Development of a 3D Perfused In Vitro System to Assess Proangiogenic Properties of Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Viability Assessment
2.3. Seeding in OrganoPlates® and Angiogenesis Assay
2.4. Nuclei and F-Actin Staining
2.5. Image Acquisition and Analysis
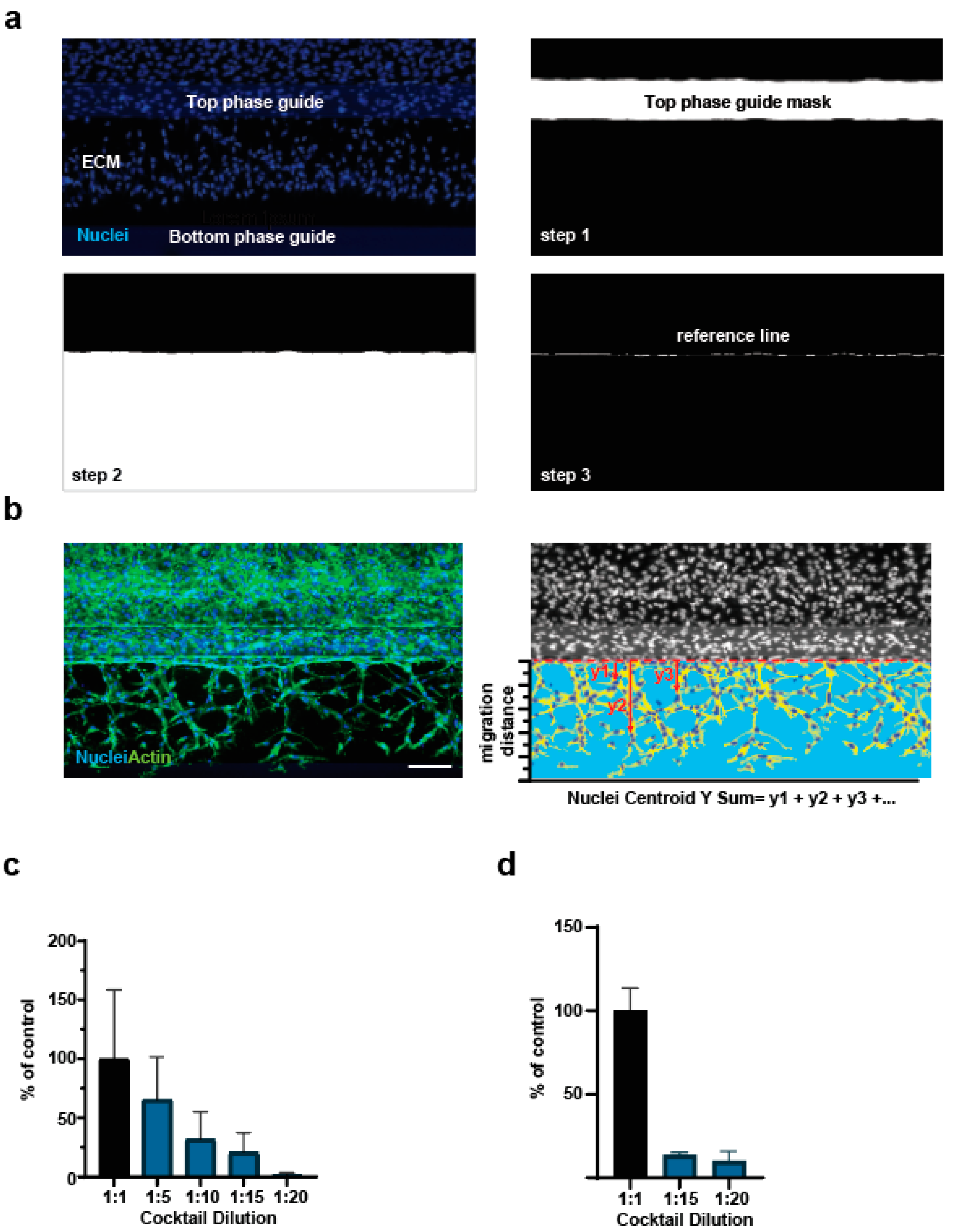
2.5.1. Sprouting Quantification
2.5.2. Nuclei Centroid Y Sum Quantification
3. Results and Discussion
3.1. Establishment of the OrganoPlate® Angiogenesis-on-Chip Assay
3.2. Refinement of Image Analysis Pipeline
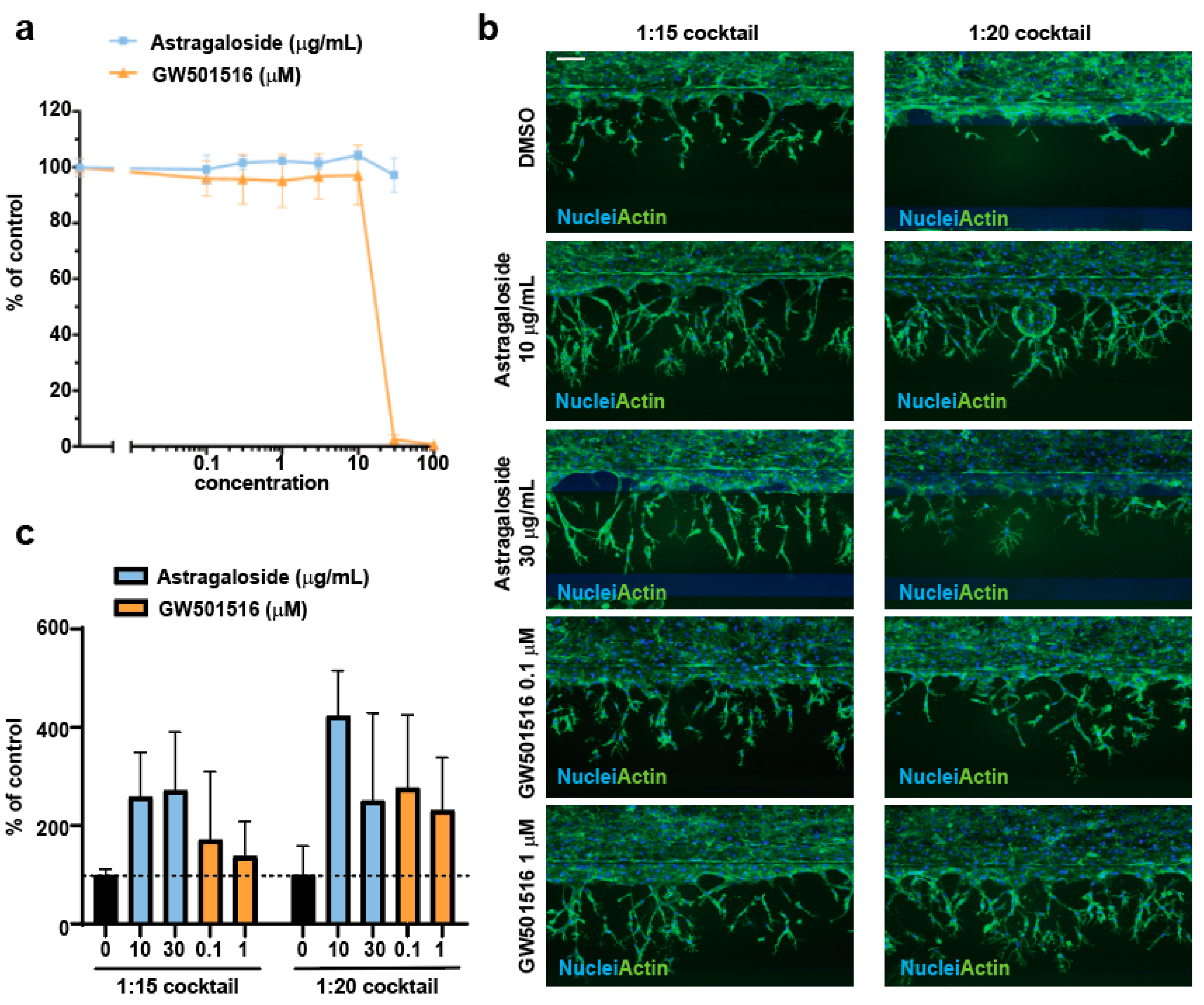
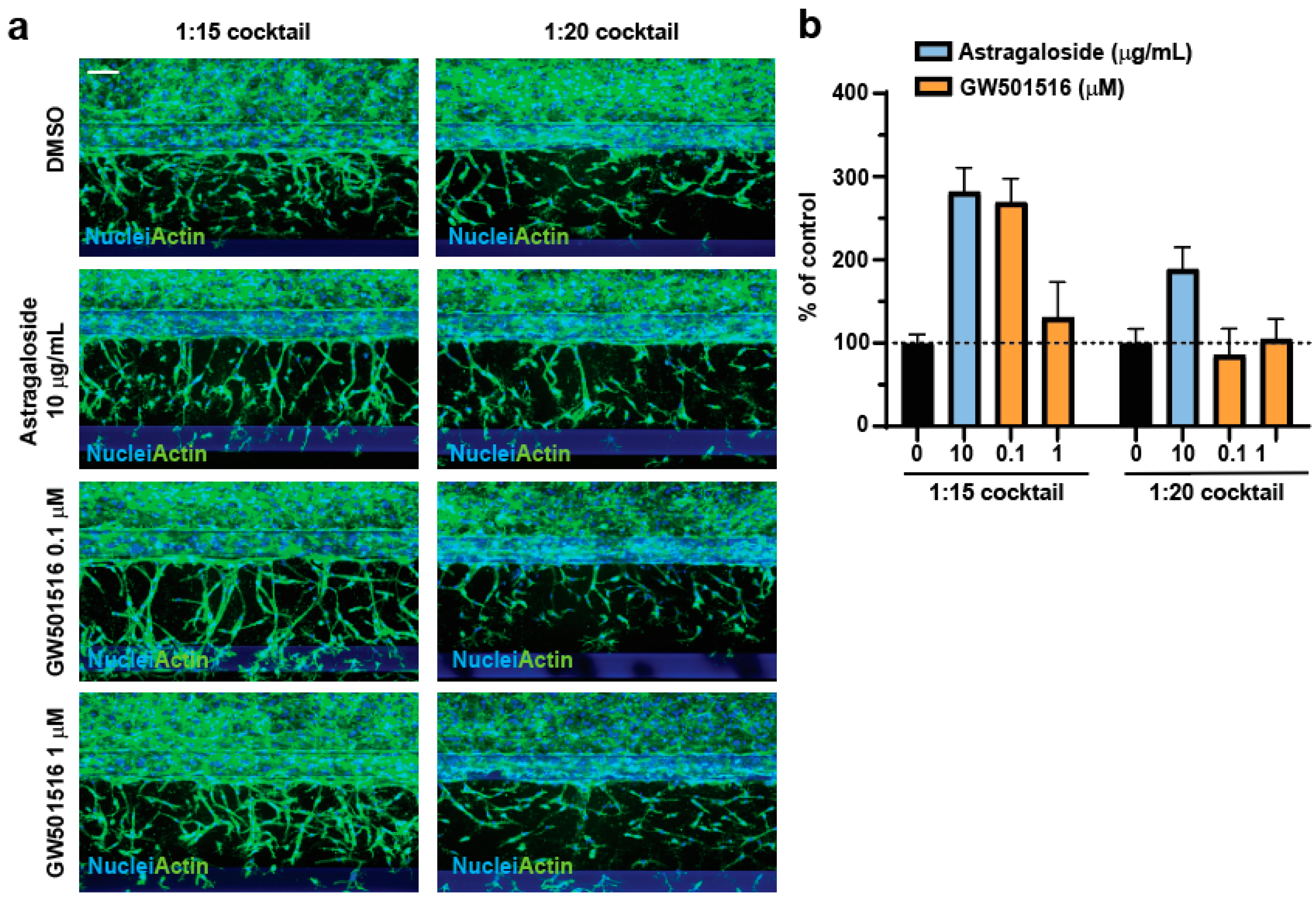
3.3. Assessment of Proangiogenic Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chung, A.S.; Ferrara, N. Developmental and pathological angiogenesis. Annu. Rev. Cell Dev. Biol. 2011, 27, 563–584. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef]
- Khan, T.A.; Sellke, F.W.; Laham, R.J. Gene therapy progress and prospects: Therapeutic angiogenesis for limb and myocardial ischemia. Gene Ther. 2003, 10, 285–291. [Google Scholar] [CrossRef]
- Quesada, A.R.; Munoz-Chapuli, R.; Medina, M.A. Anti-angiogenic drugs: From bench to clinical trials. Med. Res. Rev. 2006, 26, 483–530. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Poon, M.C.; Pu, W.T.; Han, Z.C. Therapeutic neovascularization for peripheral arterial diseases: Advances and perspectives. Histol. Histopathol. 2007, 22, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Levato, L.; Cantaffa, R.; Kropp, M.G.; Magro, D.; Piro, E.; Molica, S. Progressive peripheral arterial occlusive disease and other vascular events during nilotinib therapy in chronic myeloid leukemia: A single institution study. Eur. J. Haematol. 2013, 90, 531–532. [Google Scholar] [CrossRef] [PubMed]
- Hadzijusufovic, E.; Albrecht-Schgoer, K.; Huber, K.; Hoermann, G.; Grebien, F.; Eisenwort, G.; Schgoer, W.; Herndlhofer, S.; Kaun, C.; Theurl, M.; et al. Nilotinib-induced vasculopathy: Identification of vascular endothelial cells as a primary target site. Leukemia 2017, 31, 2388–2397. [Google Scholar] [CrossRef]
- Sahebkar, A.; Chew, G.T.; Watts, G.F. New peroxisome proliferator-activated receptor agonists: Potential treatments for atherogenic dyslipidemia and non-alcoholic fatty liver disease. Expert Opin. Pharmacother. 2014, 15, 493–503. [Google Scholar] [CrossRef]
- Piqueras, L.; Reynolds, A.R.; Hodivala-Dilke, K.M.; Alfranca, A.; Redondo, J.M.; Hatae, T.; Tanabe, T.; Warner, T.D.; Bishop-Bailey, D. Activation of PPARbeta/delta induces endothelial cell proliferation and angiogenesis. Arter. Thromb. Vasc. Biol. 2007, 27, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.D.; Du, S.; Martin, L.; Leccia, N.; Michiels, J.F.; Wagner, N. Vascular PPARbeta/delta Promotes Tumor Angiogenesis and Progression. Cells 2019, 8, 1623. [Google Scholar] [CrossRef]
- Goodwin, A.M. In vitro assays of angiogenesis for assessment of angiogenic and anti-angiogenic agents. Microvasc. Res. 2007, 74, 172–183. [Google Scholar] [CrossRef]
- Francescone, R.; Vendramini-Costa, D.B. In Vitro Models to Study Angiogenesis and Vasculature. Methods Mol. Biol. 2022, 2514, 15–28. [Google Scholar] [CrossRef]
- Irvin, M.W.; Zijlstra, A.; Wikswo, J.P.; Pozzi, A. Techniques and assays for the study of angiogenesis. Exp. Biol. Med. 2014, 239, 1476–1488. [Google Scholar] [CrossRef]
- Nowak-Sliwinska, P.; Alitalo, K.; Allen, E.; Anisimov, A.; Aplin, A.C.; Auerbach, R.; Augustin, H.G.; Bates, D.O.; van Beijnum, J.R.; Bender, R.H.F.; et al. Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenesis 2018, 21, 425–532. [Google Scholar] [CrossRef]
- van Duinen, V.; Trietsch, S.J.; Joore, J.; Vulto, P.; Hankemeier, T. Microfluidic 3D cell culture: From tools to tissue models. Curr. Opin. Biotechnol. 2015, 35, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chung, M.; Ahn, J.; Lee, S.; Jeon, N.L. Interstitial flow regulates the angiogenic response and phenotype of endothelial cells in a 3D culture model. Lab Chip 2016, 16, 4189–4199. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, W.; Lim, S.; Jeon, J.S. Vasculature-On-A-Chip for In Vitro Disease Models. Bioengineering 2017, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- van Duinen, V.; Zhu, D.; Ramakers, C.; van Zonneveld, A.J.; Vulto, P.; Hankemeier, T. Perfused 3D angiogenic sprouting in a high-throughput in vitro platform. Angiogenesis 2019, 22, 157–165. [Google Scholar] [CrossRef] [PubMed]
- van Duinen, V.; Stam, W.; Borgdorff, V.; Reijerkerk, A.; Orlova, V.; Vulto, P.; Hankemeier, T.; van Zonneveld, A.J. Standardized and Scalable Assay to Study Perfused 3D Angiogenic Sprouting of iPSC-derived Endothelial Cells In Vitro. J. Vis. Exp. 2019, 153, e59678. [Google Scholar] [CrossRef]
- van Duinen, V.; Stam, W.; Mulder, E.; Famili, F.; Reijerkerk, A.; Vulto, P.; Hankemeier, T.; van Zonneveld, A.J. Robust and Scalable Angiogenesis Assay of Perfused 3D Human iPSC-Derived Endothelium for Anti-Angiogenic Drug Screening. Int. J. Mol. Sci. 2020, 21, 4804. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.Q.; Chen, H.W.; Li, J. Astragaloside IV: An Effective Drug for the Treatment of Cardiovascular Diseases. Drug Des. Dev. Ther. 2020, 14, 3731–3746. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shen, Z.; Tang, Y.; Chen, B.; Chen, J.; Hou, J.; Li, J.; Zhang, M.; Liu, S.; Mei, Y.; et al. Astragaloside IV promotes the angiogenic capacity of adipose-derived mesenchymal stem cells in a hindlimb ischemia model by FAK phosphorylation via CXCR2. Phytomedicine 2022, 96, 153908. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, G.; Li, S.; Li, Z.H.; Lam, C.O.; Hong, S.J.; Kwan, Y.W.; Chan, S.W.; Leung, G.P.; Lee, S.M. Pro-angiogenic activity of astragaloside IV in HUVECs in vitro and zebrafish in vivo. Mol. Med. Rep. 2012, 5, 805–811. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alm, J.; Fischer, B.; Burger, A.E.; Moretti, F. Development of a 3D Perfused In Vitro System to Assess Proangiogenic Properties of Compounds. Methods Protoc. 2023, 6, 119. https://doi.org/10.3390/mps6060119
Alm J, Fischer B, Burger AE, Moretti F. Development of a 3D Perfused In Vitro System to Assess Proangiogenic Properties of Compounds. Methods and Protocols. 2023; 6(6):119. https://doi.org/10.3390/mps6060119
Chicago/Turabian StyleAlm, Johanna, Benoit Fischer, Alexandra Emanuela Burger, and Francesca Moretti. 2023. "Development of a 3D Perfused In Vitro System to Assess Proangiogenic Properties of Compounds" Methods and Protocols 6, no. 6: 119. https://doi.org/10.3390/mps6060119




