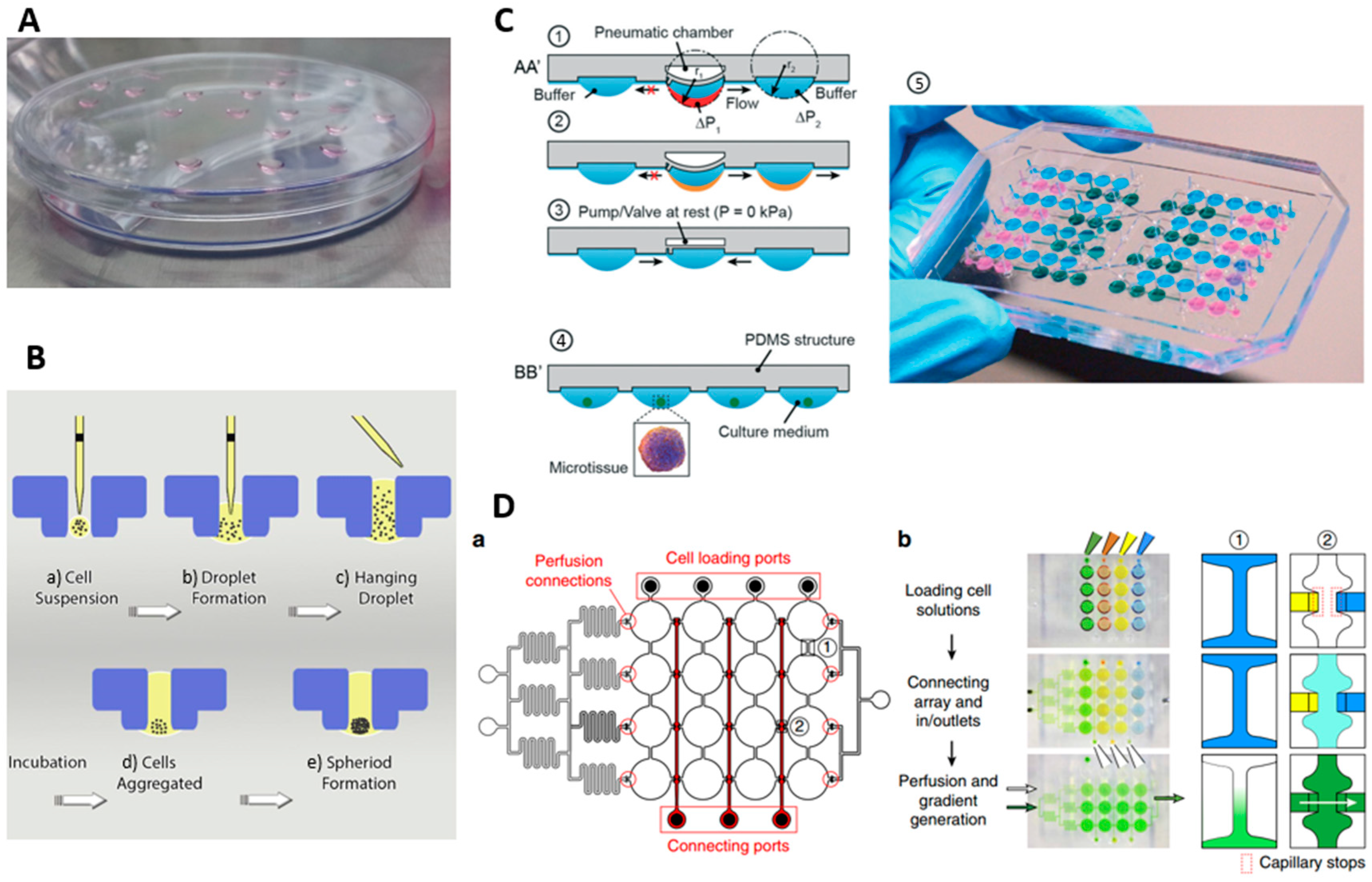
3.3. Hanging Droplet (HD) Method
4. Hydrogels in Spheroid Culture
5. Microfluidic Methods for Spheroid Culture
6. Discussion
7. Conclusions
Conflicts of Interest
References
- Schachtschneider, K.M.; Schwind, R.M.; Newson, J.; Kinachtchouk, N.; Rizko, M.; Mendoza-Elias, N.; Grippo, P.; Principe, D.R.; Park, A.; Overgaard, N.H.; et al. The Oncopig Cancer Model: An Innovative Large Animal Translational Oncology Platform. Front. Oncol. 2017, 7, 190. [Google Scholar] [CrossRef] [PubMed]
- Goers, L.; Freemont, P.; Polizzi, K.M. Co-culture systems and technologies: Taking synthetic biology to the next level. J. R. Soc. Interface 2014, 11, 20140065. [Google Scholar] [CrossRef] [PubMed]
- Trédan, O.; Galmarini, C.M.; Patel, K.; Tannock, I.F. Drug resistance and the solid tumor microenvironment. J. Natl. Cancer Inst. 2007, 99, 1441–1454. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lin, Q.; Glazer, P.M.; Yun, Z. Hypoxic tumor microenvironment and cancer cell differentiation. Curr. Mol. Med. 2009, 9, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015, 3, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.P.; Cabrera, R.M.; Segall, J.E. Tumor cell intravasation. Am. J. Physiol. Cell Physiol. 2016, 311, C1–C14. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Lou, X.; Zhang, Z.; Ingram, P.; Yoon, E. High-throughput cancer cell sphere formation for characterizing the efficacy of photo dynamic therapy in 3D cell cultures. Sci. Rep. 2015, 5, 12175. [Google Scholar] [CrossRef] [PubMed]
- Patra, B.; Peng, C.-C.; Liao, W.-H.; Lee, C.-H.; Tung, Y.-C. Drug testing and flow cytometry analysis on a large number of uniform sized tumor spheroids using a microfluidic device. Sci. Rep. 2016, 6, 21061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuchowska, A.; Jastrzebska, E.; Chudy, M.; Dybko, A.; Brzozka, Z. 3D lung spheroid cultures for evaluation of photodynamic therapy (PDT) procedures in microfluidic Lab-on-a-Chip system. Anal. Chim. Acta 2017, 990, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-T.; Chiang, C.-L.; Chang, C.-H.; Liu, H.-K.; Huang, G.-S.; Huang, R.Y.-J.; Lee, H.; Huang, C.-S.; Wo, A.M. Modeling of cancer metastasis and drug resistance via biomimetic nano-cilia and microfluidics. Biomaterials 2014, 35, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, W.; Ryu, H.; Jeon, N.L. A microfluidic platform for quantitative analysis of cancer angiogenesis and intravasation. Biomicrofluidics 2014, 8, 054102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Moshksayan, K.; Kashaninejad, N.; Warkiani, M.E.; Lock, J.G.; Moghadas, H.; Firoozabadi, B.; Saidi, M.S.; Nguyen, N.-T. Spheroids-on-a-chip: Recent advances and design considerations in microfluidic platforms for spheroid formation and culture. Sens. Actuators B Chem. 2018, 263, 151–176. [Google Scholar] [CrossRef]
- Chi, C.-W.; Ahmed, A.H.R.; Dereli-Korkut, Z.; Wang, S. Microfluidic cell chips for high-throughput drug screening. Bioanalysis 2016, 8, 921–937. [Google Scholar] [CrossRef] [PubMed]
- Ruppen, J.; Cortes-Dericks, L.; Marconi, E.; Karoubi, G.; Schmid, R.A.; Peng, R.; Marti, T.M.; Guenat, O.T. A microfluidic platform for chemoresistive testing of multicellular pleural cancer spheroids. Lab Chip 2014, 14, 1198–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, R.Z.; Chang, H.Y. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol. J. 2008, 3, 1172–1184. [Google Scholar] [CrossRef] [PubMed]
- Ingram, M.; Techy, G.; Saroufeem, R.; Yazan, O.; Narayan, K.; Goodwin, T.; Spaulding, G. Three-dimensional growth patterns of various human tumor cell lines in simulated microgravity of a NASA bioreactor. In Vitro Cell. Dev. Biol. Anim. 1997, 33, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.F.; Zhang, Y.; Ho, Y.-P.; Chiu, Y.-L.; Jung, Y.; Leong, K.W. Rapid formation of multicellular spheroids in double-emulsion droplets with controllable microenvironment. Sci. Rep. 2013, 3, 3462. [Google Scholar] [CrossRef] [PubMed]
- Kojima, N.; Takeuchi, S.; Sakai, Y. Fabrication of microchannel network in liver tissue spheroids. In Proceedings of the 16th International Conference on Miniaturized Systems for Chemistry and Life Sciences, MicroTAS, Okinawa, Japan, 28 October–1 November 2012. [Google Scholar]
- Kehat, I.; Kenyagin-Karsenti, D.; Snir, M.; Segev, H.; Amit, M.; Gepstein, A.; Livne, E.; Binah, O.; Itskovitz-Eldor, J.; Gepstein, L. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J. Clin. Investig. 2001, 108, 407–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.; Kim, C.; Yang, J.Y.; Lee, H.; Ahn, B.; Xu, L.; Kang, J.Y.; Oh, K.W. Gravity-oriented microfluidic device for uniform and massive cell spheroid formation. Biomicrofluidics 2012, 6, 014114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuda, J.; Nakazawa, K. Hepatocyte spheroid arrays inside microwells connected with microchannels. Biomicrofluidics 2011, 5, 022205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ota, H.; Yamamoto, R.; Deguchi, K.; Tanaka, Y.; Kazoe, Y.; Sato, Y.; Miki, N. Three-dimensional spheroid-forming lab-on-a-chip using micro-rotational flow. Sens. Actuators B Chem. 2010, 147, 359–365. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, D.; Liu, H.; Lin, S.; Jiang, Y. Drug cytotoxicity and signaling pathway analysis with three-dimensional tumor spheroids in a microwell-based microfluidic chip for drug screening. Anal. Chim. Acta 2015, 898, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Ruppen, J.; Wildhaber, F.D.; Strub, C.; Hall, S.R.; Schmid, R.A.; Geiser, T.; Guenat, O.T. Towards personalized medicine: Chemosensitivity assays of patient lung cancer cell spheroids in a perfused microfluidic platform. Lab Chip 2015, 15, 3076–3085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torisawa, Y.-S.; Takagi, A.; Nashimoto, Y.; Yasukawa, T.; Shiku, H.; Matsue, T. A multicellular spheroid array to realize spheroid formation, culture, and viability assay on a chip. Biomaterials 2007, 28, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Kelm, J.M.; Timmins, N.E.; Brown, C.J.; Fussenegger, M.; Nielsen, L.K. Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnol. Bioeng. 2003, 83, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Aung, A.; Theprungsirikul, J.; Lim, H.L.; Varghese, S. Chemotaxis-driven assembly of endothelial barrier in a tumor-on-a-chip platform. Lab Chip 2016, 16, 1886–1898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiswald, L.-B.; Bellet, D.; Dangles-Marie, V. Spherical cancer models in tumor biology. Neoplasia 2015, 17, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Frey, O.; Misun, P.M.; Fluri, D.A.; Hengstler, J.G.; Hierlemann, A. Reconfigurable microfluidic hanging drop network for multi-tissue interaction and analysis. Nat. Commun. 2014, 5, 4250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, K.; Lee, J.; Yarmush, M.L.; Parekkadan, B. Microcavity substrates casted from self-assembled microsphere monolayers for spheroid cell culture. Biomed. Microdevices 2014, 16, 609–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziółkowska, K.; Stelmachowska, A.; Kwapiszewski, R.; Chudy, M.; Dybko, A.; Brzózka, Z. Long-term three-dimensional cell culture and anticancer drug activity evaluation in a microfluidic chip. Biosens. Bioelectron. 2013, 40, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-A.; Kang, E.; Ju, J.; Kim, D.-S.; Lee, S.-H. Spheroid-based three-dimensional liver-on-a-chip to investigate hepatocyte–hepatic stellate cell interactions and flow effects. Lab Chip 2013, 13, 3529–3537. [Google Scholar] [CrossRef] [PubMed]
- Haisler, W.L.; Timm, D.M.; Gage, J.A.; Tseng, H.; Killian, T.; Souza, G.R. Three-dimensional cell culturing by magnetic levitation. Nat. Protoc. 2013, 8, 1940–1949. [Google Scholar] [CrossRef] [PubMed]
- Faulkner-Jones, A.; Greenhough, S.; King, J.A.; Gardner, J.; Courtney, A.; Shu, W. Development of a valve-based cell printer for the formation of human embryonic stem cell spheroid aggregates. Biofabrication 2013, 5, 015013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neto, A.; Correia, C.; Oliveira, M.; Rial-Hermida, M.; Alvarez-Lorenzo, C.; Reis, R.; Mano, J. A novel hanging spherical drop system for the generation of cellular spheroids and high throughput combinatorial drug screening. Biomater. Sci. 2015, 3, 581–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allensworth, J.L.; Evans, M.K.; Bertucci, F.; Aldrich, A.J.; Festa, R.A.; Finetti, P.; Ueno, N.T.; Safi, R.; McDonnell, D.P.; Thiele, D.J. Disulfiram (DSF) acts as a copper ionophore to induce copper-dependent oxidative stress and mediate anti-tumor efficacy in inflammatory breast cancer. Mol. Oncol. 2015, 9, 1155–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, G.Y.; Kenny, P.A.; Lee, E.H.; Bissell, M.J. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat. Methods 2007, 4, 359–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ham, S.L.; Joshi, R.; Luker, G.D.; Tavana, H. Engineered breast cancer cell spheroids reproduce biologic properties of solid tumors. Adv. Healthc. Mater. 2016, 5, 2788–2798. [Google Scholar] [CrossRef] [PubMed]
- Vadivelu, R.K.; Ooi, C.H.; Yao, R.-Q.; Velasquez, J.T.; Pastrana, E.; Diaz-Nido, J.; Lim, F.; Ekberg, J.A.; Nguyen, N.-T.; St John, J.A. Generation of three-dimensional multiple spheroid model of olfactory ensheathing cells using floating liquid marbles. Sci. Rep. 2015, 5, 15083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vrij, E.J.; Espinoza, S.; Heilig, M.; Kolew, A.; Schneider, M.; van Blitterswijk, C.; Truckenmüller, R.; Rivron, N.C. 3D high throughput screening and profiling of embryoid bodies in thermoformed microwell plates. Lab Chip 2016, 16, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Santo, V.E.; Estrada, M.F.; Rebelo, S.P.; Abreu, S.; Silva, I.; Pinto, C.; Veloso, S.C.; Serra, A.T.; Boghaert, E.; Alves, P.M. Adaptable stirred-tank culture strategies for large scale production of multicellular spheroid-based tumor cell models. J. Biotechnol. 2016, 221, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, J.; Yuhas, J. Liquid-overlay culture of cellular spheroids. In Spheroids in Cancer Research; Springer: Berlin/Heidelberg, Germany, 1984; pp. 1–23. [Google Scholar]
- Wartenberg, M.; Dönmez, F.; Ling, F.C.; Acker, H.; Hescheler, J.; Sauer, H. Tumor-induced angiogenesis studied in confrontation cultures of multicellular tumor spheroids and embryoid bodies grown from pluripotent embryonic stem cells. FASEB J. 2001, 15, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, J.; Ebner, R.; Kunz-Schughart, L.A. Experimental anti-tumor therapy in 3-D: Spheroids—Old hat or new challenge? Int. J. Radiat. Biol. 2007, 83, 849–871. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, S.L.; Hardin, J.; Amiot, B.; Argikar, U.A.; Remmel, R.P.; Rinaldo, P. Rapid, large-scale formation of porcine hepatocyte spheroids in a novel spheroid reservoir bioartificial liver. Liver Transplant. 2005, 11, 901–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammond, T.; Hammond, J. Optimized suspension culture: The rotating-wall vessel. Am. J. Physiol. Renal Physiol. 2001, 281, F12–F25. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, J.; Seidel, C.; Ebner, R.; Kunz-Schughart, L.A. Spheroid-based drug screen: Considerations and practical approach. Nat. Protoc. 2009, 4, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Sakai, Y.; Nakazawa, K. Technique for the control of spheroid diameter using microfabricated chips. Acta Biomater. 2007, 3, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Timmins, N.E.; Nielsen, L.K. Generation of multicellular tumor spheroids by the hanging-drop method. Tissue Eng. 2007, 141–151. [Google Scholar]
- Ware, M.J.; Colbert, K.; Keshishian, V.; Ho, J.; Corr, S.J.; Curley, S.A.; Godin, B. Generation of homogenous three-dimensional pancreatic cancer cell spheroids using an improved hanging drop technique. Tissue Eng. Part C Methods 2016, 22, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.-C.; Hsiao, A.Y.; Allen, S.G.; Torisawa, Y.-S.; Ho, M.; Takayama, S. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst 2011, 136, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Keller, G.M. In vitro differentiation of embryonic stem cells. Curr. Opin. Cell Biol. 1995, 7, 862–869. [Google Scholar] [CrossRef]
- Yazdi, S.R.; Shadmani, A.; Bürgel, S.C.; Misun, P.M.; Hierlemann, A.; Frey, O. Adding the ‘heart’to hanging drop networks for microphysiological multi-tissue experiments. Lab Chip 2015, 15, 4138–4147. [Google Scholar] [CrossRef] [PubMed]
- Ethier, C.R.; Simmons, C.A. Introductory Biomechanics: From Cells to Organisms; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Andersen, T.; Auk-Emblem, P.; Dornish, M. 3D cell culture in alginate hydrogels. Microarrays 2015, 4, 133–161. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Dini, S.; Dai, S.; Bi, J.; Binder, B.; Green, J.; Zhang, H. A mechanistic study on tumour spheroid formation in thermosensitive hydrogels: Experiments and mathematical modelling. RSC Adv. 2016, 6, 73282–73291. [Google Scholar] [CrossRef]
- Yamada, M.; Hori, A.; Sugaya, S.; Yajima, Y.; Utoh, R.; Yamato, M.; Seki, M. Cell-sized condensed collagen microparticles for preparing microengineered composite spheroids of primary hepatocytes. Lab Chip 2015, 15, 3941–3951. [Google Scholar] [CrossRef] [PubMed]
- Ota, H.; Kodama, T.; Miki, N. Rapid formation of size-controlled three dimensional hetero-cell aggregates using micro-rotation flow for spheroid study. Biomicrofluidics 2011, 5, 034105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.C.; Gupta, M.; Cheung, K.C. Alginate-based microfluidic system for tumor spheroid formation and anticancer agent screening. Biomed. Microdevices 2010, 12, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Lin, B.; Qin, J. Carcinoma-associated fibroblasts promoted tumor spheroid invasion on a microfluidic 3D co-culture device. Lab Chip 2010, 10, 1671–1677. [Google Scholar] [CrossRef] [PubMed]
- Inamori, M.; Mizumoto, H.; Kajiwara, T. An approach for formation of vascularized liver tissue by endothelial cell–covered hepatocyte spheroid integration. Tissue Eng. Part A 2009, 15, 2029–2037. [Google Scholar] [CrossRef] [PubMed]
- Sabhachandani, P.; Motwani, V.; Cohen, N.; Sarkar, S.; Torchilin, V.; Konry, T. Generation and functional assessment of 3D multicellular spheroids in droplet based microfluidics platform. Lab Chip 2016, 16, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Chen, M.C.; Cheung, K.C. Droplet-based microfluidic system for multicellular tumor spheroid formation and anticancer drug testing. Lab Chip 2010, 10, 2424–2432. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.S.; Kwak, B.; Han, B.; Park, K. Development of an in vitro 3D tumor model to study therapeutic efficiency of an anticancer drug. Mol. Pharm. 2013, 10, 2167–2175. [Google Scholar] [CrossRef] [PubMed]
- Eydelnant, I.A.; Li, B.B.; Wheeler, A.R. Microgels on-demand. Nat. Commun. 2014, 5, 3355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.-T.; Hejazian, M.; Ooi, C.H.; Kashaninejad, N. Recent advances and future perspectives on microfluidic liquid handling. Micromachines 2017, 8, 186. [Google Scholar] [CrossRef]
- Kashaninejad, N.; Chan, W.K.; Nguyen, N.-T. Fluid mechanics of flow through rectangular hydrophobic microchannels. In Proceedings of the ASME 9th International Conference on Nanochannels, Microchannels, and Minichannels, Edmonton, AL, Canada, 19–22 June 2011; pp. 647–655. [Google Scholar]
- Kashaninejad, N.; Kong Chan, W.; Nguyen, N.-T. Analytical modeling of slip flow in parallel-plate microchannels. Micro Nanosyst. 2013, 5, 245–252. [Google Scholar] [CrossRef]
- Kashaninejad, N.; Chan, W.K.; Nguyen, N.-T. Eccentricity effect of micropatterned surface on contact angle. Langmuir 2012, 28, 4793–4799. [Google Scholar] [CrossRef] [PubMed]
- Kashaninejad, N.; Nguyen, N.-T.; Chan, W.K. Eccentricity effects of microhole arrays on drag reduction efficiency of microchannels with a hydrophobic wall. Phys. Fluids 2012, 24, 112004. [Google Scholar] [CrossRef]
- Kashaninejad, N.; Nguyen, N.-T.; Chan, W.K. The three-phase contact line shape and eccentricity effect of anisotropic wetting on hydrophobic surfaces. Soft Matter 2013, 9, 527–535. [Google Scholar] [CrossRef]
- Velve-Casquillas, G.; Le Berre, M.; Piel, M.; Tran, P.T. Microfluidic tools for cell biological research. Nano Today 2010, 5, 28–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashaninejad, N.; Shiddiky, M.J.A.; Nguyen, N.-T. Advances in microfluidics-based assisted reproductive technology: From sperm sorter to reproductive system-on-a-chip. Adv. Biosyst. 2018, 2, 1700197. [Google Scholar] [CrossRef]
- Nguyen, N.-T.; Shaegh, S.A.M.; Kashaninejad, N.; Phan, D.-T. Design, fabrication and characterization of drug delivery systems based on lab-on-a-chip technology. Adv. Drug Deliv. Rev. 2013, 65, 1403–1419. [Google Scholar] [CrossRef] [PubMed]
- Kashaninejad, N.; Nikmaneshi, M.R.; Moghadas, H.; Kiyoumarsi Oskouei, A.; Rismanian, M.; Barisam, M.; Saidi, M.S.; Firoozabadi, B. Organ-tumor-on-a-chip for chemosensitivity assay: A critical review. Micromachines 2016, 7, 130. [Google Scholar] [CrossRef]
- Kim, S.; Kim, W.; Lim, S.; Jeon, J.S. Vasculature-on-a-chip for in vitro disease models. Bioengineering 2017, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Chung, S.; Kim, Y.E.; Lee, K.S.; Lee, S.H.; Oh, K.W.; Kang, J.Y. Generation of core-shell microcapsules with three-dimensional focusing device for efficient formation of cell spheroid. Lab Chip 2011, 11, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Hsu, H.-J.; Kaunas, R.; Kameoka, J. Collagen microsphere production on a chip. Lab Chip 2012, 12, 3277–3280. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, L.; Tian, C.; Ma, C.; Wang, J. Geometrically controlled preparation of various cell aggregates by droplet-based microfluidics. Anal. Methods 2015, 7, 10040–10051. [Google Scholar] [CrossRef]
- Alessandri, K.; Sarangi, B.R.; Gurchenkov, V.V.; Sinha, B.; Kießling, T.R.; Fetler, L.; Rico, F.; Scheuring, S.; Lamaze, C.; Simon, A. Cellular capsules as a tool for multicellular spheroid production and for investigating the mechanics of tumor progression in vitro. Proc. Natl. Acad. Sci. USA 2013, 110, 14843–14848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMillan, K.S.; McCluskey, A.G.; Sorensen, A.; Boyd, M.; Zagnoni, M. Emulsion technologies for multicellular tumour spheroid radiation assays. Analyst 2016, 141, 100–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitz, C.H.; Rowat, A.C.; Köster, S.; Weitz, D.A. Dropspots: A picoliter array in a microfluidic device. Lab Chip 2009, 9, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J. Mixed hydrogel bead-based tumor spheroid formation and anticancer drug testing. Analyst 2014, 139, 2449–2458. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Ni, C.; Grist, S.M.; Bayly, C.; Cheung, K.C. Alginate core-shell beads for simplified three-dimensional tumor spheroid culture and drug screening. Biomed. Microdevices 2015, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kwapiszewska, K.; Michalczuk, A.; Rybka, M.; Kwapiszewski, R.; Brzózka, Z. A microfluidic-based platform for tumour spheroid culture, monitoring and drug screening. Lab Chip 2014, 14, 2096–2104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patra, B.; Chen, Y.-H.; Peng, C.-C.; Lin, S.-C.; Lee, C.-H.; Tung, Y.-C. A microfluidic device for uniform-sized cell spheroids formation, culture, harvesting and flow cytometry analysis. Biomicrofluidics 2013, 7, 054114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moshksayan, K.; Saidi, M.S. Design and fabrication of a microfluidic chip for tumor spheroid formation and culture. In Proceedings of the Second National Congress on Microfluidics and Its Applications in Medicine and Engineering, Tehran, Iran, 1–2 March 2017. [Google Scholar]
- Fu, C.-Y.; Tseng, S.-Y.; Yang, S.-M.; Hsu, L.; Liu, C.-H.; Chang, H.-Y. A microfluidic chip with a U-shaped microstructure array for multicellular spheroid formation, culturing and analysis. Biofabrication 2014, 6, 015009. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.-J.; Cho, Y.-H.; Gu, J.-M.; Kim, J.; Oh, Y.-S. A multicellular spheroid formation and extraction chip using removable cell trapping barriers. Lab Chip 2010, 11, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xu, J.; Li, T.; Zhao, L.; Ma, C.; Shen, S.; Wang, J. Monitoring tumor response to anticancer drugs using stable three-dimensional culture in a recyclable microfluidic platform. Anal. Chem. 2015, 87, 9752–9760. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.Y.; Di Carlo, D.; Lee, L.P. Microfluidic self-assembly of tumor spheroids for anticancer drug discovery. Biomed. Microdevices 2008, 10, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Ota, H.; Miki, N. Microfluidic experimental platform for producing size-controlled three-dimensional spheroids. Sens. Actuators A Phys. 2011, 169, 266–273. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, F.; Qiu, T.; Xie, L.; Xing, W.; Cheng, J. Rapid fabrication of a microdevice with concave microwells and its application in embryoid body formation. Biomicrofluidics 2012, 6, 016504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Wang, J.-C.; Wang, J. Controllable organization and high throughput production of recoverable 3D tumors using pneumatic microfluidics. Lab Chip 2015, 15, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Kim, M.-C.; Thorsen, T.; Wang, Z. A self-contained microfluidic cell culture system. Biomed. Microdevices 2009, 11, 1233–1237. [Google Scholar] [CrossRef] [PubMed]
- Barisam, M.; Saidi, M.; Kashaninejad, N.; Nguyen, N.-T. Prediction of necrotic core and hypoxic zone of multicellular spheroids in a microbioreactor with a u-shaped barrier. Micromachines 2018, 9, 94. [Google Scholar] [CrossRef]
- Barisam, M.; Saidi, M.; Kashaninejad, N.; Vadivelu, R.; Nguyen, N.-T. Numerical simulation of the behavior of toroidal and spheroidal multicellular aggregates in microfluidic devices with microwell and U-shaped barrier. Micromachines 2017, 8, 358. [Google Scholar] [CrossRef]
- Anada, T.; Fukuda, J.; Sai, Y.; Suzuki, O. An oxygen-permeable spheroid culture system for the prevention of central hypoxia and necrosis of spheroids. Biomaterials 2012, 33, 8430–8441. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, J.; Nakazawa, K. Orderly arrangement of hepatocyte spheroids on a microfabricated chip. Tissue Eng. 2005, 11, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, T.; Yamazoe, H.; Mochizuki, N.; Khademhosseini, A.; Suzuki, H.; Fukuda, J. Preparation of arrays of cell spheroids and spheroid-monolayer cocultures within a microfluidic device. J. Biosci. Bioeng. 2010, 110, 572–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.Y.; Kim, J.; Lee, S.-H.; Kim, D.-S. Lab on a chip-based hepatic sinusoidal system simulator for optimal primary hepatocyte culture. Biomed. Microdevices 2016, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wu, M.; Guo, F.; Li, P.; Chan, C.Y.; Mao, Z.; Li, S.; Ren, L.; Zhang, R.; Huang, T.J. Rapid formation of size-controllable multicellular spheroids via 3D acoustic tweezers. Lab Chip 2016, 16, 2636–2643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsiao, A.Y.; Torisawa, Y.-S.; Tung, Y.-C.; Sud, S.; Taichman, R.S.; Pienta, K.J.; Takayama, S. Microfluidic system for formation of PC-3 prostate cancer co-culture spheroids. Biomaterials 2009, 30, 3020–3027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moghadas, H.; Saidi, M.S.; Kashaninejad, N.; Kiyoumarsioskouei, A.; Nguyen, N.-T. Fabrication and characterization of low-cost, bead-free, durable and hydrophobic electrospun membrane for 3D cell culture. Biomed. Microdevices 2017, 19, 74. [Google Scholar] [CrossRef] [PubMed]
- Moghadas, H.; Saidi, M.S.; Kashaninejad, N.; Nguyen, N.-T. A high-performance polydimethylsiloxane electrospun membrane for cell culture in lab on a chip. Biomicrofluidics 2018, 12, 024117. [Google Scholar] [CrossRef] [PubMed]
- Rousset, N.; Monet, F.; Gervais, T. Simulation-assisted design of microfluidic sample traps for optimal trapping and culture of non-adherent single cells, tissues, and spheroids. Sci. Rep. 2017, 7, 245. [Google Scholar] [CrossRef] [PubMed]
- Alexander, F.; Eggert, S.; Wiest, J. A novel lab-on-a-chip platform for spheroid metabolism monitoring. Cytotechnology 2017, 70, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Misun, P.M.; Rothe, J.; Schmid, Y.R.; Hierlemann, A.; Frey, O. Multi-analyte biosensor interface for real-time monitoring of 3D microtissue spheroids in hanging-drop networks. Microsyst. Nanoeng. 2016, 2, 16022. [Google Scholar] [CrossRef] [Green Version]
- Schmid, Y.R.; Bürgel, S.C.; Misun, P.M.; Hierlemann, A.; Frey, O. Electrical impedance spectroscopy for microtissue spheroid analysis in hanging-drop networks. ACS Sens. 2016, 1, 1028–1035. [Google Scholar] [CrossRef]
- Aleksandrova, A.; Pulkova, N.; Gerasimenko, T.; Anisimov, N.Y.; Tonevitskaya, S.; Sakharov, D. Mathematical and experimental model of oxygen diffusion for HepaRG cell spheroids. Bull. Exp. Biol. Med. 2016, 160, 857–860. [Google Scholar] [CrossRef] [PubMed]
- Astolfi, M.; Péant, B.; Lateef, M.; Rousset, N.; Kendall-Dupont, J.; Carmona, E.; Monet, F.; Saad, F.; Provencher, D.; Mes-Masson, A.-M. Micro-dissected tumor tissues on chip: An ex vivo method for drug testing and personalized therapy. Lab Chip 2016, 16, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Grimes, D.R.; Kelly, C.; Bloch, K.; Partridge, M. A method for estimating the oxygen consumption rate in multicellular tumour spheroids. J. R. Soc. Interface 2014, 11, 20131124. [Google Scholar] [CrossRef] [PubMed]
- Moshksayan, K.; Kashaninejad, N.; Saidi, M.S. Mathematical analysis of a conventional microfluidic device culturing tumor spheroids. In Proceedings of the International Congress on Cancer Prevention & Early Detection Integration of Research & Action, Tehran, Iran, 28–30 January 2017. [Google Scholar]
- Moshksayan, K.; Kashaninejad, N.; Saidi, M.S. Numerical investigation of the effects of functional parameters in hypoxia initiation within a cell spheroid cultured in a microfluidic chip. In Proceedings of the 25th Annual International Conference on Mechanical Engineering held by ISME, Tehran, Iran, 2–4 May 2017; pp. 861–862. [Google Scholar]
- Moghadas, H.; Saidi, M.S.; Kashaninejad, N.; Nguyen, N.-T. Challenge in particle delivery to cells in a microfluidic device. Drug Deliv. Transl. Res. 2017, 8, 830–842. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Fluri, D.A.; Marchan, R.; Boonen, K.; Mohanty, S.; Singh, P.; Hammad, S.; Landuyt, B.; Hengstler, J.G.; Kelm, J.M. 3D spherical microtissues and microfluidic technology for multi-tissue experiments and analysis. J. Biotechnol. 2015, 205, 24–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maschmeyer, I.; Lorenz, A.K.; Schimek, K.; Hasenberg, T.; Ramme, A.P.; Hübner, J.; Lindner, M.; Drewell, C.; Bauer, S.; Thomas, A. A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Lab Chip 2015, 15, 2688–2699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munaz, A.; Vadivelu, R.K.; St John, J.A.; Nguyen, N.-T. A lab-on-a-chip device for investigating the fusion process of olfactory ensheathing cell spheroids. Lab Chip 2016, 16, 2946–2954. [Google Scholar] [CrossRef] [PubMed]
- Au, S.H.; Chamberlain, M.D.; Mahesh, S.; Sefton, M.V.; Wheeler, A.R. Hepatic organoids for microfluidic drug screening. Lab Chip 2014, 14, 3290–3299. [Google Scholar] [CrossRef] [PubMed]
- Eydelnant, I.A.; Li, B.B.; Wheeler, A.R. Virtual microwells for three-dimensional cell culture on a digital microfluidic platform. In Proceedings of the IEEE 25th International Conference on Micro Electro Mechanical Systems (MEMS), Paris, France, 29 January–2 February 2012; pp. 898–901. [Google Scholar]
- Aijian, A.P.; Garrell, R.L. Digital microfluidics for automated hanging drop cell spheroid culture. J. Lab. Autom. 2015, 20, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Ng, A.H.; Fobel, R.; Wheeler, A.R. Digital microfluidics. Annu. Rev. Anal. Chem. 2012, 5, 413–440. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.H.; Li, B.B.; Chamberlain, M.D.; Wheeler, A.R. Digital microfluidic cell culture. Annu. Rev. Biomed. Eng. 2015, 17, 91–112. [Google Scholar] [CrossRef] [PubMed]
- McMillan, K.S.; Boyd, M.; Zagnoni, M. Transitioning from multi-phase to single-phase microfluidics for long-term culture and treatment of multicellular spheroids. Lab Chip 2016, 16, 3548–3557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albanese, A.; Lam, A.K.; Sykes, E.A.; Rocheleau, J.V.; Chan, W.C. Tumour-on-a-chip provides an optical window into nanoparticle tissue transport. Nat. Commun. 2013, 4, 2718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.; Bang, J.H.; Kim, Y.E.; Lee, S.H.; Kang, J.Y. On-chip anticancer drug test of regular tumor spheroids formed in microwells by a distributive microchannel network. Lab Chip 2012, 12, 4135–4142. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-T.; Chiang, C.-L.; Huang, R.Y.-J.; Lee, H.; Wo, A.M. Configurable 2D and 3D spheroid tissue cultures on bioengineered surfaces with acquisition of epithelial–mesenchymal transition characteristics. NPG Asia Mater. 2012, 4, e27. [Google Scholar] [CrossRef]
- St-Georges-Robillard, A.; Masse, M.; Kendall-Dupont, J.; Strupler, M.; Patra, B.; Jermyn, M.; Mes-Masson, A.-M.; Leblond, F.; Gervais, T. Spectroscopic imaging system for high-throughput viability assessment of ovarian spheroids or microdissected tumor tissues (MDTs) in a microfluidic chip. In Proceedings of the SPIE BiOS, San Francisco, CA, USA, 29 February 2016; Volume 9689. [Google Scholar]
- Anada, T.; Masuda, T.; Honda, Y.; Fukuda, J.; Arai, F.; Fukuda, T.; Suzuki, O. Three-dimensional cell culture device utilizing thin membrane deformation by decompression. Sens. Actuators B Chem. 2010, 147, 376–379. [Google Scholar] [CrossRef]
- Torisawa, Y.-S.; Mosadegh, B.; Luker, G.D.; Morell, M.; O’Shea, K.S.; Takayama, S. Microfluidic hydrodynamic cellular patterning for systematic formation of co-culture spheroids. Integr. Biol. Quant. Biosci. Nano Macro 2009, 1, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, F.; Kinne, R.K. Short exposure to millimolar concentrations of ethanol induces apoptotic cell death in multicellular HepG2 spheroids. J. Cancer Res. Clin. Oncol. 2000, 126, 305–310. [Google Scholar] [CrossRef] [PubMed]



| Reference | Year | Cell Type | Channel Dimensions | Hydrogel Type | Spheroid Formation Time | Spheroid or Droplet Diameter (µm) | Cells in Each Spheroid | Cell Density (cells/mL) | Media Flow Rate | 3D Culture Formation Method | Standard Deviation of Spheroid Size | Throughput |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| McMillan et al. [126] | 2016 | human glioblastoma cell line (UVW) | - | Alginate | Less than one day | - | - | 3 × 106 | The medium was refreshed every 2 days | Single emulsion CS/O | - | 48 |
| McMillan et al. [83] | 2016 | UVW | - | - | 24 h | 300–575 | 500–1500 | 5 × 106 | Daily Refreshment | Single emulsion CS/O | - | 2000 |
| Wang et al. [85] | 2014 | human cervical carcinoma (HeLa) | - | Alginate and Matrigel | 4 days | - | - | 107 | - | Double Emulsion CS/O and Gel/O | - | - |
| Sabhachandani et al. [63] | 2016 | breast cancer cell lines (MCF-7) and fibroblast cell lines (HS-5) | - | Alginate | 3 to 4 h | 170 (optimum) | - | 107 (mono) 7.5 × 106 (co) | 20 μL/h (equivalent to 230 μm/s) | Single emulsion O/Gel | - | 1000 |
| Chan et al. [18] | 2013 | mesenchymal stem cells, HepG2, PMEF and Caco-2 | - | alginate | 150 min | 36 to 84 | - | 2, 5, 10 and 20 million cells/mL | - | Double emulsion CS/O/CM | - | - |
| Yu et al. [86] | 2015 | MCF-7 | - | alginate | - | 183 | - | 107 | - | Double Emulsion CS/Gel/O | 4% | - |
| Yu et al. [64] | 2010 | LCC6/Her-2 breast tumour cells | - | alginate | 4 days for spheroid and | 250 | 100 | 107 | 0.25 µL/min | Single emulsion CS/O and Gel/O | - | 28 |
| Alessandri et al. [82] | 2013 | CT26 mouse colon and HeLa cells and murine sarcoma S180 | - | Collagen, alginate | - | 100–150 | - | - | - | Double Emulsion CS/IS/Gel | - | 1000 droplet/s |
| Yamada et al. [58] | 2015 | NIH-3T3 cells and HepG2 cells | diameter = 200 μm, depth = 300 μm | Collagen I | 1 day | - | - | 2 × 105 | - | Flat bottom microwells | - | - |
| Liu et al. [96] | 2015 | human glioma (U251) cells | - | - | - | 120–200 after 10 days | 200–400 | 5 × 106 | at a very slow perfusion rate (5 μL/min) | U-shaped microstructures | - | 360 |
| Wu et al. [93] | 2008 | MCF-7 breast tumour cells | - | - | 7 to 11 h | 50 | 10 | 106 | 0.05~10 μL·min−1 (0.02 to 4 mm/s) | U-shaped microstructures | - | 7500 per cm2 |
| Shin et al. [65] | 2013 | MCF-7 breast tumour cells | - | matrigel and a gelatin hydrogel | 3 days | 50 | Less than 20 | 106 | 30 µL/h, equivalent to 278 µm/s | Cell suspension 50 µm wells (50 µm height) | - | - |
| Albanese et al. [127] | 2013 | MDA-MB-435 cells | - | - | 3 days | 260–280 | 750–1500 | - | 50 and 450 mL/h produced a 75–675 mm/s fluid velocity | Hanging droplet plates | - | - |
| Kwapiszewska et al. [87] | 2014 | HT-29 colon carcinoma and Hep-G2 liver carcinoma | - | - | 48 h | Almost 50 | - | 1–5 × 106 | 4.5 μL/min for 15 min daily | in hemispherical bottom micro wells | Up to 30% | 216 |
| Aung et al. [28] | 2016 | human umbilical HUVECs and MCF-7 breast tumour cells | - | gelatin methacrylate (GelMA) | 20 h | 200 | - | - | 10 to 40 μL/h | In Petri dish and cultured on an orbital shaker | - | - |
| Ruppen et al. [25] | 2015 | lung adenocarcinoma + malignant pleural mesothelioma+ pericytes | Micro-well diameter: 500 µm Well height: 600 µm | - | 48 h | 325 and 210 | 1250 312 | - | Changed once a day | Cell sedimentation in round and flat-bottom wells in the chip | 35 to 45 µm | 8 in each unit |
| Jin et al. [91] | 2010 | non-small lung cancer cells, H1650 | - | - | 24 h | 197 | - | - | - | U-shaped microstructures | 11.7 micron | 4 |
| Torisawa et al. [26] | 2007 | MCF-7, HepG2 | - | - | 2 days for MCF-7 and 3days for HepG2 | - | 370 for HepG2 with 3 × 106 | 1, 3, 10 × 106 | - | Pyramidal structures which have a hole at their vertex | - | 16 |
| Kim et al. [118] | 2015 | Human colorectal tumour and Primary rat liver | - | - | - | 180 | 250 | - | 13 µL/min. hydrostatic | Hanging droplet of Human colorectal tumour | - | 8 |
| Ziółkowska et al. [32] | 2013 | HT-29 human carcinoma cells | Well: 200, 150 Channel: 50, 1000 | - | 48 to 72 h | - | 100 | 1.5 × 106 | 4.5 µL/min | Flat bottom microwells | not exceeding 20% in cell numbers | 45 |
| Lee et al. [33] | 2013 | Hepatocytes and hepatic stellate cells (HSCs) | Well: 500, 400 | - | - | 200 to 375 | - | 2 × 106 | 5.53 mm/h or approximately 1.5 µm/s | Concave bottom microwells | - | 50 |
| Choong Kim et al. [79] | 2011 | mouse embryonic carcinoma | - | - | 3 day | 158 | 178 | 5 × 105 | 0.2 mL/h for cell seeding | Flat bottom Microwell trapping | 4.50% | 60 |
| Ota et al. [23] | 2010 | Human hepatocellular liver carcinoma cells | - | - | 120 s | 130–430 µm | 1000 for 180 micron spheroid | 6.9 × 106 | 0.4 ± 0.05 mL/min. | microrotation | 13.2% in 150–200 µm and 17.2% in 130–430 µm | 1 |
| Choong Kim et al. [128] | 2012 | MCF-7 | - | - | 3 days | 188 | 200 | - | 0.2 mL/h for cell seeding | Flat bottom Microwell trapping | 6.06 µm | 80 |
| Ota et al. [94] | 2011 | Hep-G2 | - | - | 120 s | 134 ± 25, 180 ± 30 and 237 ± 40 µm | - | 2–5–13 × 106 | 1.2 mL/min | microrotation | 18.7%, 16.6% and 16.9% | 15 |
| Ota et al. [59] | 2011 | Hep-G2 and endothelial cells | - | collagen | 120 s | 97–226 | - | 145, 290, 480 and 675 × 104/mL | 1.2 mL/min | microrotation | 17%, 18.7%, 16.6% and 16.9% | 15 |
| Patra et al. [8] | 2016 | human hepatocellular carcinoma cells (HepG2) | Chanel: 250 | - | 24 h | 130 and 212 | - | - | 100 µL/min for cell seeding and changed every 12 h by adding 1 mL of fresh culture media | Flat bottom well | 6% for small and 3% for large spheroids | 5000 |
| Well: 200 × 200 × 250 and 300 × 300 × 250 | ||||||||||||
| Kangsun Lee et al. [21] | 2012 | human embryonic kidney 293 cells (HEK 293) | - | - | Less than one day | Less than 300 µm for retrieval | - | 1–2–4 × 106 | - | sedimentation | 5.5%, 7.2% and 8.9% for 1, 2 and 4 × 106 | 50 |
| Kuo et al. [129] | 2012 | human epithelial ovarian cancer cells (SKOV3) | - | - | 48 h | 75 | - | 1.5 × 104 | Hydrostatic flow for trapping and media change for culture | Trapping behind a porous membrane | Min of 7.6% | - |
| Patra et al. [88] | 2013 | murine ES cell, HepG2, African green monkey kidney epithelial fibroblast (COS-7) | Channel: 150, 1400, 25,000 Well: 200 × 200, 250 | - | 24 h for COS-7, 1 day for HepG2, 16 h for ES | COS-7 and HepG2 spheroids are 80 and 200 µm | - | HepG2 and COS-7 cell 107 and 105 respectively | 1 µL/min for cell seeding and 20 µL/min for culture refreshment every 48 h | Flat bottom well | standard deviations of 4 and 10 µm, respectively | 5000 |
| Chen et al. [7] | 2015 | T47D, MCF-7 and SUM159 (breast cancer) | Channel: 100 Well: 250, 400 and 450, 400 | - | 1 day | - | - | 5 × 106 | 300 μL per minute for cell seeding | Flat bottom well | 10% | 1024 within an area of 2 by 2 cm |
| Yongli Chen et al. [24] | 2015 | HCT116, T47D breast cancer and HepG2 | Channel: 100, 3000, 9500 Well: 500, 200 | - | 24 h | - | - | 106 | - | Flat bottom well | - | 120 |
| Choi et al. [103] | 2016 | Hepatocytes | Channel: 100, 4000 | - | - | - | - | 1 × 106 | 4.2 µm/(0.12 µL/min) | Concave bottom microwells | - | 50 |
| Robillard et al. [130] | 2016 | ovarian cancer cell line OV90 | Channel: 500, 2000 Well: 450 × 450 × 500 | - | - | 170 | - | 5 × 105 cells/mL | The medium was changed Each day | Flat bottom microwells | - | 120 |
| Anada et al. [131] | 2010 | Human osteosarcoma MG63, HepG2 | Well: 1000, 500 | - | 1 day | 150 to 320 after 5 days of culture | - | 1.25 × 105 to 8 × 106 | - | Pneumatic concave wells | 5–8% | 1535 |
| Fukuda and Nakazawa [22] | 2011 | Hepatocytes of Wistar rat | Open Channel: 100, 100 Well: 300, 400 | - | 2 day | 150 | - | 2.5 × 106 | - | Flat bottom microwells | - | 1575 |
| Xu et al. [95] | 2012 | P19 cells | - | - | 1 day | 100 to 450 | - | 2–20 × 104 cells mL−1 | 2 mm/ to rinse excess cells, 6 or 0.5 mm/sec for spheroid retrieval | Concave bottom microwells | - | 880 |
| Zhang et al. [97] | 2009 | BALB/3T3 (murine embryonic fibroblast) cell line. | - | - | - | 90 | 85 ± 6.3 | 107 | 1 µL/min for 10 min every 6 h | U-shaped microstructures | - | 512 totally (8 in each chamber) |
| Chien-Yu Fu et al. [90] | 2014 | HepG2 and Balb/c 3T3 fibroblast cells | - | - | 1 day | - | - | 8.4 × 106 | 1.5 µL/min for long-term perfusion | U-shaped microstructures | - | 56 |
| Tung et al. [52] | 2011 | COS7, ES-D3 and human epithelial carcinoma cell | - | - | 1 day | - | 300, 1500 and 7500 | - | - | Novel Hanging droplet method (3d-biomatrix, perfecta 3d) | - | 384 |
| Santo et al. [42] | 2016 | MCF7, H1650, H157, HT29, Human Dermal Fibroblasts (hDFs) | - | - | - | 100 to 800 | - | 0.2 × 106 & 0.5 × 106 | - | Stirred tank | Up to about 40% | - |
| Torisawa et al. [132] | 2009 | Fibroblast COS-7; HepG2; ATCC; Breast cancer MDA-MB-231 | - | - | - | - | - | 105 | Hydrostatic-driven flow, medium daily exchanged | Patterning on semi-porous membranes | - | - |
| Hsiao et al. [105] | 2009 | prostate cancer cells osteoblasts and endothelial cells | - | - | 1 day | 86 | - | - | Hydrostatic-driven flow, medium daily exchanged | Patterning on semi-porous membranes | 12 µm | 28 |
| Chen et al. [104] | 2016 | HEK 293, SH-FY5Y, HepG2 and HeLa cells | - | - | 1 day | 30 to 100 | - | 2–17 × 106 | medium daily exchanged in Petri dish | Acoustic tweezers | - | 150 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moshksayan, K.; Kashaninejad, N.; Saidi, M.S. Inventions and Innovations in Preclinical Platforms for Cancer Research. Inventions 2018, 3, 43. https://doi.org/10.3390/inventions3030043
Moshksayan K, Kashaninejad N, Saidi MS. Inventions and Innovations in Preclinical Platforms for Cancer Research. Inventions. 2018; 3(3):43. https://doi.org/10.3390/inventions3030043
Chicago/Turabian StyleMoshksayan, Khashayar, Navid Kashaninejad, and Mohammad Said Saidi. 2018. "Inventions and Innovations in Preclinical Platforms for Cancer Research" Inventions 3, no. 3: 43. https://doi.org/10.3390/inventions3030043






