Analytical Methods for Nanomaterial Determination in Biological Matrices
Abstract
:1. Introduction
1.1. Nanomaterials
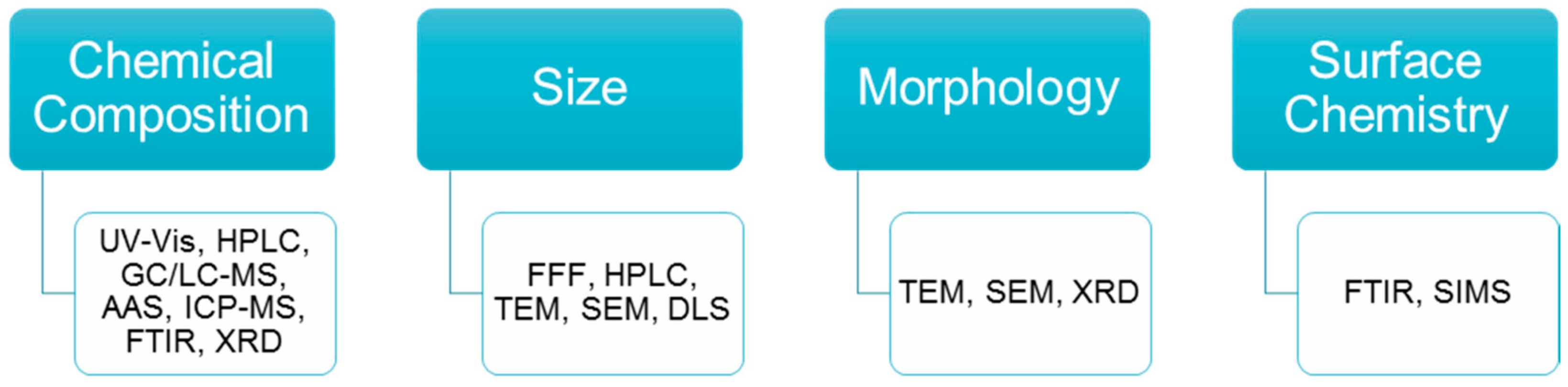
1.2. Chemical Composition
2. Occurrence of Nanomaterials in Biological Samples
Nanoparticles in Biological Systems
3. Bioanalytical Methods for the Determination of Nanoparticles
3.1. Electron Microscopy
3.2. Optical Microscopy
3.3. Light Scattering
3.4. X-ray Tomography
3.5. Spectroscopic
4. Sample Preparation
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dolez, P.I. Nanomaterials Definitions, Classifications, and Applications. In Nanoengineering; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3–40. ISBN 978-0-444-62747-6. [Google Scholar]
- Ligler, F.S.; White, H.S. Nanomaterials in Analytical Chemistry. Anal. Chem. 2013, 85, 11161–11162. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, H.S.N.; Liyanage, S.H.; Rathnayake, K.; Patel, U.; Yan, M. Analytical Methods for Characterization of Nanomaterial Surfaces. Anal. Chem. 2021, 93, 1889–1911. [Google Scholar] [CrossRef] [PubMed]
- Scida, K.; Stege, P.W.; Haby, G.; Messina, G.A.; García, C.D. Recent applications of carbon-based nanomaterials in analytical chemistry: Critical review. Anal. Chim. Acta 2011, 691, 6–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valentini, F.; Palleschi, G. Nanomaterials and Analytical Chemistry. Anal. Lett. 2008, 41, 479–520. [Google Scholar] [CrossRef]
- Lorente, A.I.L.; Simonet, Β.Μ.; Valcarel, M. Determination of nanoparticles in biological matrices. Front Biosci. 2012, E4, 1024–1042. [Google Scholar] [CrossRef]
- Albanese, A.; Tang, P.S.; Chan, W.C.W. The Effect of Nanoparticle Size, Shape, and Surface Chemistry on Biological Systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.; Ghadiri, R.; Weigel, T.; Aumann, A.; Gurevich, E.; Esen, C.; Medenbach, O.; Cheng, W.; Chichkov, B.; Ostendorf, A. Comparison of in Situ and Ex Situ Methods for Synthesis of Two-Photon Polymerization Polymer Nanocomposites. Polymers 2014, 6, 2037–2050. [Google Scholar] [CrossRef]
- Fritea, L.; Banica, F.; Costea, T.; Moldovan, L.; Dobjanschi, L.; Muresan, M.; Cavalu, S. Metal Nanoparticles and Carbon-Based Nanomaterials for Improved Performances of Electrochemical (Bio)Sensors with Biomedical Applications. Materials 2021, 14, 6319. [Google Scholar] [CrossRef]
- Crevillen, A.G.; Escarpa, A.; García, C.D. Carbon-Based Nanomaterials in Analytical Chemistry; Chapter 1. In Carbon-Based Nanomaterials in Analytical Chemistry; The Royal Society of Chemistry: London, UK, 2019; pp. 1–36. ISBN 978-1-78801-102-0. [Google Scholar]
- Kurakula, M.; Sobahi, T.; El-Helw, A.; Abdelaal, M. Development and Validation of a RP-HPLC Method for Assay of Atorvastatin and Its Application in Dissolution Studies on Thermosensitive Hydrogel-Based Nanocrystals. Trop. J. Pharm. Res. 2014, 13, 1681. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Chang, Y.; Chen, Y. Toxicity and Bioaccumulation of TiO2 Nanoparticle Aggregates in Daphnia Magna. Chemosphere 2010, 78, 209–215. [Google Scholar] [CrossRef]
- Summers, H. Can Cells Reduce Nanoparticle Toxicity? Nano Today 2010, 5, 83–84. [Google Scholar] [CrossRef]
- do Nascimento, G.M.; de Oliveira, R.C.; Pradie, N.A.; Lins, P.R.G.; Worfel, P.R.; Martinez, G.R.; Di Mascio, P.; Dresselhaus, M.S.; Corio, P. Single-Wall Carbon Nanotubes Modified with Organic Dyes: Synthesis, Characterization and Potential Cytotoxic Effects. J. Photochem. Photobiol. A Chem. 2010, 211, 99–107. [Google Scholar] [CrossRef]
- Park, E.-J.; Bae, E.; Yi, J.; Kim, Y.; Choi, K.; Lee, S.H.; Yoon, J.; Lee, B.C.; Park, K. Repeated-Dose Toxicity and Inflammatory Responses in Mice by Oral Administration of Silver Nanoparticles. Environ. Toxicol. Pharmacol. 2010, 30, 162–168. [Google Scholar] [CrossRef]
- Ye, Y.; Liu, J.; Chen, M.; Sun, L.; Lan, M. In Vitro Toxicity of Silica Nanoparticles in Myocardial Cells. Environ. Toxicol. Pharmacol. 2010, 29, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Eidi, H.; Joubert, O.; Attik, G.; Duval, R.E.; Bottin, M.C.; Hamouia, A.; Maincent, P.; Rihn, B.H. Cytotoxicity Assessment of Heparin Nanoparticles in NR8383 Macrophages. Int. J. Pharm. 2010, 396, 156–165. [Google Scholar] [CrossRef]
- Holgate, S.T. Exposure, Uptake, Distribution and Toxicity of Nanomaterials in Humans. J. Biomed. Nanotechnol. 2010, 6, 1–19. [Google Scholar] [CrossRef]
- Baun, A.; Hartmann, N.B.; Grieger, K.; Kusk, K.O. Ecotoxicity of Engineered Nanoparticles to Aquatic Invertebrates: A Brief Review and Recommendations for Future Toxicity Testing. Ecotoxicology 2008, 17, 387–395. [Google Scholar] [CrossRef]
- Chen, K.L.; Elimelech, M. Relating Colloidal Stability of Fullerene (C60) Nanoparticles to Nanoparticle Charge and Electrokinetic Properties. Environ. Sci. Technol. 2009, 43, 7270–7276. [Google Scholar] [CrossRef]
- Metcalfe, C.; Bennett, E.; Chappell, M.; Steevens, J.; Depledge, M.; Goss, G.; Goudey, S.; Kaczmar, S.; O’Brien, N.; Picado, A.; et al. Smarten. In Nanomaterials: Risks and Benefits; Linkov, I., Steevens, J., Eds.; NATO Science for Peace and Security Series C: Environmental Security; Springer: Dordrecht, The Netherlands, 2009; pp. 95–109. ISBN 978-1-4020-9490-3. [Google Scholar]
- Carl Englert, B. Nanomaterials and the Environment: Uses, Methods and Measurement. J. Environ. Monit. 2007, 9, 1154. [Google Scholar] [CrossRef]
- Hassellφv, M.; Readman, J.W.; Ranville, J.F.; Tiede, K. Nanoparticle Analysis and Characterization Methodologies in Environmental Risk Assessment of Engineered Nanoparticles. Ecotoxicology 2008, 17, 344–361. [Google Scholar] [CrossRef]
- Grieger, K.D.; Baun, A.; Owen, R. Redefining Risk Research Priorities for Nanomaterials. J. Nanopart. Res. 2010, 12, 383–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhry, Q.; Scotter, M.; Blackburn, J.; Ross, B.; Boxall, A.; Castle, L.; Aitken, R.; Watkins, R. Applications and Implications of Nanotechnologies for the Food Sector. Food Addit. Contam. Part A 2008, 25, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Stamm, H.; Gibson, N.; Anklam, E. Detection of Nanomaterials in Food and Consumer Products: Bridging the Gap from Legislation to Enforcement. Food Addit. Contam. Part A 2012, 29, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Tiede, K.; Hassellöv, M.; Breitbarth, E.; Chaudhry, Q.; Boxall, A.B.A. Considerations for Environmental Fate and Ecotoxicity Testing to Support Environmental Risk Assessments for Engineered Nanoparticles. J. Chromatogr. A 2009, 1216, 503–509. [Google Scholar] [CrossRef]
- Pena, M.D.P.S.; Gottipati, A.; Tahiliani, S.; Neu-Baker, N.M.; Frame, M.D.; Friedman, A.J.; Brenner, S.A. Hyperspectral Imaging of Nanoparticles in Biological Samples: Simultaneous Visualization and Elemental Identification: Hyperspectral Mapping in Biological Samples. Microsc. Res. Tech. 2016, 79, 349–358. [Google Scholar] [CrossRef]
- Brar, S.K.; Verma, M. Measurement of Nanoparticles by Light-Scattering Techniques. TrAC Trends Anal. Chem. 2011, 30, 4–17. [Google Scholar] [CrossRef]
- Rauscher, H.; Mech, A.; Gibson, N.; Gilliland, D.; Held, A.; Kestens, V.; Koeber, R.; Linsinger, T.P.J.; Stefaniak, E.A.; European Commission; et al. Identification of Nanomaterials through Measurements: Points to Consider in the Assessment of Particulate Materials According to the European Commission’s Recommendation on a Definition of Nanomaterial; Publications Office of the European Union: Luxembourg, 2019; ISBN 978-92-76-10371-4. [Google Scholar]
- Correia, M.; Loeschner, K. Detection of Nanoplastics in Food by Asymmetric Flow Field-Flow Fractionation Coupled to Multi-Angle Light Scattering: Possibilities, Challenges and Analytical Limitations. Anal Bioanal. Chem. 2018, 410, 5603–5615. [Google Scholar] [CrossRef] [Green Version]
- Deering, C.E.; Tadjiki, S.; Assemi, S.; Miller, J.D.; Yost, G.S.; Veranth, J.M. A Novel Method to Detect Unlabeled Inorganic Nanoparticles and Submicron Particles in Tissue by Sedimentation Field-Flow Fractionation. Part. Fibre Toxicol. 2008, 5, 18. [Google Scholar] [CrossRef] [Green Version]
- Servin, A.D.; Castillo-Michel, H.; Hernandez-Viezcas, J.A.; De Nolf, W.; De La Torre-Roche, R.; Pagano, L.; Pignatello, J.; Uchimiya, M.; Gardea-Torresdey, J.; White, J.C. Bioaccumulation of CeO2 Nanoparticles by Earthworms in Biochar-Amended Soil: A Synchrotron Microspectroscopy Study. J. Agric. Food Chem. 2018, 66, 6609–6618. [Google Scholar] [CrossRef]
- Da Silva, G.H.; Clemente, Z.; Khan, L.U.; Coa, F.; Neto, L.L.R.; Carvalho, H.W.P.; Castro, V.L.; Martinez, D.S.T.; Monteiro, R.T.R. Toxicity Assessment of TiO2-MWCNT Nanohybrid Material with Enhanced Photocatalytic Activity on Danio Rerio (Zebrafish) Embryos. Ecotoxicol. Environ. Saf. 2018, 165, 136–143. [Google Scholar] [CrossRef]
- Mahmoud, N.N.; Harfouche, M.; Alkilany, A.M.; Al-Bakri, A.G.; El-Qirem, R.A.; Shraim, S.A.; Khalil, E.A. Synchrotron-Based X-Ray Fluorescence Study of Gold Nanorods and Skin Elements Distribution into Excised Human Skin Layers. Colloids Surf. B Biointerfaces 2018, 165, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Lombi, E.; Scheckel, K.G.; Kempson, I.M. In Situ Analysis of Metal(Loid)s in Plants: State of the Art and Artefacts. Environ. Exp. Bot. 2011, 72, 3–17. [Google Scholar] [CrossRef]
- McRae, R.; Bagchi, P.; Sumalekshmy, S.; Fahrni, C.J. In Situ Imaging of Metals in Cells and Tissues. Chem. Rev. 2009, 109, 4780–4827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, B.; Loeschner, K.; Hadrup, N.; Mortensen, A.; Sloth, J.J.; Bender Koch, C.; Larsen, E.H. Quantitative Characterization of Gold Nanoparticles by Field-Flow Fractionation Coupled Online with Light Scattering Detection and Inductively Coupled Plasma Mass Spectrometry. Anal. Chem. 2011, 83, 2461–2468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henss, A.; Otto, S.-K.; Schaepe, K.; Pauksch, L.; Lips, K.S.; Rohnke, M. High Resolution Imaging and 3D Analysis of Ag Nanoparticles in Cells with ToF-SIMS and Delayed Extraction. Biointerphases 2018, 13, 03B410. [Google Scholar] [CrossRef] [Green Version]
- Peters, R.J.B.; Rivera, Z.H.; van Bemmel, G.; Marvin, H.J.P.; Weigel, S.; Bouwmeester, H. Development and Validation of Single Particle ICP-MS for Sizing and Quantitative Determination of Nano-Silver in Chicken Meat. Anal Bioanal. Chem. 2014, 406, 3875–3885. [Google Scholar] [CrossRef]
- Vidmar, J.; Buerki-Thurnherr, T.; Loeschner, K. Comparison of the Suitability of Alkaline or Enzymatic Sample Pre-Treatment for Characterization of Silver Nanoparticles in Human Tissue by Single Particle ICP-MS. J. Anal. At. Spectrom. 2018, 33, 752–761. [Google Scholar] [CrossRef] [Green Version]
- Loeschner, K.; Harrington, C.F.; Kearney, J.-L.; Langton, D.J.; Larsen, E.H. Feasibility of Asymmetric Flow Field-Flow Fractionation Coupled to ICP-MS for the Characterization of Wear Metal Particles and Metalloproteins in Biofluids from Hip Replacement Patients. Anal. Bioanal. Chem 2015, 407, 4541–4554. [Google Scholar] [CrossRef]
- Arslan, Z.; Ates, M.; McDuffy, W.; Agachan, M.S.; Farah, I.O.; Yu, W.W.; Bednar, A.J. Probing Metabolic Stability of CdSe Nanoparticles: Alkaline Extraction of Free Cadmium from Liver and Kidney Samples of Rats Exposed to CdSe Nanoparticles. J. Hazard. Mater. 2011, S0304389411005498. [Google Scholar] [CrossRef] [Green Version]
- Gajdosechova, Z.; Lawan, M.M.; Urgast, D.S.; Raab, A.; Scheckel, K.G.; Lombi, E.; Kopittke, P.M.; Loeschner, K.; Larsen, E.H.; Woods, G.; et al. In Vivo Formation of Natural HgSe Nanoparticles in the Liver and Brain of Pilot Whales. Sci. Rep. 2016, 6, 34361. [Google Scholar] [CrossRef] [Green Version]
- Gray, E.P.; Coleman, J.G.; Bednar, A.J.; Kennedy, A.J.; Ranville, J.F.; Higgins, C.P. Extraction and Analysis of Silver and Gold Nanoparticles from Biological Tissues Using Single Particle Inductively Coupled Plasma Mass Spectrometry. Environ. Sci. Technol. 2013, 47, 14315–14323. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Trujilloa, S.; Jiménez-Morenoa, M.; Ríos, A.; del Carmen Rodríguez Martín-Doimeadios, R. A Simple Analytical Methodology for Platinum Nanoparticles Control in Complex Clinical Matrices via SP-ICP-MS. Talanta 2021, 231, 122370. [Google Scholar] [CrossRef] [PubMed]
- Abdolahpur Monikh, F.; Chupani, L.; Zuskovα, E.; Peters, R.; Vancovα, M.; Vijver, M.G.; Porcal, P.; Peijnenburg, W.J.G.M. Method for Extraction and Quantification of Metal-Based Nanoparticles in Biological Media: Number-Based Biodistribution and Bioconcentration. Environ. Sci. Technol. 2019, 53, 946–953. [Google Scholar] [CrossRef]
- Bocca, B.; Battistini, B.; Petrucci, F. Silver and Gold Nanoparticles Characterization by SP-ICP-MS and AF4-FFF-MALS-UV-ICP-MS in Human Samples Used for Biomonitoring. Talanta 2020, 220, 121404. [Google Scholar] [CrossRef] [PubMed]
- Witzler, M.; Küllmer, F.; Günther, K. Validating a Single-Particle ICP-MS Method to Measure Nanoparticles in Human Whole Blood for Nanotoxicology. Analytical Letters 2018, 51, 587–599. [Google Scholar] [CrossRef]
- Ramos, K.; Ramos, L.; Gómez-Gómez, M.M. Simultaneous Characterisation of Silver Nanoparticles and Determination of Dissolved Silver in Chicken Meat Subjected to in Vitro Human Gastrointestinal Digestion Using Single Particle Inductively Coupled Plasma Mass Spectrometry. Food Chem. 2017, 221, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wu, Y.; Wang, J.; Shang, X.; Jiang, X. Simultaneous Preconcentration of Cadmium and Lead in Water Samples with Silica Gel and Determination by Flame Atomic Absorption Spectrometry. J. Environ. Sci. 2013, 25, S45–S49. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Li, Y.; Jiang, Y.; Yan, X.-P. Magnetic Immobilization of Amine-Functionalized Magnetite Microspheres in a Knotted Reactor for on-Line Solid-Phase Extraction Coupled with ICP-MS for Speciation Analysis of Trace Chromium. J. Anal. At. Spectrom. 2010, 25, 1467. [Google Scholar] [CrossRef]
- Xie, L.; Jiang, R.; Zhu, F.; Liu, H.; Ouyang, G. Application of Functionalized Magnetic Nanoparticles in Sample Preparation. Anal. Bioanal. Chem. 2014, 406, 377–399. [Google Scholar] [CrossRef]
- Afkhami, A.; Saber-Tehrani, M.; Bagheri, H.; Madrakian, T. Flame Atomic Absorption Spectrometric Determination of Trace Amounts of Pb(II) and Cr(III) in Biological, Food and Environmental Samples after Preconcentration by Modified Nano-Alumina. Microchim. Acta 2011, 172, 125–136. [Google Scholar] [CrossRef]
- Huang, C.; Hu, B.; Jiang, Z. Simultaneous Speciation of Inorganic Arsenic and Antimony in Natural Waters by Dimercaptosuccinic Acid Modified Mesoporous Titanium Dioxide Micro-Column on-Line Separation and Inductively Coupled Plasma Optical Emission Spectrometry Determination. Spectrochim. Acta Part B At. Spectrosc. 2007, 62, 454–460. [Google Scholar] [CrossRef]
- Manzoori, J.L.; Amjadi, M.; Hallaj, T. Preconcentration of Trace Cadmium and Manganese Using 1-(2-Pyridylazo)-2-Naphthol-Modified TiO2 Nanoparticles and Their Determination by Flame Atomic Absorption Spectrometry. Int. J. Environ. Anal. Chem. 2009, 89, 749–758. [Google Scholar] [CrossRef]
- He, Q.; Chang, X.; Huang, X.; Hu, Z. Determination of Trace Elements in Food Samples by ICP-AES after Preconcentration with p-Toluenesulfonylamide Immobilized on Silica Gel and Nanometer SiO2. Microchim. Acta 2008, 160, 147–152. [Google Scholar] [CrossRef]
- Saleh, T.A. Trends in the Sample Preparation and Analysis of Nanomaterials as Environmental Contaminants. Trends Environ. Anal. Chem. 2020, 28, e00101. [Google Scholar] [CrossRef]
- López-Sanz, S.; Guzmán Bernardo, F.J.; Rodríguez Martín-Doimeadios, R.C.; Ríos, A. Analytical metrology for nanomaterials: Present achievements and future challenges. Anal. Chim. Acta 2019, 1059, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.; Ma, S.; Schmalzried, T.; Amstutz, H.C. Tissue Digestion for Wear Debris Particle Isolation. J. Biomed. Mater. Res. 1994, 28, 523–526. [Google Scholar] [CrossRef]
| Analyte | Matrix | Analytical Method | LOD | Ref |
|---|---|---|---|---|
| metal particles and metalloproteins | biofluids | AF4-ICP-MS | [42] | |
| Au NPs | Rat liver | AF4-ICP-MS | [48] | |
| Cd, Pb, Hg | Cultured cells | ETV-ICP-MS | Cd (0.72 ng/L), Hg (0.86 ng/L), Pb (1.12 ng/L) | [39] |
| Ag NPs | Chicken meat | sp-ICP-MS | [40] | |
| Ag NPs | Human tissue | sp-ICP-MS | [41] | |
| CdSe | Rat liver and kidney | sp-ICP-MS | [43] | |
| HgSe NPs | Whale liver and brain | sp-ICP-MS | [44] | |
| Ag NPs, Au NPs | Beef, Daphnia magna, Lumbriculus variegatus | sp-ICP-MS | [45] | |
| Platinum nanoparticles PTNPs | human urine, blood serum | SP-ICP-MS | 1.9 × 105 particles/L | [47] |
| Silver nanoparticles | human tissue | (SP-ICP-MS) | [46] | |
| Silver and gold nanoparticles | human samples | SP-ICP-MS, AF4-FFF-MALS-UV-ICP-MS | 0.0006 ng/mL | [48] |
| silver nanoparticles | chicken meat subjected to in vitro human | SP-ICP-MS | 0.5 ng/L | [50] |
| gastrointestinal digestion | ||||
| saliva, gastric and intestinal digestions. | ||||
| Au NPs | human blood | SP-ICP-MS | Ag 15 ng/L | [49] |
| Ag NPs | Au 25 ng/L | |||
| Al(III) | Hair and scallop reference | ICP-OES | 60 pg/mL | [51] |
| materials, spiked water samples, | ||||
| human urine | ||||
| Cr(III), Cr(VI) | Drinking water | ICP-MS | Cr(III) 1.5 ng/L | [52] |
| Cr(VI) 2.1 ng/L | ||||
| Cd(II) | Water, wastewater, biological | FAAS | 0.14 lg/L | [53] |
| and food samples | ||||
| Pb(II), Cr(III) | Various water, food, industrial | FAAS | Pb(II) 0.43 lg/L | [54] |
| effluent, and urine samples | Cr(III) 0.55 lg/L | |||
| As(III), As(V), Sb(III), Sb(V) | natural waters | ICP-OES | on-line: As(III) 0.53 ng/mL, | [55] |
| As(V) 0.49 ng/mL | ||||
| Sb(III)0.77 ng/mL, | ||||
| Sb(V) 0.71 ng/mL | ||||
| off-line: As(III) 0.11 ng/mL | ||||
| As(III) 0.11 ng/mL, | ||||
| As(V) 0.10 ng/mL | ||||
| Sb(III)0.15 ng/mL, | ||||
| Sb(V) 0.13 ng/mL | ||||
| Mn, Cd | Water samples | FAAS | Mn (1.0 ng/mL), | [56] |
| Cd (0.96 ng/mL) | ||||
| Cd, Cr, Cu, Mn | Environmental samples | ICP-OES | Cd (48 ng/L), Cr | [57] |
| Cr 36 ng/L | ||||
| Cu 21 ng/L | ||||
| Mn 24 ng/L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vladitsi, M.; Nikolaou, C.; Kalogiouri, N.P.; Samanidou, V.F. Analytical Methods for Nanomaterial Determination in Biological Matrices. Methods Protoc. 2022, 5, 61. https://doi.org/10.3390/mps5040061
Vladitsi M, Nikolaou C, Kalogiouri NP, Samanidou VF. Analytical Methods for Nanomaterial Determination in Biological Matrices. Methods and Protocols. 2022; 5(4):61. https://doi.org/10.3390/mps5040061
Chicago/Turabian StyleVladitsi, Magdalini, Charalampia Nikolaou, Natasa P. Kalogiouri, and Victoria F. Samanidou. 2022. "Analytical Methods for Nanomaterial Determination in Biological Matrices" Methods and Protocols 5, no. 4: 61. https://doi.org/10.3390/mps5040061








