Protocol of Co-Culture of Human Osteoblasts and Osteoclasts to Test Biomaterials for Bone Tissue Engineering
Abstract
:1. Introduction
2. Experimental Design
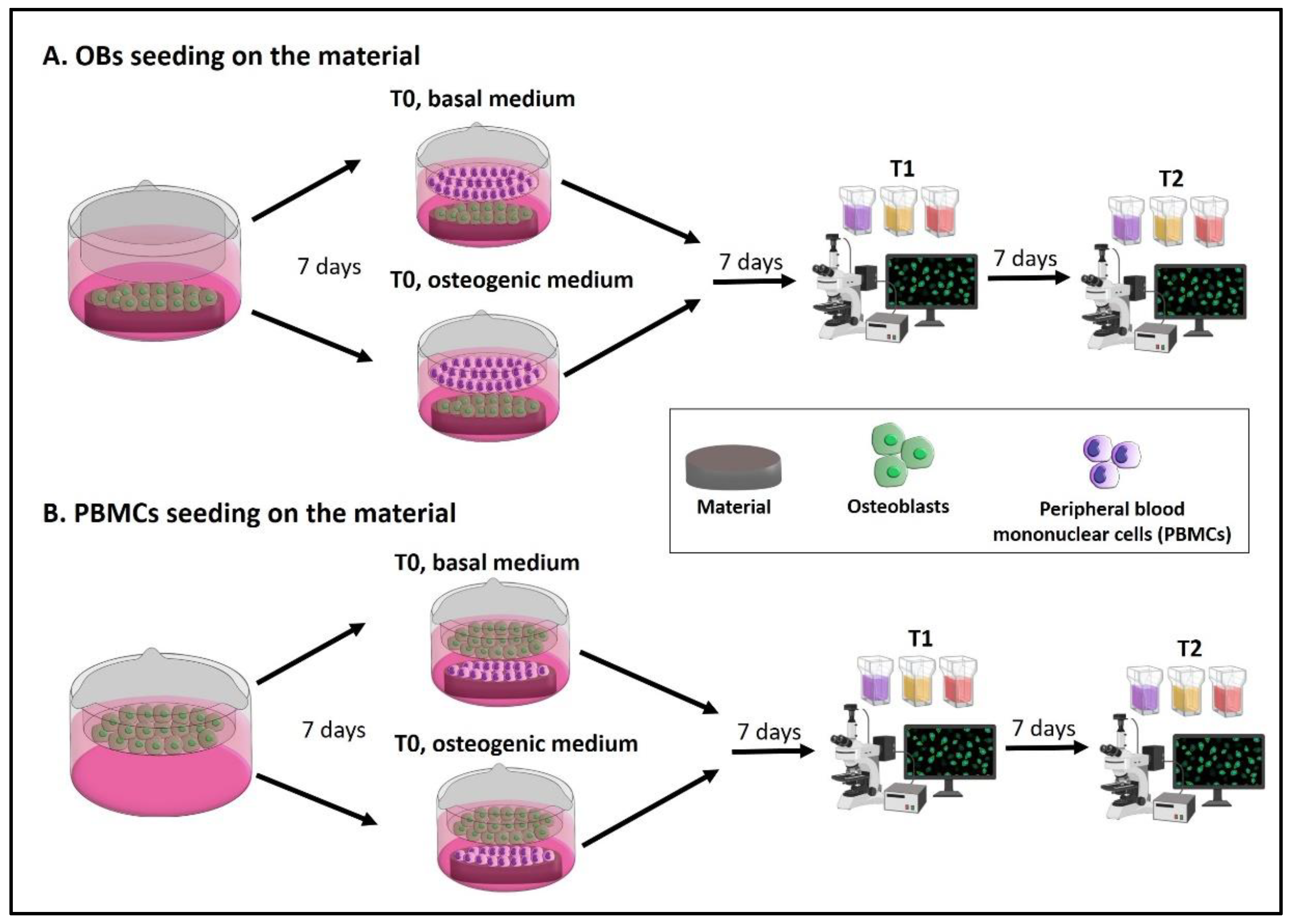
- A.
- The seeding of OBs on the material with PBMCs in the indirect co-culture. Seed OBs on the material and wait 7 days until cell confluence, then seed PBMCs into the transwell to begin the indirect co-culture (T0). The indirect co-culture is maintained in osteogenic medium or basal medium for 14 days (T2), with the intermediate step of 7 days (T1) to check cell behavior.
- B.
- The seeding of PBMCs on the material with OBs in the indirect co-culture. Seed OBs into the transwell and wait 7 days until cell confluence, then seed PBMCs on the material to begin the indirect co-culture (T0). The indirect co-culture is maintained in osteogenic medium or basal medium for 14 days (T2), with the intermediate step of 7 days (T1) to check cell behavior.
2.1. Materials: Reagents and Reagents Set Up (for Composition of Solutions, See Section 5) (All the Solutions, Instruments and Tissue Culture Plastics Must Be Sterile)
- Wash buffer: 1× phosphate buffered saline (PBS).
- Fixative solution n.1.
- Fixative solution n.2.
- Fixative solution n.3.
- Fixative solution n.4.
- Ficoll-Paque PLUS.
- Ca+2-free complete culture medium for OBs.
- Complete culture medium for OBs.
- Complete culture medium for PBMCs.
- Cell freezing medium.
- Basal medium.
- Osteogenic medium.
- Differentiation medium for PBMCs.
- Trypsin-EDTA solution.
- Alizarin Red S (ARS) staining solution.
- Von Kossa staining solution.
- Fluorescein isothiocyanate (FITC)-phalloidin (Sigma-Aldrich, P-5282, Milan, Italy) staining solution.
- Leukocyte Alkaline phosphatase assay (ALP staining) (Sigma-Aldrich, 86R, Milan, Italy).
- Acid phosphatase leukocyte assay (Tartrate-resistant acid phosphatase—TRAP staining) (Sigma-Aldrich, 387A, Milan, Italy).
- Nuclear staining.
- Erythrosin B staining solution 1×. Instead of Erythrosin B, Trypan blue dye may be applied. Please note that the operating principle is the same: both dyes stain dead cells only, since the dyes cannot penetrate the cell membrane of live cells to enter the cytoplasm. Under light microscopy, count only non-stained cells (viable).
- EDTA-based buffer for the lysis of red blood cells (RBCs).
- Tris buffer + 0.1% Triton X-100 pH 7.5.
- P-nitrophenyl phosphate liquid substrate system (PNP) (Sigma-Aldrich, N7653, Milan, Italy).
- 10% cetylpyridinium solution.
- 0.1 M sodium cacodylate buffer (Thermo Fisher Scientific, 15463149, Milan, Italy).
- 1% osmium tetroxide solution.
- Live dead assay (LIVE/DEAD™ Cell Imaging Kit (488/570), Invitrogen, Thermo Fisher Scientific, Milan, Italy).
2.2. Equipment
- Tissue culture plastic 25 cm2 and 75 cm2 flask for isolation and expansion of OBs, (75 cm2 Euroclone, ET7076, Milan, Italy; 25 cm2 Corning, Life Sciences, Falcon, 353108, Turin, Italy).
- 8-well glass chamber-slides for PBMCs and OBs single-culture (SARSTEDT, 94.6170.802, Nümbrecht, Germany), 0.8 cm2 growth area.
- Tissue culture plastics 24-well plate for the co-culture (Falcon, 353047, Turin, Italy).
- Transwell device: insert for 24-well tissue culture plate, with polyethylene terephthalate (PET) membrane and polystyrene (PS) as frame material, transparent, sterile, non-pyrogenic/endotoxin-free, non-cytotoxic, with a flat base, 0.4 µm pore size, 0.3 cm2 growth area, 0.1–0.4 mL working volume (SARSTEDT, 83.3932.041, Nümbrecht, Germany). Please note that is important that the transwell fit into the well of the 24-well tissue culture plates and the plate can be closed.
- Tissue culture (TC) coverslip for 24-well tissue culture plates (13 mm diameter): polyethylene terephthalate, glycol-modified (PET-G), transparent, sterile, non-pyrogenic/endotoxin-free, non-cytotoxic (SARSTEDT, 83.1840.002, Nümbrecht, Germany).
- Horizontal laminar flow station (Heraguard, Thermo Fisher Scientific, Milan, Italy).
- CO2 incubator.
- 50-mL Falcon tubes.
- Pipettes.
- p200 and p1000 pipet tips.
- Centrifuge.
- Fluorescent optical microscope (Nikon Eclipse E800) equipped with NIS-Element image software BR4.00.00 (Nikon), NIS-Element image software Advanced Research (Version 5.30) (Nikon, Amsterdam, Netherlands).
- Inverted phase contrast microscope.
- Camera (microscope-connected camera).
- Image analysis software.
- Hemocytometer.
- Spectrophotometer for microplates (Infinite F200 PRO, TECAN, Mannedorf, Switzerland).
3. Procedure
3.1. Osteoblasts Isolation Procedure
- (a)
- Wash bone fragments in PBS for 5 min twice and place them in 25 cm2 culture flask with at least 5 up to 10 mL of Ca+2-free complete culture medium for OBs (to inhibit fibroblast growth). Culture OBs at 37 °C, 5% CO2, and 95% humidity with Ca+2-free complete culture medium changes every 3 days.
CRITICAL STEP: use a minimum volume of medium in order to maintain the bone fragments covered by medium but adherent to the flask bottom.
- (b)
- At confluence (usually after 2–3 weeks), wash OBs with PBS, detach them from the flask by trypsin-EDTA digestion, and subculture by reseeding in two new 75 cm2 flasks. At confluence OBs may be detached and used (passage 1).
- (c)
- Count OBs: stain 20 μL of cell suspension with 20 μL of erythrosine B solution, mix gently, and collect 10 μL to be counted in hemocytometer under the inverted microscope.
- I.
- To store OBs in liquid nitrogen, centrifuge the residual OBs at 1050 rpm (RCF 132) for 30 min (Centrifuge Eppendorf 5810). Resuspend the pellet in 1 mL of cell freezing medium and transfer the cell suspension into a cryogenic vial. Use 1 mL of cell freezing solution for each 25 cm2 or 75 cm2 flask.
- II.
- Keep the cryogenic vial at −80 °C for 3 days.
- III.
- Store the cryogenic vial in liquid nitrogen.
3.2. Peripheral Blood Mononuclear Cells Isolation Procedure
- (a)
- Dilute the buffy coat sample with an equal amount of 1× PBS (1:1 final dilution).
- (b)
- Pour 3 mL of Ficoll-Paque PLUS at the bottom of 15 mL tubes. Subsequently gently add 4 mL of diluted blood overlaying the Ficoll layer, being careful not to break the gradient layer. When working with 50 mL tubes, the Ficoll:blood ratio is usually 15:20 mL; these volumes may be increased, but the efficiency of PBMC isolation can be negatively affected.
- (c)
- Gradient centrifugation: centrifuge the tubes at room temperature (RT) and 2000 rpm (RCF 479) for 30 min with the breaks off.
CRITICAL STEP turning off the break enables the PBMCs to stay onto the gradient layer without being forced into the erythrocytes layer, while keeping the break on during the deceleration could disrupt the density gradient since a rapid and turbulent vortex is generated in the middle of the tube. This vortex picks up the thin layer of cells and redistributes them in the supernatant.
- (d)
- At the end of the first gradient centrifugation, PBMCs form a “ring” between Ficoll and plasma, as illustrated in Figure 2. Harvest each layer of PBMCs and transfer it into a new tube. Then, add 15 mL (or 50 mL) of PBS.
CRITICAL STEP harvest the PBMCs ring with a 1000 µL pipette tip.
- (e)
- Centrifuge the tubes at RT and 1600 rpm (RCF 306) for 20 min keeping the breaks off.
- (f)
- Harvest each PBMC-pellet and transfer to new tubes. Then, add 15 mL (or 50 mL) of PBS.
CRITICAL STEP harvest the PBMCs ring with a 1000 µL pipette tip.
- (g)
- Centrifuge the tubes at RT and 1600 rpm (RCF 306) for 10 min keeping off the breaks.
- (h)
- Harvest each pellet and transfer into a new tube. Then, add 15 mL (or 50 mL) of PBS.
CRITICAL STEP harvest the PBMCs ring with a 1000 µL pipette tip.
- (i)
- Centrifuge the tubes at RT and 1400 rpm (RCF 234) for 10 min keeping the breaks off.
- (j)
- Remove the supernatant and resuspend the pellet in DMEM-HG (about 30 mL).
- (k)
- Count PBMCs: dilute 50 μL of cell suspension in 450 μL of PBS, then mix gently, stain with 500 μL of 1× erythrosin B staining solution and mix gently again. Count the viable cells (viable cells are not pink-stained) in 10 μL of cell suspension in a hemocytometer under the inverted microscope.

3.3. Indirect OBs/PBMCs Co-Culture and Single-Culture Controls
- (a)
- Seed 5 × 103 OBs in complete DMEM-LG into transwells seven days before the start of the co-culture, to achieve a confluent monolayer at day 7. Change the medium twice a week.
- (b)
- Equip the bottom of the multiwell plate with a proper-sized TC coverslip previously pre-wetted with complete DMEM-HG for 2 h at least.
- (c)
- Seed 6 × 106/well PBMCs in complete DMEM-HG freshly isolated from buffy coat samples in the proper-sized TC coverslip at the bottom of the 24-multiwell plate, starting the co-culture (time 0).
- (d)
- Incubate at 37 °C, 5% CO2 and 95% humidity to allow cell settling; the following day remove the old medium and refresh with the basal medium or osteogenic medium, with medium change twice a week.
- (e)
- Maintain the indirect co-culture for 7 and 14 days in basal medium or osteogenic medium.
- (a)
- Seed 1.5 × 104/well OBs in complete DMEM-LG in 8 well chamber-slides.
- (b)
- Incubate at 37 °C to allow cell settling; the following day remove the old medium and refresh with the basal medium or osteogenic medium, with medium change twice a week.
- (c)
- Maintain OBs in culture in basal and osteogenic medium, to be tested at 7 and 14 days.
- (a)
- Seed 2.4 × 106/well PBMCs in complete DMEM-HG in 8 well chamber-slides.
- (b)
- Incubate overnight at 37 °C, 5% CO2 and 95% humidity to allow cell settling; the following day, remove the non-adherent PBMCs by changing the old medium with the differentiation medium for PBMCs. Refresh the medium twice a week.
- (c)
- Maintain PBMCs culture in differentiation medium to be tested at 7 and 14 days..
3.4. Cell Biochemistry—Cytochemical Staining Protocols
- (a)
- Remove the medium and separate the transwell (with OBs seeded) and the TC coverslip (with PBMCs seeded) of the same well and rinse the cell layers with PBS.
- (b)
- For each cytochemical staining, fix the cell layer with the appropriate fixative solution as follows:
- I.
- For Alizarin Red S staining use 3.7% paraformaldehyde solution in PBS 1× for 20 min at RT.
- II.
- For von Kossa staining use ice-cooled methanol kept at −20 °C for 5 min.
- III.
- For Alkaline phosphatase (ALP) staining use fixative solution n.3 for 30 min at RT.
- IV.
- For tartrate-resistant acid phosphatase (TRAP) staining use fixative solution n.2 for 20 min.
- V.
- For phalloidin-tetramethylrhodamine B isothiocyanate (TRITC) or phalloidin-fluorescein isothiocyanate (FITC) use fixative solution n.1 for 5 min.
- (c)
- Wash the cell layer with PBS 1× for 5–10 min for ARS, von Kossa, TRAP, and phalloidin stainings or distilled water for 45 min for ALP staining.
- (d)
- Stain the cell layer.
- I.
- For Alizarin Red S staining: add Alizarin Red S staining solution for 1 h at RT.
- II.
- For Von Kossa staining: add 2% AgNO3 solution for 1 h at RT under light and wash twice with distilled water. Add 2.5% sodium thiosulfate solution for 5 min and wash twice with distilled water. Lastly, add 0.33% neutral red solution for 1 h. The different solutions must be added in the order reported.
- III.
- For Alkaline phosphatase (ALP) staining: add ALP stain solution for 15 min at RT avoiding light exposure.
CRITICAL STEP Incubate the staining solution in dark conditions to minimize potential artifacts.
- IV.
- For tartrate-resistant acid phosphatase (TRAP) and Hoechst staining: add HEPES-Triton solution for 5 min to permeabilize cell membrane, wash with PBS; incubate cell monolayer with TRAP staining solution for 1 h at 37 °C in dark conditions.
CRITICAL STEP Incubate the staining solution at 37 °C in dark conditions to minimize potential artifacts.
- After a fast rinse with distilled water, stain nuclei with Hoechst staining for 10 min in the dark.
CRITICAL STEP Incubate the staining solution in dark conditions to minimize potential artifacts.
- V.
- For phalloidin-tetramethylrhodamine B isothiocyanate (TRITC) or phalloidin-fluorescein isothiocyanate (FITC): add TritonX 0.5% in PBS for 10 min to permeabilize cell membrane, incubate cell monolayer with phalloidin-FITC or phalloidin-TRITC staining solution in PBS for 45 min in the dark. After a fast rinse with PBS, stain nuclei with Hoechst staining for 10 min in the dark.
CRITICAL STEP Incubate the staining solution in dark conditions to minimize potential artifacts.
- (e)
- After staining, wash cells for 5–10 min with PBS 1× in the case of ALP or with distilled water when using von Kossa, TRAP, phalloidin, and Alizarin Red S stainings; for Alizarin Red S staining rinse with distilled water until the washing solution becomes clear.
- (f)
- Mount glass coverslip using 70% glycerol solution or specific aqueous mounting media.
3.5. Material Testing with the Indirect Co-Culture
- (a)
- The material is dipped in complete culture medium for OBs or PBMCs: the presence of FBS in the medium, whatever it is, is needed for the adsorption of serum proteins to the material.
- (b)
- Incubate at 37 °C overnight.
- (c)
- Remove the complete culture medium.
- (a)
- Prepare the OB suspension according to the material surface area and the seeding density/cm2 reported in Section 3.3—“a” (5 × 103/0.3 cm2). To cover the entire surface with the cell suspension, resuspend the final number of OBs in a proper medium volume using the complete culture medium for OBs.
- (b)
- Incubate the OB-laden material for 7 days to achieve a confluent monolayer, with medium change every 3 days.
- (c)
- Seed PBMCs in the transwell with a cell seeding density as reported in Section 3.3—“c” (9 × 105 PBMCs/transwell (3 × 106/cm2)). Use complete culture medium for PBMCs.
- (d)
- Incubate the PBMC-loaded transwell for 3 h at 37 °C.
- (e)
- Insert the transwell in the well using sterile tweezers.
- (f)
- Add basal medium or osteogenic medium both into the well and transwell.
- (a)
- Seed OBs in the transwell with a density as reported in Section 3.3—“a”, that is 5 × 103 OBs/transwell (5 × 103/0.3 cm2) using complete culture medium.
- (b)
- Incubate OBs for 7 days to achieve a confluent monolayer, with medium change every 3 days.
- (c)
- Prepare the PBMC suspension according to the material surface area and the seeding density/cm2 reported in Section 3.3—“c” (3 × 106/cm2). To cover the entire surface with cell suspension resuspend the final number of PBMCs in a proper medium volume using the complete culture medium for PBMCs.
- (d)
- Incubate the PBMCs-laden material for 3 h at 37 °C.
- (e)
- Insert the OBs-loaded transwell into the well using sterile tweezers.
- (f)
- Add basal medium or osteogenic medium to the well and transwell.
- (a)
- ALP staining can be replaced by ALP quantification in the OB lysates and cell culture supernatant.
- To measure ALP in the cell lysate, after the removal of the cell culture medium, wash the sample twice with PBS and add 200 µL of Tris buffer + 0.1% Triton X-100 pH 7.5. Following incubation for 10 min at 37 °C, mix 100 µL of the cell lysate with 100 µL of PNP (final volume 200 µL). Transfer 100 µL of the mixture in a well of a 96 well-plate and read the absorbance at λ 405 nm with a microplate spectrophotometer.
- To measure ALP in the supernatant, harvest 100 µL of 3 day-aged cell culture medium for each sample and add 100 µL of PNP (final volume 200 µL). After transferring 100 µL of the mixture in a 96 well-plate, the absorbance is read at λ 405 nm with a microplate spectrophotometer.
- An example of ALP content in the OB lysate and supernatant derived from the indirect co-culture within Coll_MBG and Coll_HA is shown in Figure 14.
- (b)
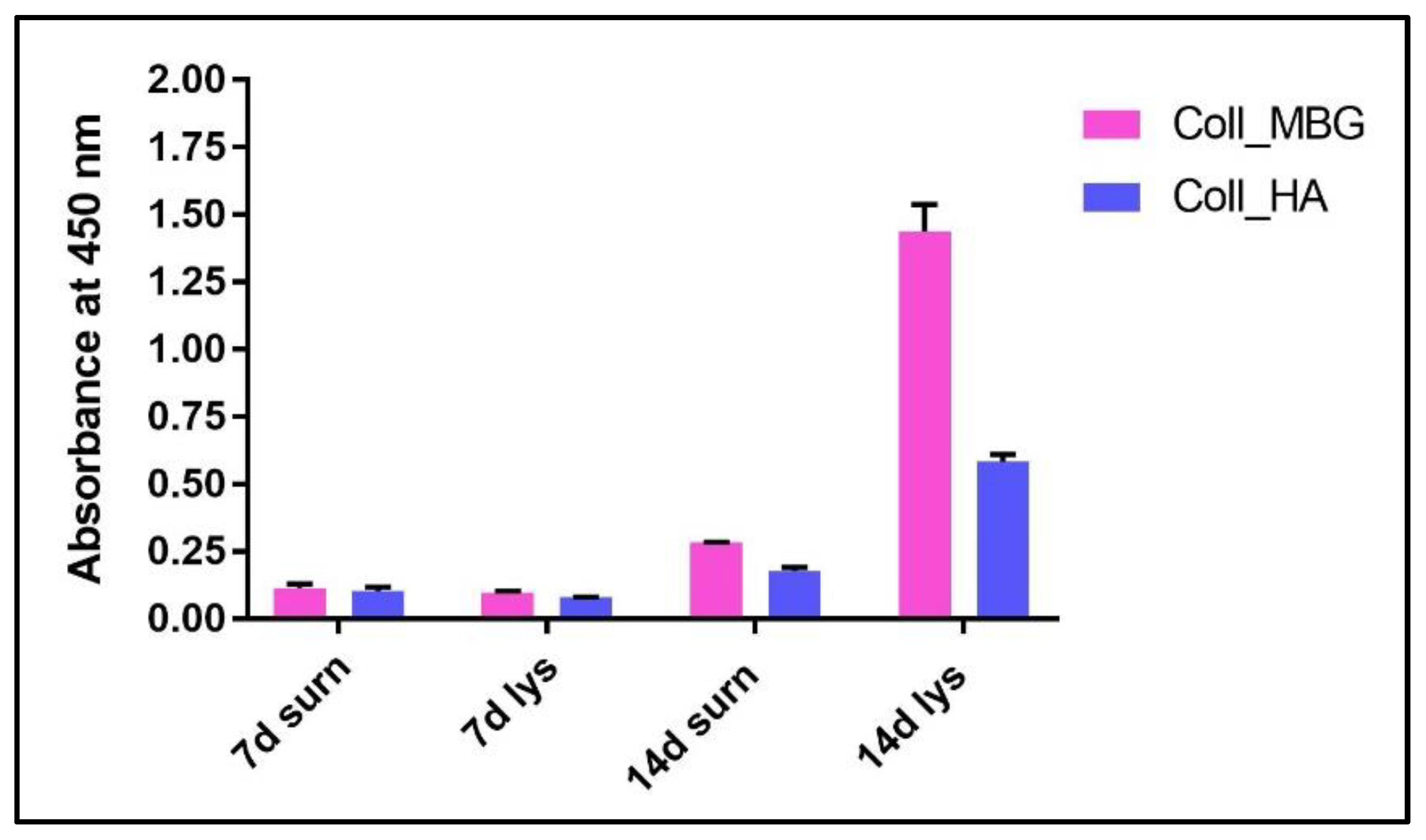
- ARS staining on OBs is performed as described in Section 3.4 “Cell Biochemistry—Cytochemical staining protocols” to be followed by stain elution: incubate each sample with 500 µL of 10% cetylpyridinium solution at room temperature for 5 min under orbital shaking. Transfer 100 µL of the solution in a 96-well plate and read the absorbance at λ 570 nm with a microplate spectrophotometer. An example of ARS eluate reading on OBs derived from the indirect co-culture with Coll_MBG and Coll_HA is shown in Figure 15.
- (c)
- TRAP stain for PBMCs can be substituted with ELF97, a fluorescent method for TRAP-positive granule detection.
- After the removal of the cell culture medium, the samples are rinsed twice with PBS and cells are fixed with fixative solution n.2 for 20 min. Following a wash of the cell layer with PBS 1× for 5–10 min, the samples are stained with 500 µL (or a final volume able to cover the sample) of ELF97 200 µM for 15 min avoiding light exposure. After rinsing the cell layer with PBS 1× the cell layer is observed under a fluorescent microscope.
- (d)
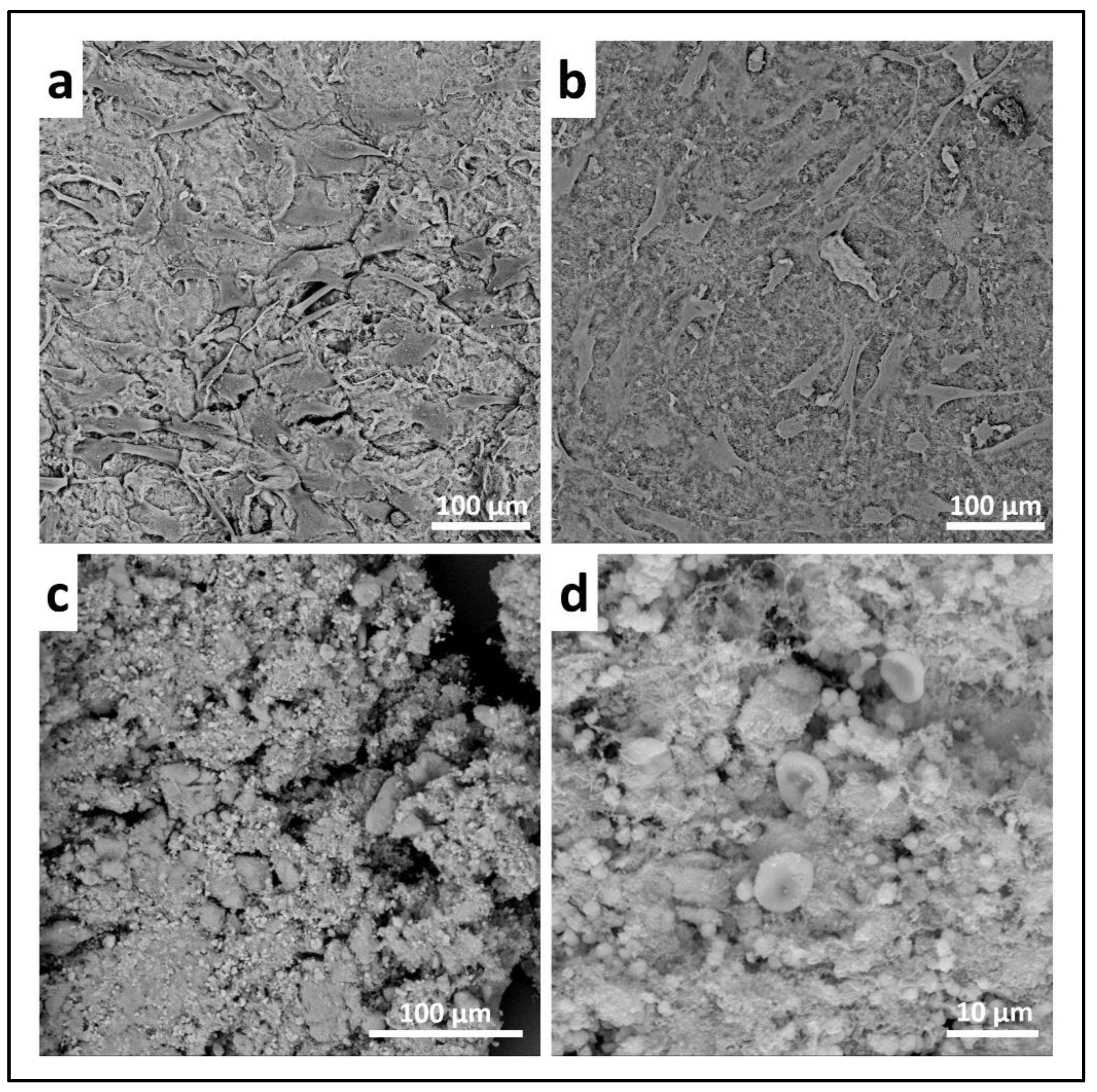
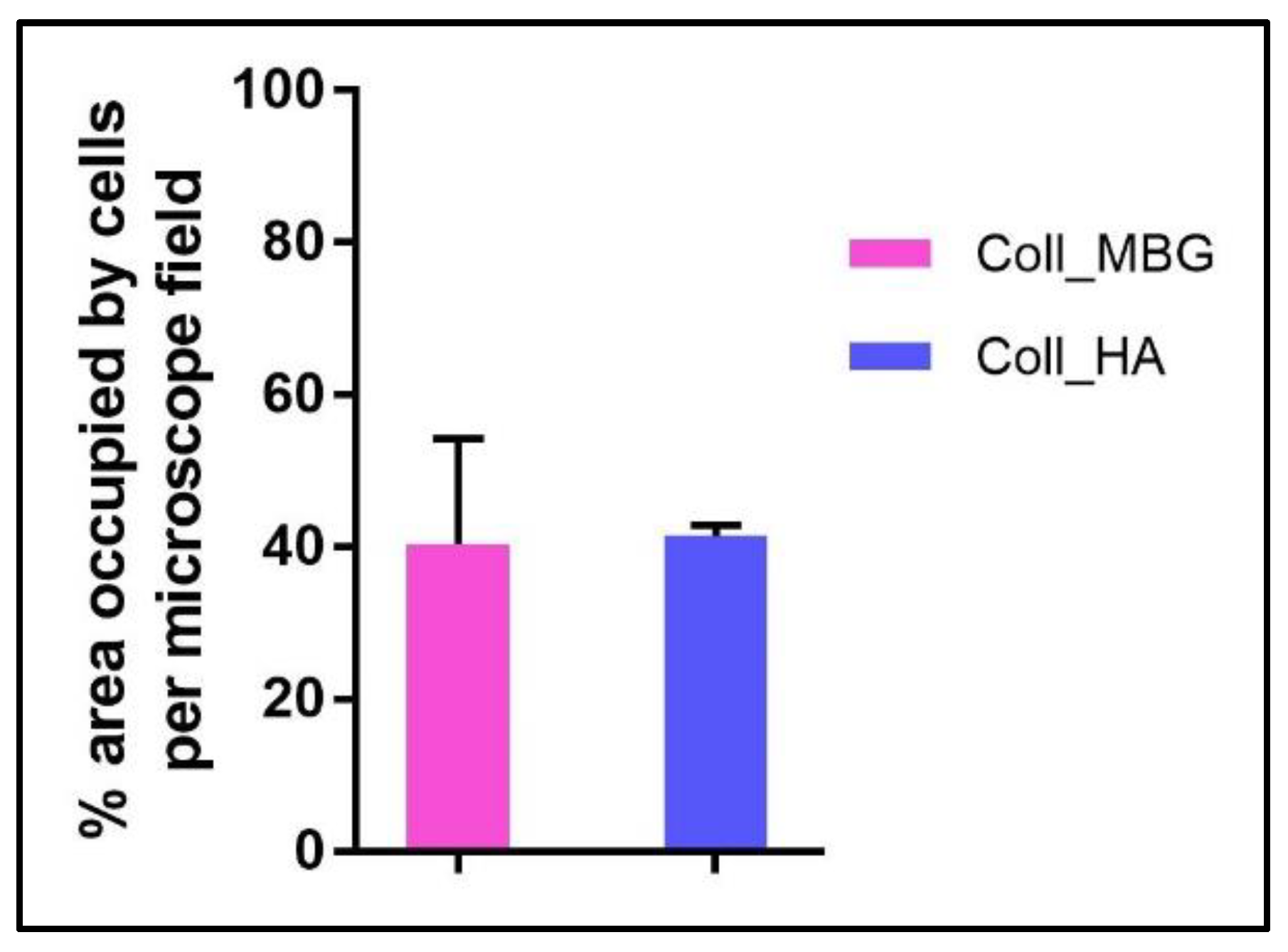
- Scanning electron microscopy (SEM) may be applied to observe the morphology of cells seeded on the material. After the removal of the cell culture medium, the samples are rinsed with PBS and fixed with fixative solution n.4 at 4 °C for 1 h. Following a wash with 0.1 M sodium cacodylate buffer for 15 min at room temperature, the samples are post-fixed with 1% osmium tetroxide solution at 4 °C for 1 h. After another wash with 0.1 M sodium cacodylate buffer for 15 min at room temperature, the samples are dehydrated in graded alcohol series: EtOH 25%, EtOH 50%, EtOH 70%, EtOH 80%, EtOH95% for 15 min, then EtOH 100% twice for 20 min with final dehydration twice in hexamethyldisilane (Electron Microscopy Sciences, Hatfield, PA, 16700) for 10 min at room temperature. Sputter-coated samples are examined by SEM. Representative images by SEM of cell adhesion on Coll_MBG and Coll_HA are reported in Figure 16, where OBs and PBMCs seeded on the two materials are shown.
- (e)
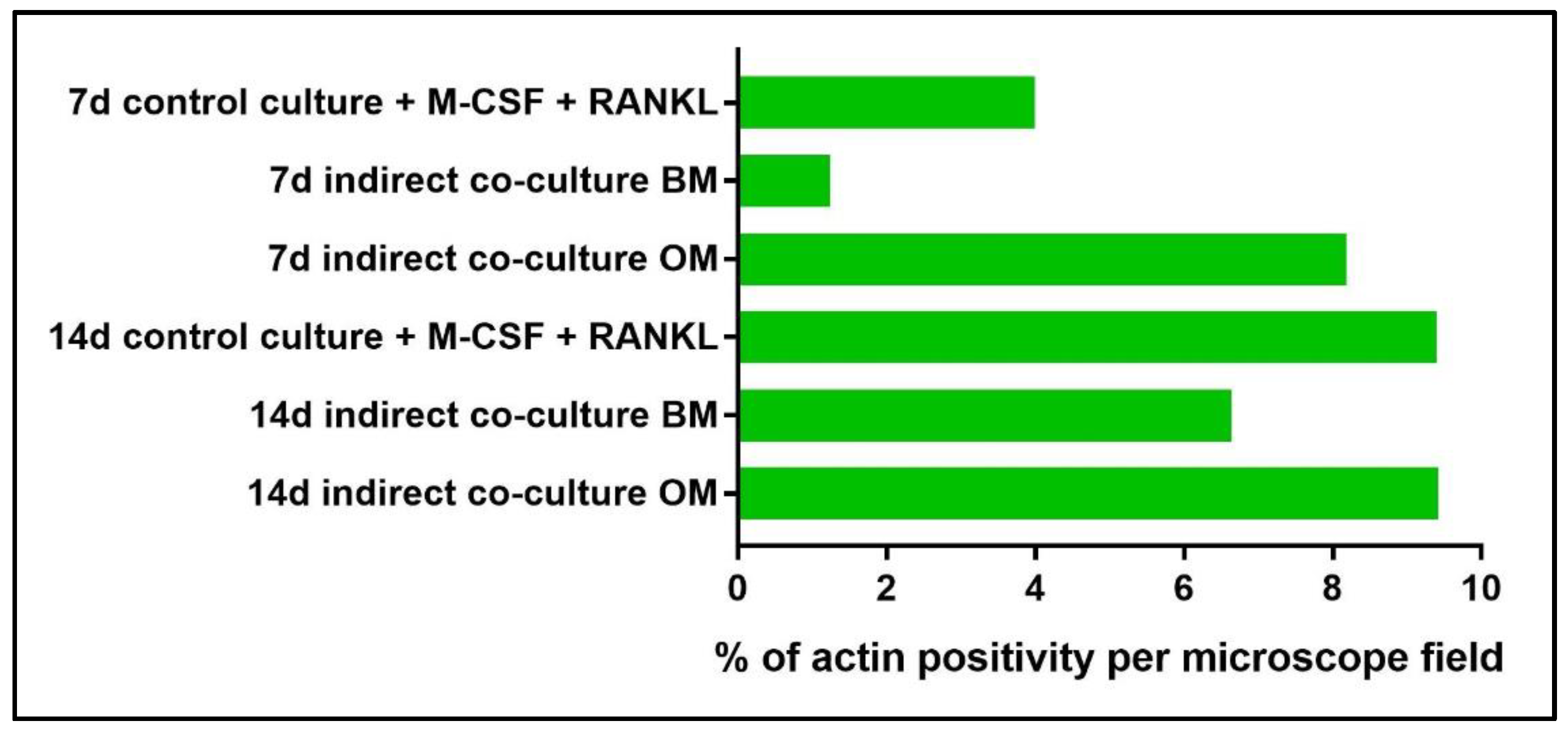
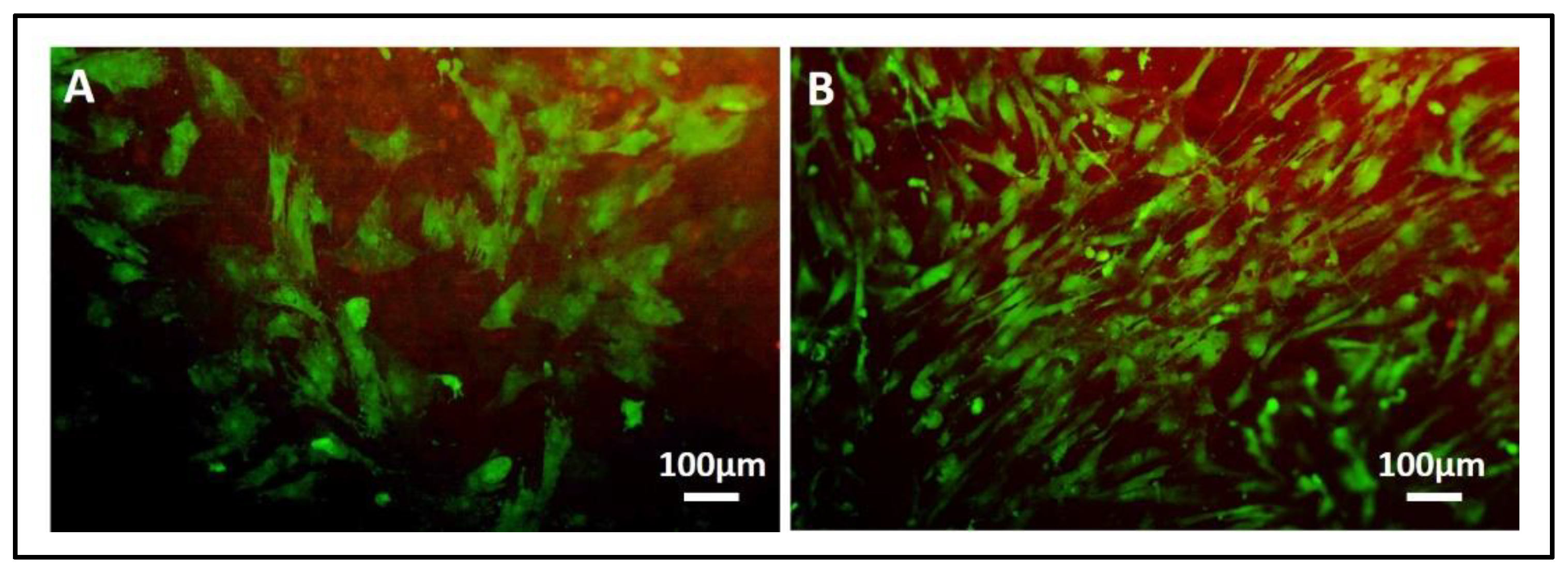
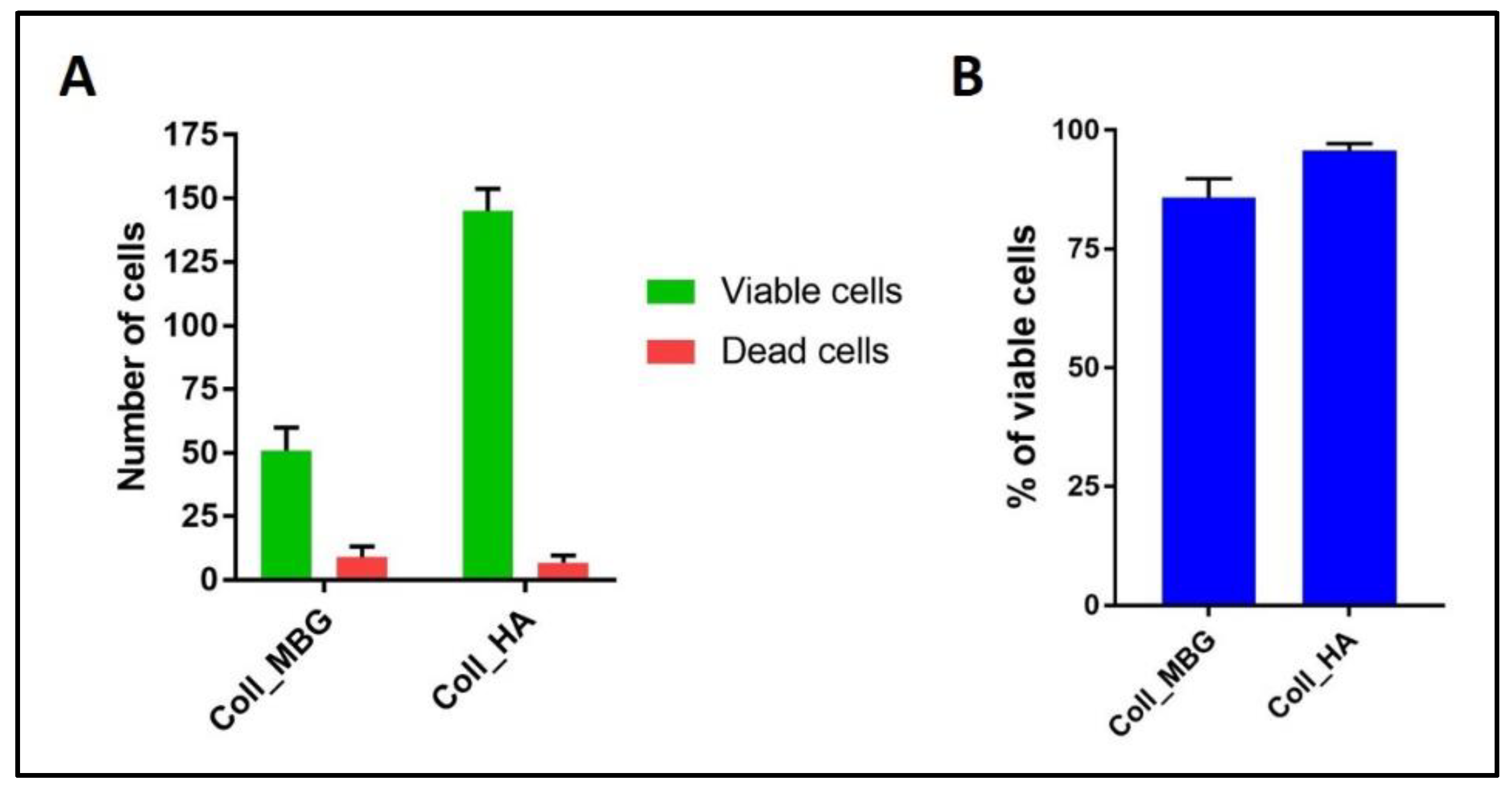
- In addition, cell viability can be evaluated with live-dead assay, a fluorochrome-based method that allows the observation of viable cells seeded on the material. Briefly, after the removal of the cell culture medium and a wash with PBS 1×, the samples are incubated with the live-dead staining solution in an orbital shaker for 20 min at 37 °C, protected from light. The staining solution is prepared by mixing the two vials with 1 mL of culture medium without FBS. After rinsing with PBS, the cells are observed under an optical fluorescent microscope (Nikon Eclipse E800) and the images acquired at different magnifications using the NIS-Element imaging software BR4.00.00 (Nikon) (Figure 17).
3.6. Timing and Trouble Shooting
3.7. Statistical Analysis
4. Expected Results
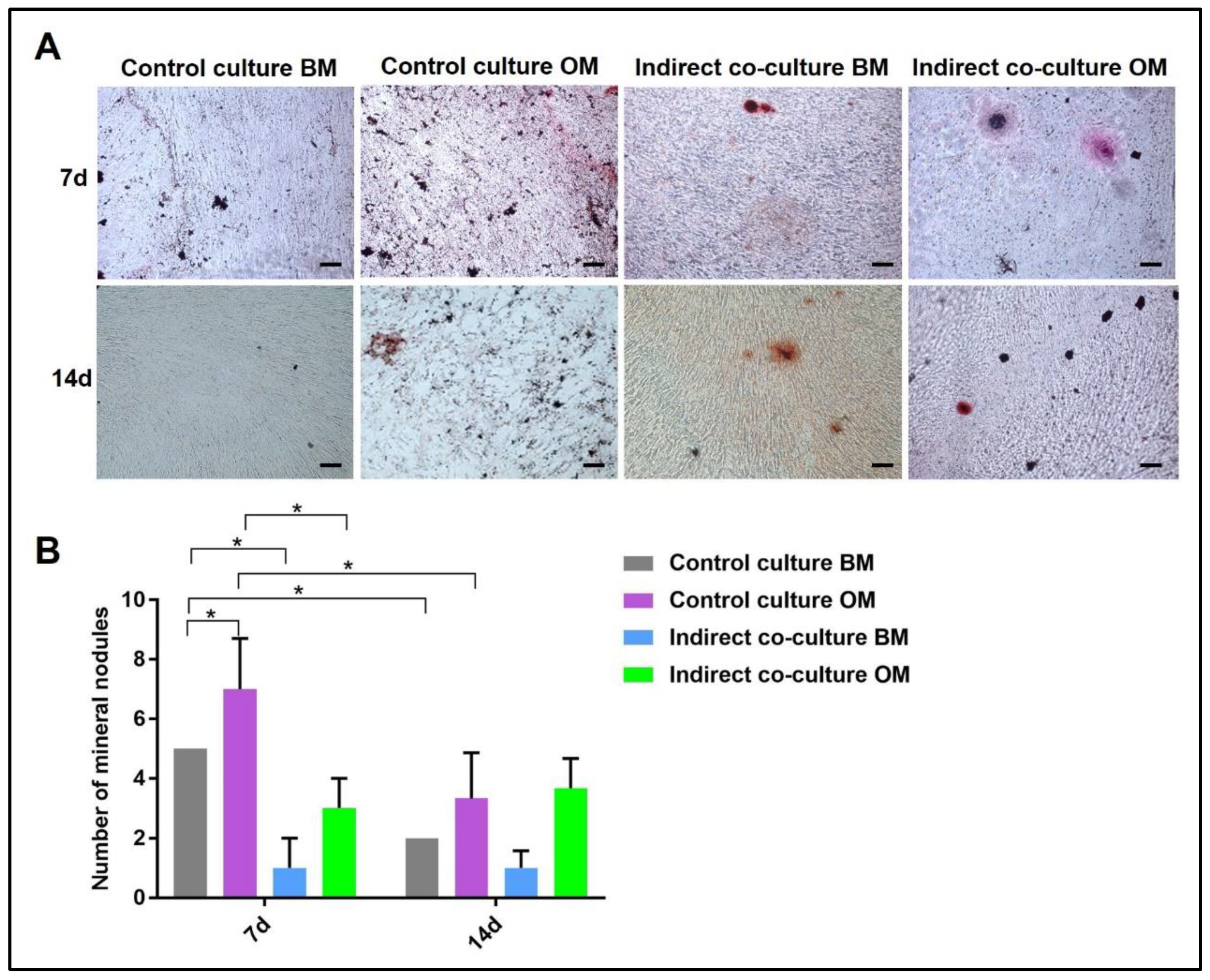
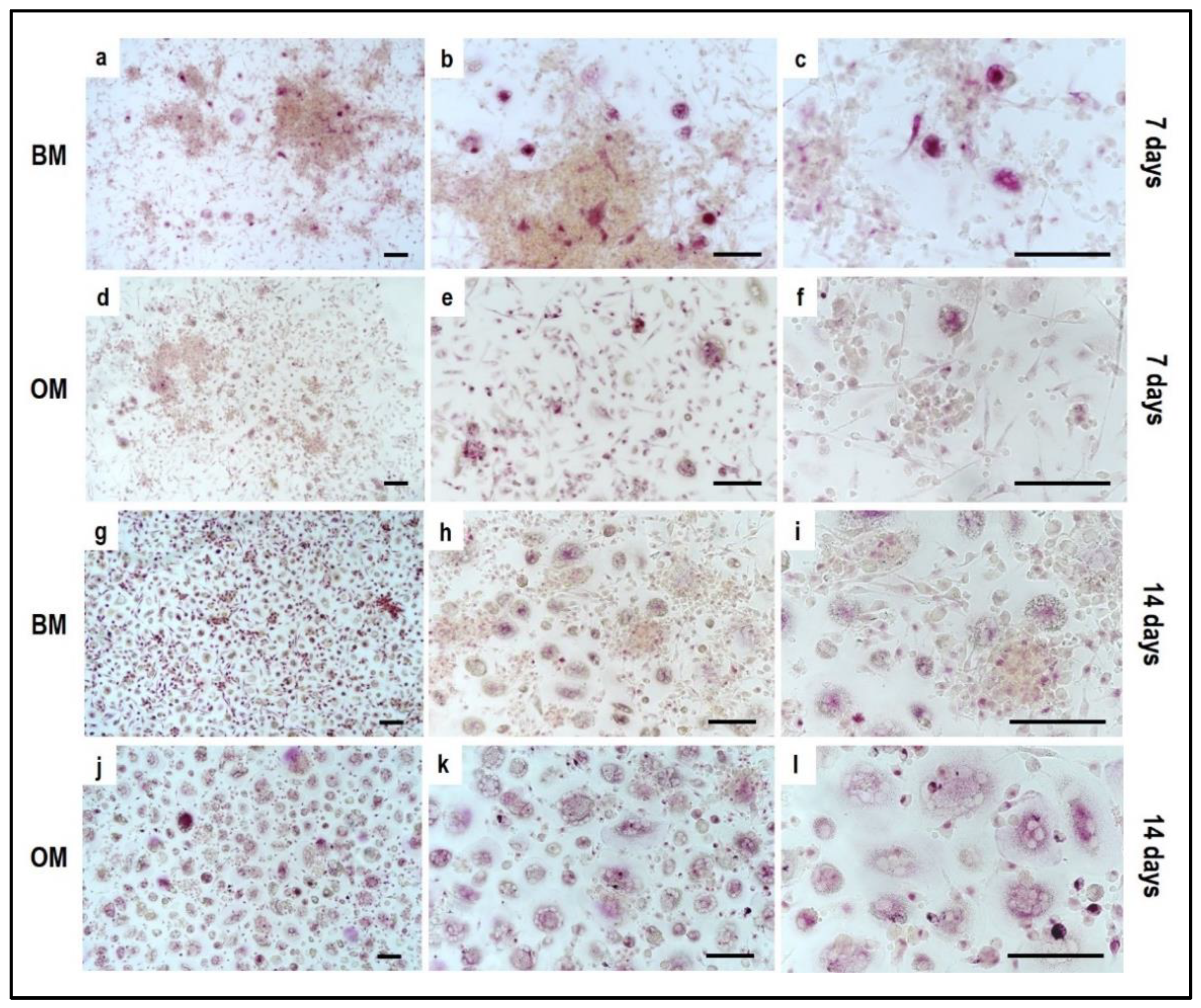
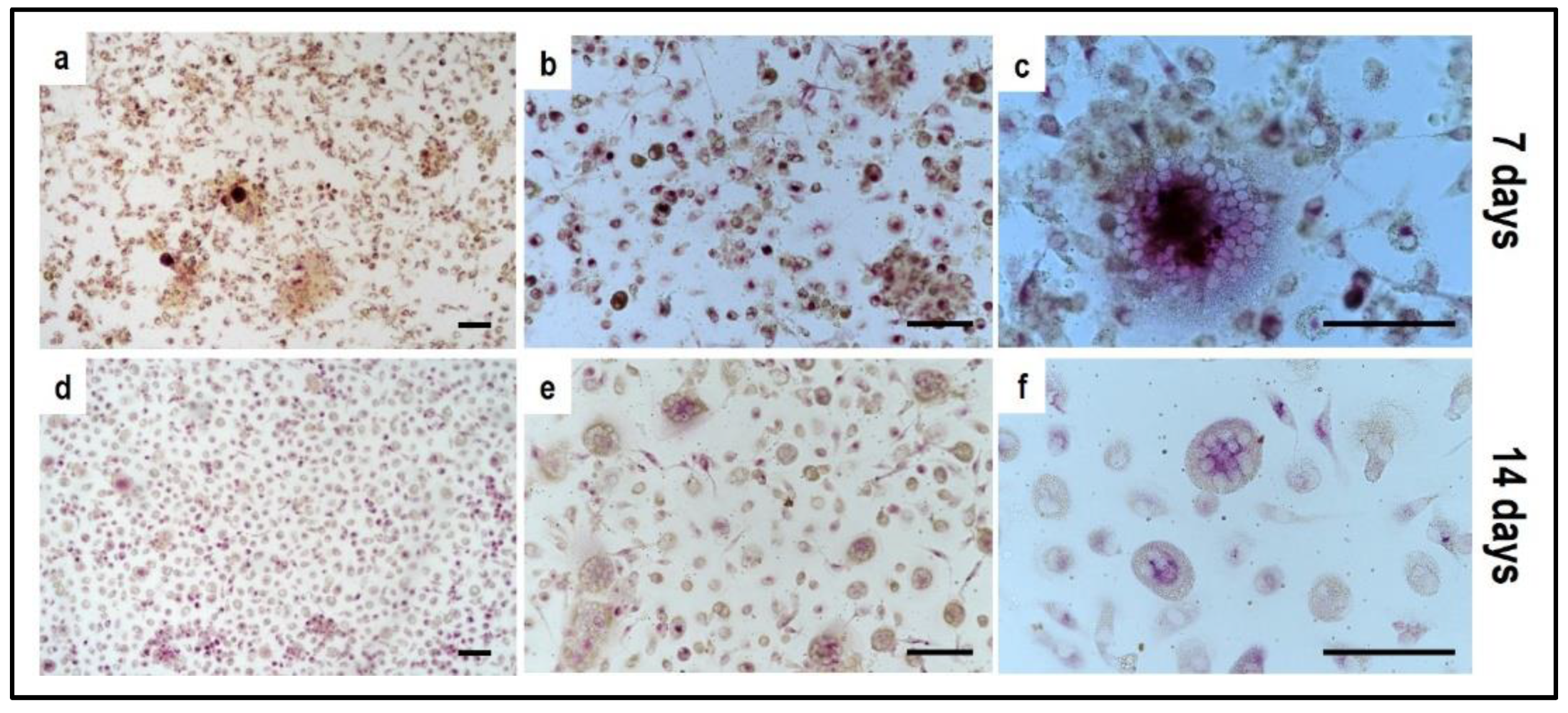
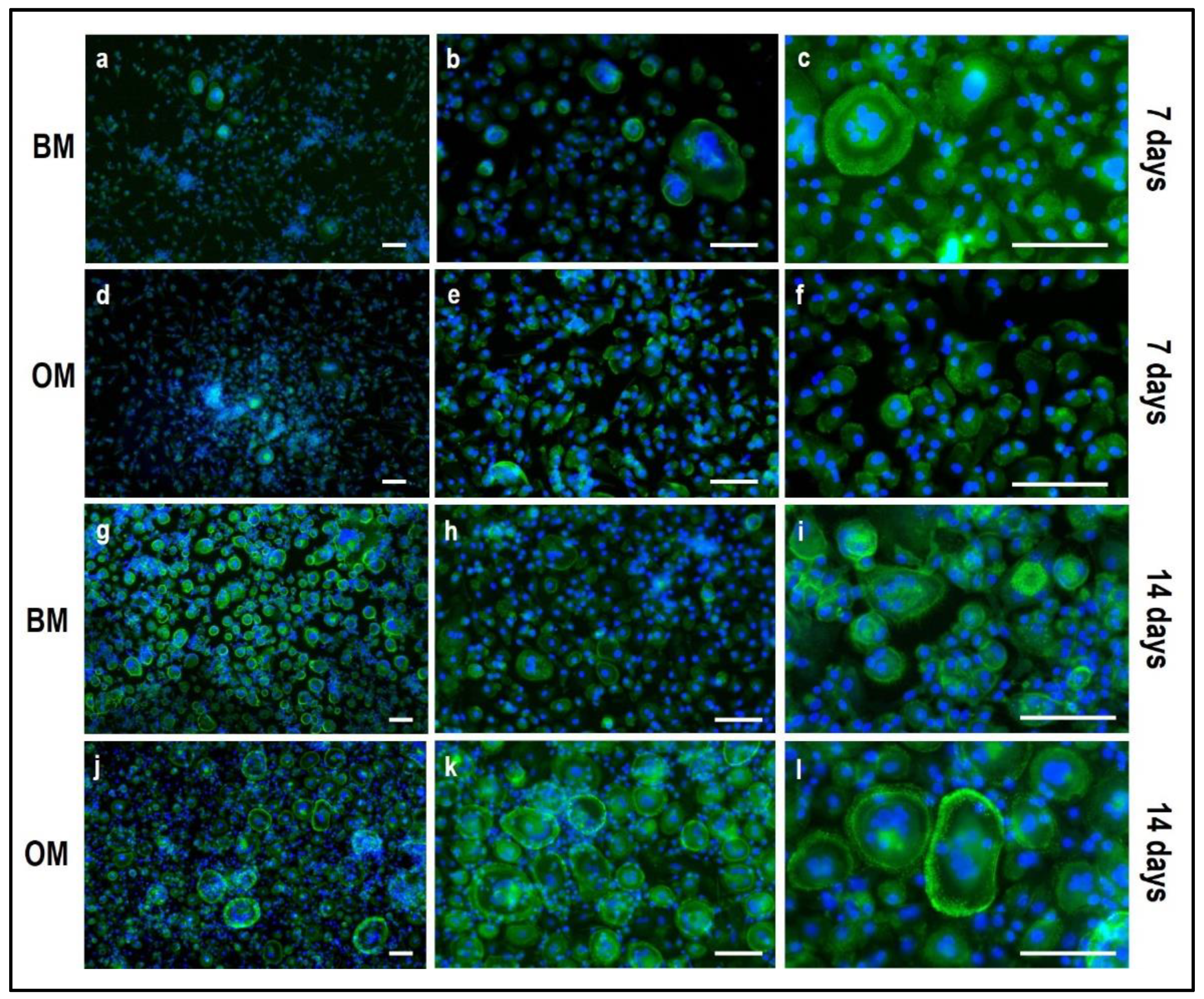
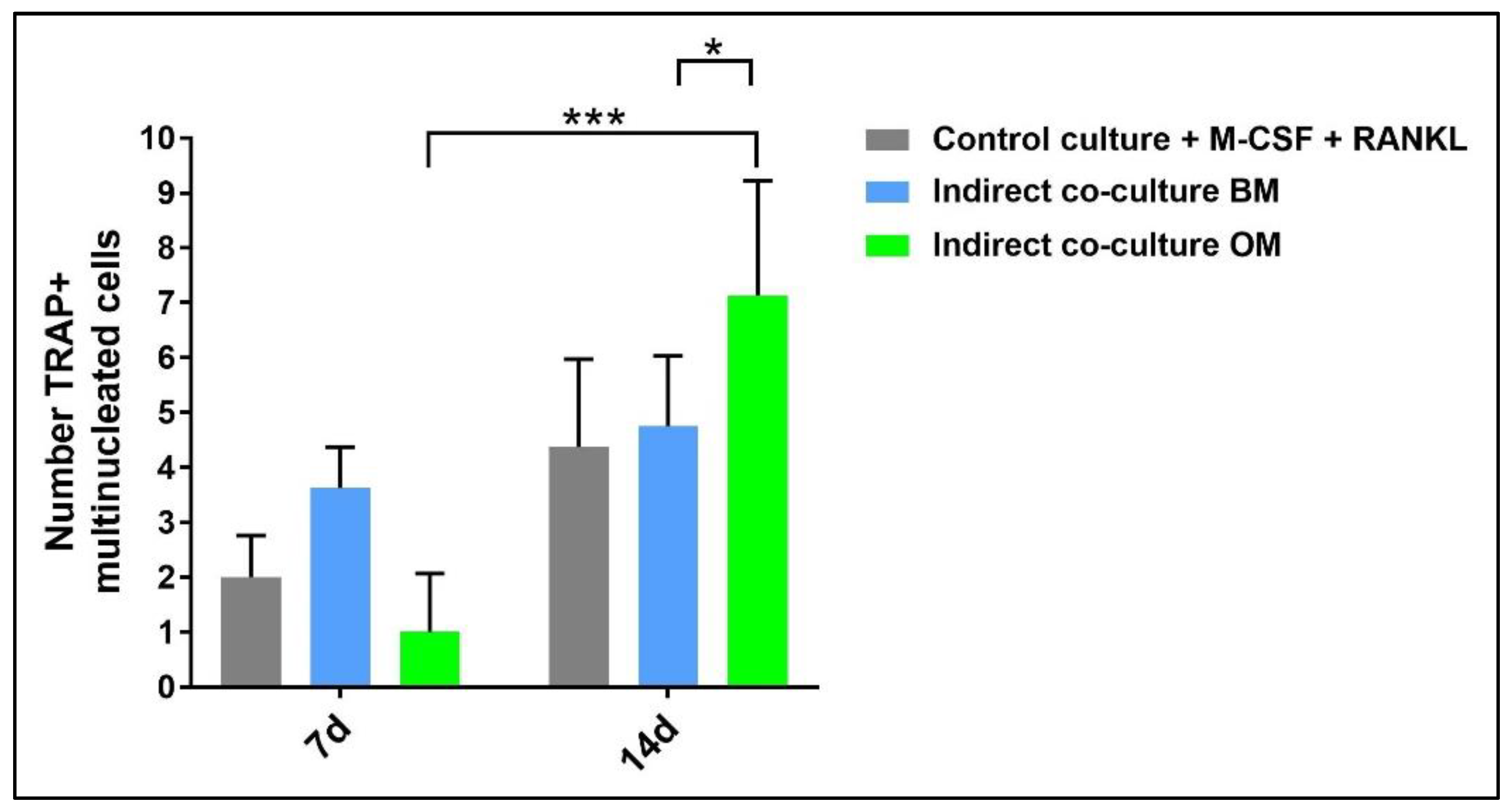
4.1. Indirect hOB/PBMC Co-Culture
- -
- If the material is 2D and translucent, the previously reported methods can be applied without any additional precaution, as reported in Section 3.4 “Cell Biochemistry–Cytochemical Staining Protocols”.
- -
- If the material is 3D and non-translucent, we suggest new assays to be performed (see Section 3.5 “Material Testing with the Indirect Co-Culture”), where the authors show alternative assays performed on two non-translucent collagen-based hydrogels.
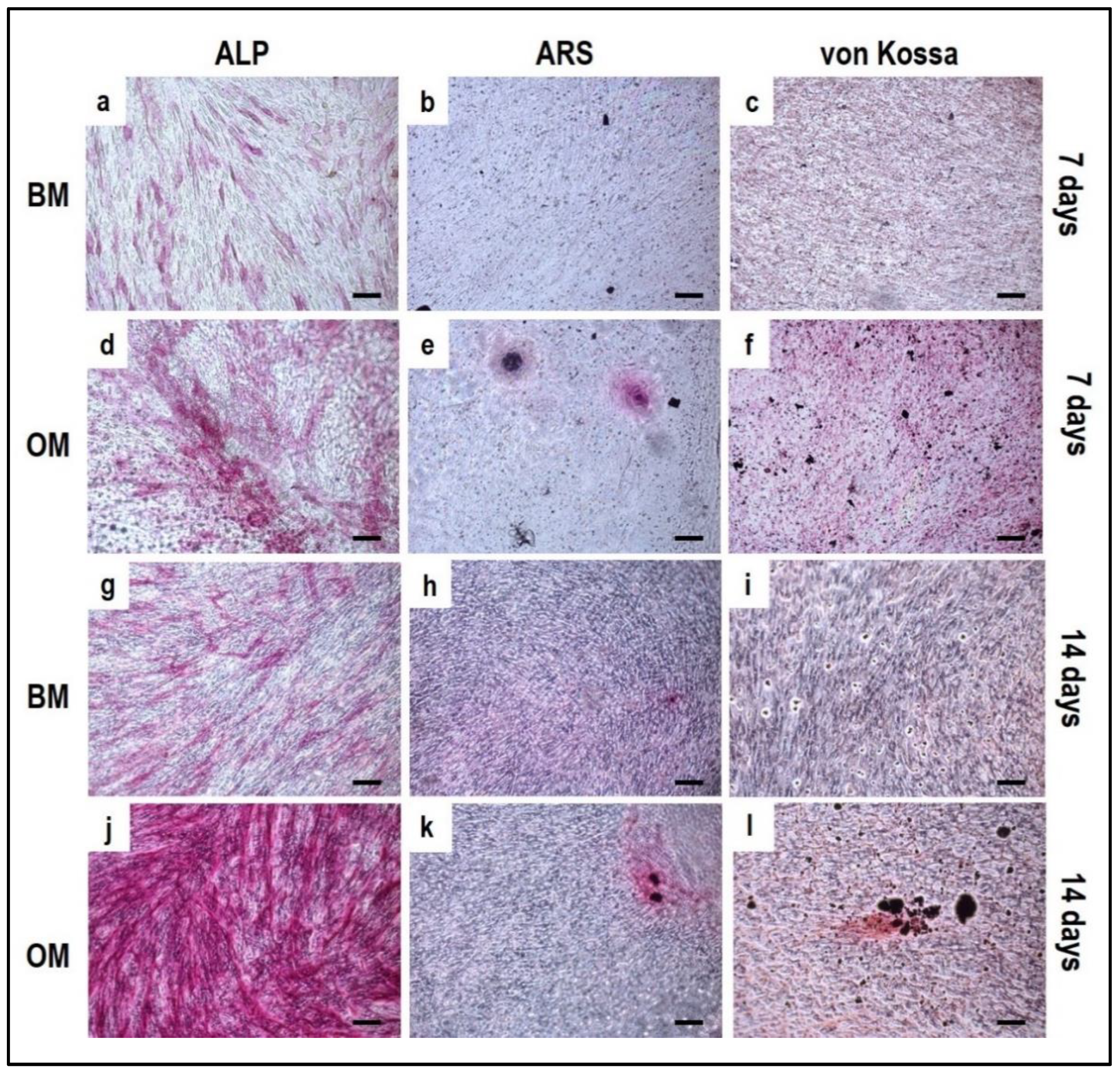
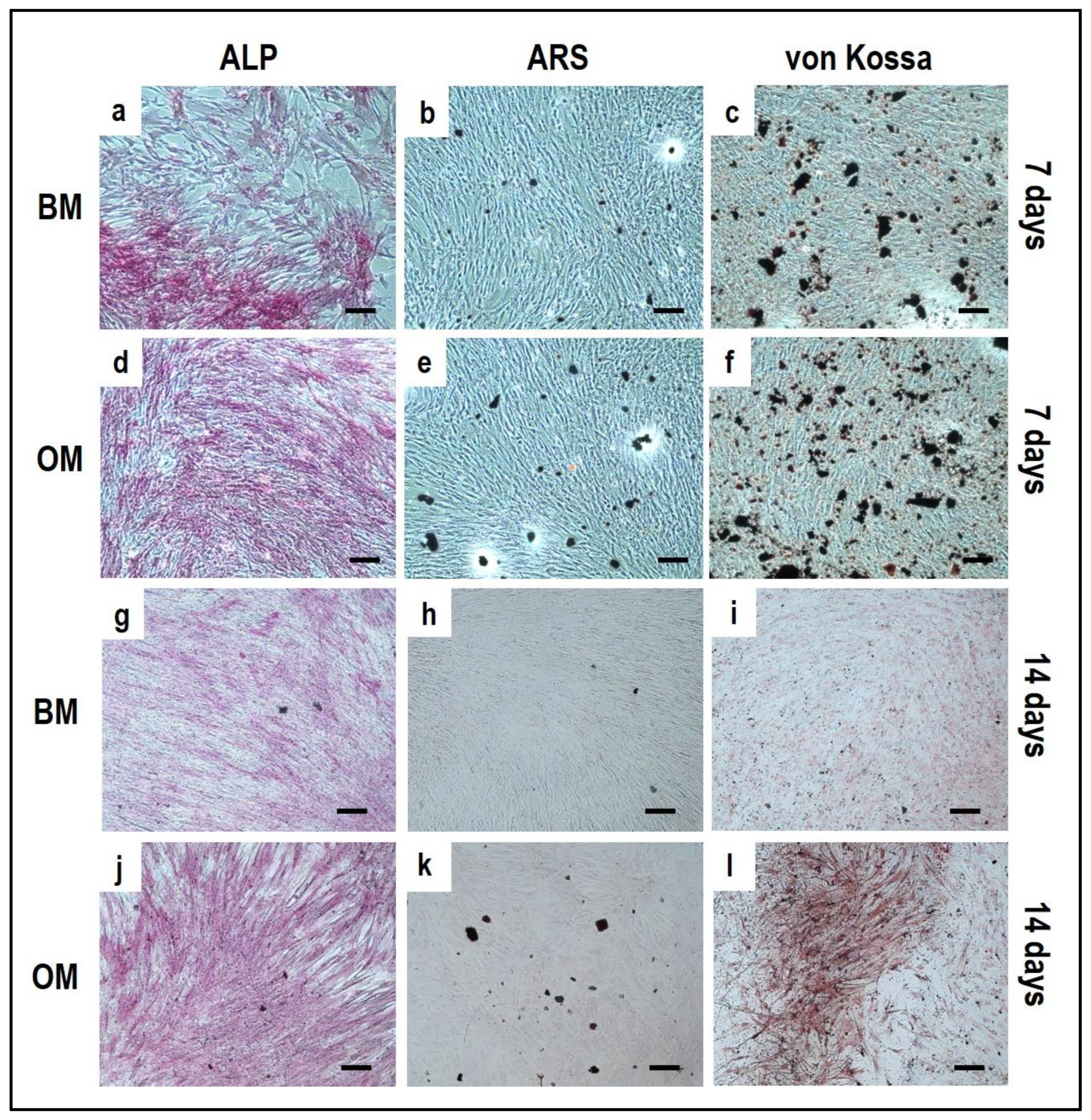
4.2. PBMCs Single-Culture as Differentiation Control
4.3. OBs Single-Culture as Differentiation Control
5. Reagents Setup
- Fixative solution n.1: 3.7% paraformaldehyde (PFA) in PBS: to prepare 25 mL of fixative solution, dissolve 0.925 g of PFA (Sigma-Aldrich, P6148, Milan, Italy) in 15 mL of pre-heated (60 °C) PBS 1×, clarify with 1–2 drops of NaOH, cool in ice, measure pH 7.6, and bring to the final volume of 25 mL with PBS. Store eventually at –20 °C.
- Fixative solution n.2: 3% paraformaldehyde (PFA) with 2% sucrose (Electron Microscopy Sciences, 21,600, Hatfield, PA, USA) in PBS: to prepare 25 mL of fixative solution, dissolve 0.75 g of PFA (Sigma-Aldrich, P6148, Milan, Italy) in 15 mL of pre-heated (60 °C) PBS 1×, clarify with 1–2 drops of NaOH, cool in ice, dissolve 0.5 g of sucrose, measure pH 7.6, and bring to the final volume of 25 mL with PBS. Store eventually at –20 °C.
- Fixative solution n.3: for a final volume of 10 mL, mix 2.5 mL citrate solution, 7 mL acetone, and 0.8 mL 3.7% formaldehyde to be kept for 30 min at RT. All the previous reagents are included in the leukocyte alkaline phosphatase assay kit (Sigma-Aldrich, 86R, Milan, Italy).
- Fixative solution n.4: 2% glutaraldehyde (MERCK, Sigma-Aldrich, 4239, Milan, Italy) in 0.2 M sodium cacodylate buffer.
- 0.2 M sodium cacodylate buffer: to prepare 1000 mL, dissolve 42.8 g of sodium cacodylate (Electron Microscopy Sciences, 12310, Hatfield, PA, USA) in 800 mL of distillate water and measure pH 7.4 with the addition, if required, of HCl 37%. Bring the final volume to 1000 mL.
- 1% osmium tetroxide solution: dissolve 500 mg of osmium tetroxide (Electron Microscopy Sciences, 19100, Hatfield, PA, USA) in 25 mL of distillate water and then dilutes 1:1 with 0.2 M sodium cacodylate buffer.
- Complete culture medium for PBMCs: Dulbecco’s modification of Eagle’s medium with high glucose (Euroclone, ECB7501L, Milan, Italy) (DMEM-HG) supplemented with 10% fetal bovine serum (FBS) (Sigma-Aldrich, F7524, Milan, Italy) and 1% penicillin-streptomycin (Euroclone, ECB3001D, Milan, Italy).
- Ficoll-Paque PLUS, 1.078 g/mL (Cytiva, Sigma-Aldrich, GE17144002, Milan, Italy).
- Ca+2-free complete culture medium for OBs: Dulbecco’s modification of Eagle’s medium (D-MEM) (Gibco, Thermo Fisher Scientific, 31600-083, Milan, Italy) supplemented with 10% fetal bovine serum (FBS) (Sigma-Aldrich, F7524, Milan, Italy) and 1% penicillin-streptomycin (Euroclone, ECB3001D, Milan, Italy).
- Complete culture medium for OBs: Dulbecco’s modification of Eagle’s medium with low glucose (DMEM-LG) (Sigma-Aldrich, D2902, Milan, Italy) supplemented with 10% fetal bovine serum (FBS) (Sigma-Aldrich, F7524, Milan, Italy) and 1% penicillin-streptomycin (Euroclone, ECB3001D, Milan, Italy).
- Cell freezing medium: 90% of fetal bovine serum (FBS) and 10% of dimethylsulfoxide (DMSO).
- Basal medium: 1:1 of complete DMEM-LG/complete DMEM-HG medium.
- Osteogenic medium: 1:1 of complete DMEM-LG/complete DMEM-HG medium supplemented with 5 mM β-glycerophosphate (Sigma-Aldrich, G9422, Milan, Italy),10-7 M dexamethasone (Sigma-Aldrich, d-2915, Milan, Italy) and 50 µM ascorbic acid (Sigma-Aldrich, A8960, Milan, Italy).
- Differentiation medium for PBMCs: DMEM-HG supplemented with 10% fetal bovine serum (FBS) characterized (Euroclone, CHA115L, Milan, Italy), 1% penicillin-streptomycin (Euroclone, ECB3001D, Milan, Italy), containing 25 ng/mL monocyte/macrophage colony-stimulating factor (M-CSF) (Peprotech, 300-25, London, UK) and 50 ng/mL receptor activator of NF-κB ligand (RANKL) (Peprotech, 310-01, London, UK). For the first four days of culture, only M-CSF is added, while from the fourth day to the end of the culture period RANK-L is added, too.
- Trypsin-EDTA solution: trypsin 0,05%, EDTA 0,02% in PBS w/o calcium w/o magnesium w/o phenol red (Euroclone, ECB3052D, Milan, Italy).
- Alizarin Red S (ARS) staining solution: 2% (w/v) Alizarin Red S (Sigma-Aldrich, A5533, Milan, Italy) in H2O, pH 4.2.
- Von Kossa staining solution: solution 1 (2% silver nitrate): 2 g AgNO3 (Sigma-Aldrich, S0139, Milan, Italy) in 100 mL deionized H2O; solution 2 (2.5% sodium thiosulfate): 2.5 g sodium thiosulfate (Carlo Erba, 483,827, Milan, Italy) in 100 mL deionized H2O; solution 3 (0.33% nuclear fast red solution): 0.33 g nuclear fast red (Carlo Erba, 476,951, Milan, Italy) in 100 mL deionized H2O.
- Fluorescein isothiocyanate (FITC)-phalloidin (Sigma-Aldrich, P-5282, Milan, Italy) staining solution: 1 mg/mL in PBS.
- Nuclear staining: 1.25 μg/mL Hoechst 33528 (Sigma-Aldrich, 861405, Milan, Italy) in H2O.
- Erythrosin B staining solution 1×: 0.4 g of Erythrosin B (Sigma-Aldrich, e-9259, Milan, Italy), 0.81 g sodium chloride, 0.06 g sodium phosphate monobasic in 100 mL H2O. Boil the solution. Once cooled down, dilute 1:10 with dH2O before use.
- EDTA-based buffer for the lysis of red blood cells (RBCs): dissolve in distilled water 0.037 g/L ethylenediaminetetraacetic acid disodium salt dihydrate (EDTA) (Sigma-Aldrich, E5134, Milan, Italy), 1 g/L potassium bicarbonate (Sigma-Aldrich, P9144, Milan, Italy), 8.26 g/L ammonium chloride (Carlo Erba, 314002, Milan, Italy). Add 450 µL of this solution to the 50 µL of RBC suspension diluted in 450 µL of PBS and incubate for 10 min at 37 °C avoiding light exposure.
- Tris buffer + 0.1% Triton X-100 pH 7.5: prepare Tris buffer (10 mM, pH 7.5) containing 0.1% Triton X-100.
- 10% cetylpyridinium solution: dissolve 10 g of cetylpyridinium (Sigma-Aldrich, C0732, Milan, Italy) in 100 mL of distilled water.
- Live-Dead staining solution: mix vial 1 and vial 2 and add 1 mL of culture medium without FBS.
6. Conclusions and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sims, N.A.; Martin, T.J. Coupling Signals between the Osteoclast and Osteoblast: How are Messages Transmitted between These Temporary Visitors to the Bone Surface? Front. Endocrinol. 2015, 6, 41. [Google Scholar] [CrossRef] [Green Version]
- Sims, N.A.; Martin, T.J. Coupling the activities of bone formation and resorption: A multitude of signals within the basic multicellular unit. Bonekey Rep. 2014, 3, 481. [Google Scholar] [CrossRef] [Green Version]
- Ruggiu, A.; Tortelli, F.; Komlev, V.S.; Peyrin, F.; Cancedda, R. Extracellular matrix deposition and scaffold biodegradation in an in vitro three-dimensional model of bone by X-ray computed microtomography. J. Tissue. Eng. Regen. Med. 2014, 8, 557–565. [Google Scholar] [CrossRef]
- Hammerl, A.; Diaz Cano, C.; De-Juan-Pardo, E.; van Griensven, M.; Poh, P. A Growth Factor-Free Co-Culture System of Osteoblasts and Peripheral Blood Mononuclear Cells for the Evaluation of the Osteogenesis Potential of Melt-Electrowritten Polycaprolactone Scaffolds. Int. J. Mol. Sci. 2019, 20, 1068. [Google Scholar] [CrossRef] [Green Version]
- Domaschke, H.; Gelinsky, M.; Burmeister, B.; Fleig, R.; Hanke, T.; Reinstorf, A.; Pompe, W.; Rösen-Wolff, A. In Vitro Ossification and Remodeling of Mineralized Collagen I Scaffolds. Tissue Eng. 2006, 12, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Borciani, G.; Montalbano, G.; Baldini, N.; Cerqueni, G.; Vitale-Brovarone, C.; Ciapetti, G. Co–culture systems of osteoblasts and osteoclasts: Simulating in vitro bone remodeling in regenerative approaches. Acta Biomater. 2020, 108, 22–45. [Google Scholar] [CrossRef] [PubMed]
- Scheinpflug, J.; Pfeiffenberger, M.; Damerau, A.; Schwarz, F.; Textor, M.; Lang, A.; Schulze, F. Journey into Bone Models: A Review. Genes 2018, 9, 247. [Google Scholar] [CrossRef] [Green Version]
- Wilkesmann, S.; Fellenberg, J.; Nawaz, Q.; Reible, B.; Moghaddam, A.; Boccaccini, A.R.; Westhauser, F. Primary osteoblasts, osteoblast precursor cells or osteoblast-like cell lines: Which human cell types are (most) suitable for characterizing 45S5-bioactive glass? J. Biomed. Mater. Res. Part A 2020, 108, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Czekanska, E.M.; Stoddart, M.J.; Ralphs, J.R.; Richards, R.G.; Hayes, J.S. A phenotypic comparison of osteoblast cell lines versus human primary osteoblasts for biomaterials testing. J. Biomed. Mater. Res. Part A 2014, 102, 2636–2643. [Google Scholar] [CrossRef] [PubMed]
- Hoemann, C.D.; El-Gabalawy, H.; McKee, M.D. In Vitro osteogenesis assays: Influence of the primary cell source on alkaline phosphatase activity and mineralization. Pathol. Biol. 2009, 57, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, D.; Jourdain, M.-L.; Braux, J.; Guillaume, C.; Gangloff, S.C.; Jacquot, J.; Velard, F. An Optimized Method to Generate Human Active Osteoclasts From Peripheral Blood Monocytes. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolin, V.; Bareggi, R.; Baldini, G.; Bortul, R.; Martinelli, B.; Narducci, P. Effects of neridronic acid on osteoclasts derived by physiological dual-cell cultures. Acta Histochem. 2007, 109, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Maria, S.; Swanson, M.H.; Enderby, L.T.; D’Amico, F.; Enderby, B.; Samsonraj, R.M.; Dudakovic, A.; van Wijnen, A.J.; Witt-Enderby, P.A. Melatonin-micronutrients Osteopenia Treatment Study (MOTS): A translational study assessing melatonin, strontium (citrate), vitamin D3 and vitamin K2 (MK7) on bone density, bone marker turnover and health related quality of life in postmenopausal osteopen. Aging 2017, 9, 256–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolci, L.S.; Panzavolta, S.; Torricelli, P.; Albertini, B.; Sicuro, L.; Fini, M.; Bigi, A.; Passerini, N. Modulation of Alendronate release from a calcium phosphate bone cement: An in vitro osteoblast-osteoclast co-culture study. Int. J. Pharm. 2019, 554, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Hayden, R.S.; Vollrath, M.; Kaplan, D.L. Effects of clodronate and alendronate on osteoclast and osteoblast co-cultures on silk-hydroxyapatite films. Acta Biomater. 2014, 10, 486–493. [Google Scholar] [CrossRef] [Green Version]
- Penolazzi, L.; Lolli, A.; Sardelli, L.; Angelozzi, M.; Lambertini, E.; Trombelli, L.; Ciarpella, F.; Vecchiatini, R.; Piva, R. Establishment of a 3D-dynamic osteoblasts-osteoclasts co-culture model to simulate the jawbone microenvironment in vitro. Life Sci. 2016, 152, 82–93. [Google Scholar] [CrossRef]
- Nicolin, V.; Baldini, G.; Bareggi, R.; Zweyer, M.; Zauli, G.; Vaccarezza, M.; Narducci, P. Morphological features of osteoclasts derived from a co-culture system. J. Mol. Histol. 2006, 37, 171–177. [Google Scholar] [CrossRef]
- Midha, S.; van den Bergh, W.; Kim, T.B.; Lee, P.D.; Jones, J.R.; Mitchell, C.A. Bioactive Glass Foam Scaffolds are Remodelled by Osteoclasts and Support the Formation of Mineralized Matrix and Vascular Networks In Vitro. Adv. Healthc. Mater. 2013, 2, 490–499. [Google Scholar] [CrossRef]
- Schröder, H.C.; Wang, X.H.; Wiens, M.; Diehl-Seifert, B.; Kropf, K.; Schloßmacher, U.; Müller, W.E.G. Silicate Modulates the Cross-Talk Between Osteoblasts (SaOS-2) and Osteoclasts (RAW 264.7 Cells): Inhibition of Osteoclast Growth and Differentiation. J. Cell. Biochem. 2012, 113, 3197–3206. [Google Scholar] [CrossRef]
- Jolly, J.J.; Chin, K.-Y.; Farhana, M.F.N.; Alias, E.; Chua, K.H.; Hasan, W.N.W.; Ima-Nirwana, S. Optimization of the Static Human Osteoblast/Osteoclast Co-culture System. Iran. J. Med. Sci. 2018, 43, 208–213. [Google Scholar]
- Borciani, G.; Montalbano, G.; Melo, P.; Baldini, N.; Ciapetti, G.; Vitale Brovarone, C. Assessment of Collagen-Based Nanostructured Biomimetic Systems with a Co-Culture of Human Bone-Derived Cells. Cells 2021, 11, 26. [Google Scholar] [CrossRef] [PubMed]




| Step | Timing |
|---|---|
| OB isolation and expansion | 3–4 weeks until confluent OB monolayer |
| PBMCs isolation | 5 h |
| Material preparation and cell seeding | 2 days |
| Cell biochemistry | 1 days |
| Step | Problems | Solution |
|---|---|---|
| 3.1 | Cell aggregates are seen before seeding step in the wells | Make sure that cells are well resuspended within the culture medium |
| 3.1 | Low number of viable OBs | Check viability before starting the protocol Detach a 2nd flask of OBs |
| 3.2 (d, f, h) | Harvesting only the white ring of PBMCs is difficult | Harvest also the plasma surrounding the white ring (see Figure 2) |
| 3.2 (k) | PBMCs count hard to accomplish | Further dilute the cell suspension with PBS |
| 3.2–3.3 | Low seeding density of PBMCs compromises the success of the co-culture system | Process a new buffy coat if available |
| 3.3 | Difficult manipulation of the indirect co-culture set up | Remove the transwell with OBs seeded by means of sterile pliers, lay down it in a sterile multiwell plate with complete medium; then seed PBMCs at the bottom of the well previously equipped with sterile and pre-wetted TC coverslip. Lastly, insert again the transwell with OBs seeded in co-culture with the PBMCs (see Figure 3). A schematic timeline of the co-culture maintenance is shown in Figure 1. |
| 3.5 | Difficult manipulation of the material with cells seeded on the top | Remove the material from the well (to be observed under a microscope) with a spatula, lifting it from the bottom |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borciani, G.; Montalbano, G.; Baldini, N.; Vitale-Brovarone, C.; Ciapetti, G. Protocol of Co-Culture of Human Osteoblasts and Osteoclasts to Test Biomaterials for Bone Tissue Engineering. Methods Protoc. 2022, 5, 8. https://doi.org/10.3390/mps5010008
Borciani G, Montalbano G, Baldini N, Vitale-Brovarone C, Ciapetti G. Protocol of Co-Culture of Human Osteoblasts and Osteoclasts to Test Biomaterials for Bone Tissue Engineering. Methods and Protocols. 2022; 5(1):8. https://doi.org/10.3390/mps5010008
Chicago/Turabian StyleBorciani, Giorgia, Giorgia Montalbano, Nicola Baldini, Chiara Vitale-Brovarone, and Gabriela Ciapetti. 2022. "Protocol of Co-Culture of Human Osteoblasts and Osteoclasts to Test Biomaterials for Bone Tissue Engineering" Methods and Protocols 5, no. 1: 8. https://doi.org/10.3390/mps5010008








