Leaf Mesophyll Mitochondrial Polarization Assessment in Arabidopsis thaliana
Abstract
:1. Introduction
2. Experimental Design
2.1. Materials
- Carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP) (Sigma-Aldrich. St. Louis, MO, USA; Cat. no.: C2920).
- Silicon Oil (Sigma-Aldrich. St. Louis, MO, USA; Cat. no.: 378437).
- Perfluorodecalin (PFD), (Sigma-Aldrich. St. Louis, MO, USA; Cat. no.: P9900).
- Murashige and Skoog (MS) Basal Medium, (Sigma-Aldrich. St. Louis, MO, USA; Cat. no.: M5519).
- 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimi- dazolylcarbocyanine iodide (JC-1), (Sigma-Aldrich. St. Louis, MO, USA; Cat. no.: T4069).
- MitoTracker Green FM, (Invitrogen. Waltham, MA, USA; Cat.no.: M7514).
- Mannitol.
- Ethylenediaminetetraacetic acid EDTA.
- K2HPO4.
- Tris.
- NaClO solution.
- Tween.
- Scotch® Transparent Tape.
- Dissecting scissors (Sigma-Aldrich. St. Louis, MO, USA; Cat. no.: Z265969).
- 10 cm Petri plates.
- Agar.
- Pipettes.
- Eppendorf tubes.
- Microscope slides.
- Precision tweezers.
- Coverslips.
- Arabidopsis thaliana Col-0 ecotype seeds (ABRC. Columbus, OH, USA; Cat. no: CS66459).
- Sunshine Mix 3 (Sungro Horticulture).
- Miracle-Gro 8 and Miracle Gro Perlite (The Scotts Miracle-Gro Company, Marysville, OH, USA. Model: 70752300).
2.2. Equipment
- Ambi Hi-Lo incubation chamber (Lab-Line Instruments Inc. Melrose Park, IL, USA, Cat. no.: 3554-35).
- AmScope T-600C Epifluorescence microscope (AmScope. Irvine, CA, USA; Cat. no.: T600, SKU FK-EPI-NL).
- Corning LSE low-speed orbital shaker, w/flat platform (Sigma-Aldrich. St. Louis, MO, USA, Cat. No.: CLS6780FP).
- Autoclave.
- Laminar flow hood for MS medium preparation.
3. Procedure
3.1. A. thaliana Growth Conditions (Time to Completion: 3 Weeks)
- Steps 2 to 4 should be performed under sterile conditions.
- Place 10–20 seeds in an Eppendorf tube filled with 1 mL of sterilization solution (3.7% sodium hypochlorite, 0.02% Tween) for 10 min.
- Wash seeds three times with sterile deionized water.
- Place seeds in a Petri dish containing sterile ½x MS agar medium [7].
- Place the Petri dish in an incubator at 22 °C in a 16 h/8 h light/dark photoperiod.
- Once the seedlings have achieved desired development, transfer to soil or desired substrate (we typically use peat moss Sunshine Mix 3 (Sungro) plus Miracle-Gro-type minerals in a 2:1:1 ratio).
CRITICAL STEP. Allow plant growth for no more than 2 to 3 weeks from germination (longer times will result in peeling-resistant leaves).
- Leaves from plants grown on Petri dishes can be alternatively used for epidermis removal.
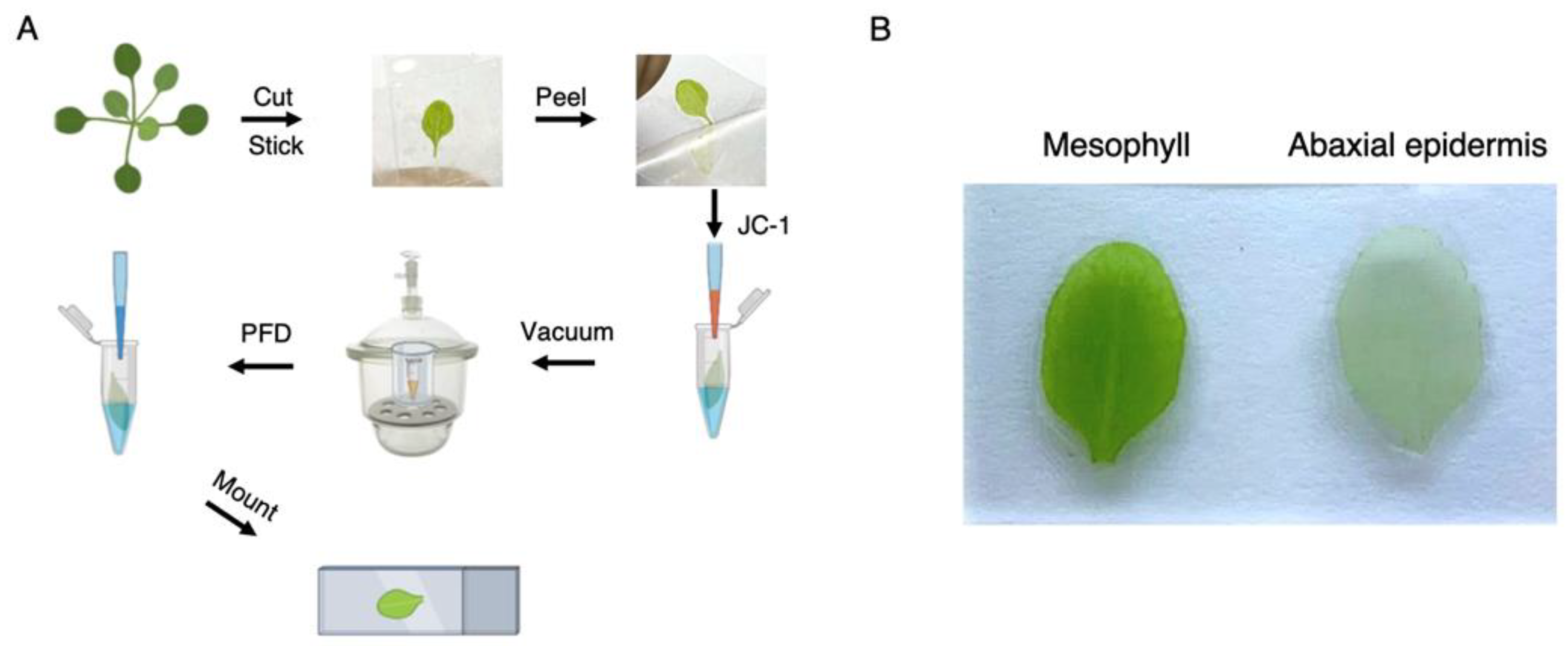
3.2. Leaf Epidermis Removal (Time to Completion: 3 Weeks)
- Select (~0.5 cm) leaves from 3-week-old plants.
- Adhere a piece of Scotch® Transparent Tape over each leaf adaxial epidermis (Figure 1A).
- Adhere a piece of Scotch® Transparent Tape to the abaxial epidermis.
- Remove air bubbles and enhance epidermis adhesion by gently pressing the leaf with one finger.
- Pull away the Scotch® Transparent Tape slowly. The lower epidermis will be detached.
- Cut excess tape with scissors to match mesophyll perimeter.
- All further steps should be performed without any pause.
3.3. Leaf Mesophyll Staining (Time to Completion: 45 min)
- Place one peeled leaf mesophyll inside an Eppendorf tube containing 1 mL Stain buffer (see Section 5 for buffer preparation instructions).
- Infiltrate under mild vacuum conditions for 1 min in the absence of light. Make sure the tube cap remains open during the process. Optional: Longer vacuum times can be used to ensure better penetration if needed.
- Close tube cap and place it in a shaker at 60 rpm for 30 min in the absence of light. Make sure tube contents mix thoroughly.
- Pipette out or vacuum aspirate Stain buffer without disturbing the leaf (see Section 3.5 for more details).
- Add 1 mL Wash buffer. (See Section 5 for buffer preparation instructions).
- Wash the leaf five times with 1 mL Wash buffer.
CRITICAL STEP Make sure all stain remnants are washed away in the sample and the tube, otherwise keep washing the sample.
- Remove Wash buffer and add 100 μL PFD above the leaf level.
3.4. Leaf Mesophyll Mounting (Time to Completion: 10 min)
- Place the leaf with the aid of precision tweezers on a microscope slide and add an even layer of silicon oil around each leaf to form a gasket. Add ~100 μL PFD to fill the gasket.
- Place a coverslip above, making sure no air bubbles remain inside.
- Press gently to minimize sample volume.
- Seal coverslip with transparent nail polish. This is especially relevant when imaging in inverted microscopes.
- Image samples at 405 nm excitation for enhanced J-aggregate detection at 575–630 nm emission. Use 505–550 nm BP filter for the green channel and 575–630 nm filter for the red channel.
3.5. Additional Notes
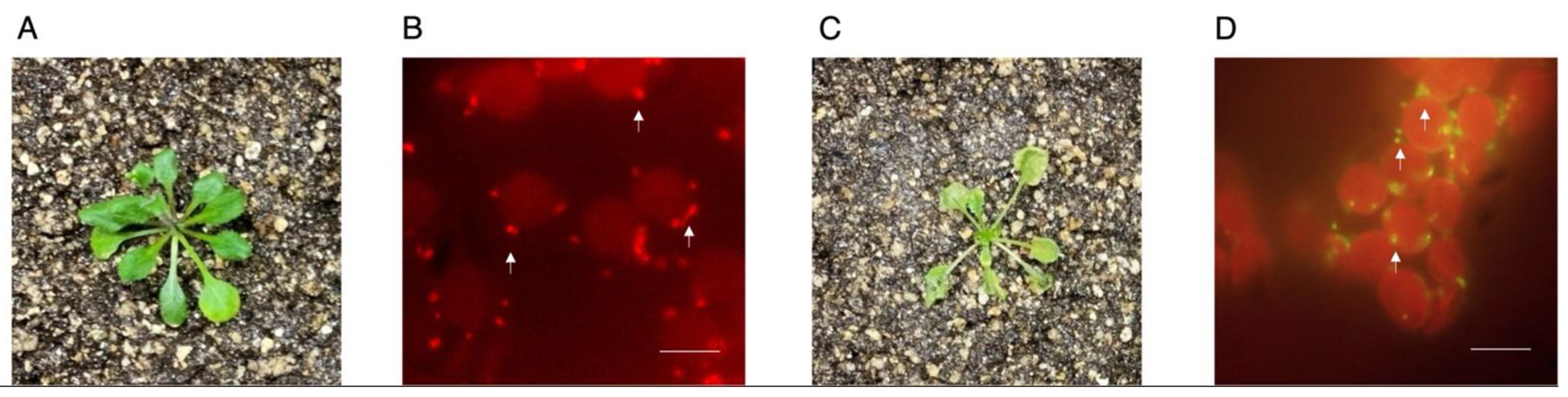
4. Expected Results
4.1. Leaf Epidermis Removal and Mesophyll Staining
4.2. Mitochondrial Polarization Assessment in Heat-Shock-Treated Plants
5. Reagents Setup
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seymour, R.S. Diffusion pathway for oxygen into highly thermogenic florets of the arum lily Philodendron selloum. J. Exp. Bot. 2001, 52, 1465–1472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seymour, R.S.; Gibernau, M. Respiration of thermogenic inflorescences of Philodendron melinonii: Natural pattern and responses to experimental temperatures. J. Exp. Bot. 2008, 59, 1353–1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, K.; Seymour, R. Expression of uncoupling protein and alternative oxidase depends on lipid or carbohydrate substrates in thermogenic plants. Biol. Lett. 2005, 1, 427–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vacca, R.A.; Valenti, D.; Bobba, A.; De Pinto, M.C.; Merafina, R.S.; De Gara, L.; Passarella, S.; Marra, E. Proteasome function is required for activation of programmed cell death in heat shocked tobacco Bright-Yellow 2 cells. FEBS Lett. 2007, 581, 917–922. [Google Scholar] [CrossRef] [Green Version]
- Vacca, R.A.; Valenti, D.; Bobba, A.; Merafina, R.S.; Passarella, S.; Marra, E. Cytochrome c Is Released in a Reactive Oxygen Species-Dependent Manner and Is Degraded via Caspase-Like Proteases in Tobacco Bright-Yellow 2 Cells en Route to Heat Shock-Induced Cell Death. Plant Physiol. 2006, 141, 208–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vacca, R.A.; de Pinto, M.C.; Valenti, D.; Passarella, S.; Marra, E.; De Gara, L. Production of Reactive Oxygen Species, Alteration of Cytosolic Ascorbate Peroxidase, and Impairment of Mitochondrial Metabolism Are Early Events in Heat Shock-Induced Programmed Cell Death in Tobacco Bright-Yellow 2 Cells. Plant Physiol. 2004, 134, 1100–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oikawa, K.; Imai, T.; Thagun, C.; Toyooka, K.; Yoshizumi, T.; Ishikawa, K.; Kodama, Y.; Numata, K. Mitochondrial movement during its association with chloroplasts in Arabidopsis thaliana. Commun. Biol. 2021, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Scott, I.; Logan, D.C. Mitochondrial morphology transition is an early indicator of subsequent cell death in Arabidopsis. New Phytol. 2007, 177, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Xing, D.; Li, L.; Zhang, L. Implication of reactive oxygen species and mitochondrial dysfunction in the early stages of plant programmed cell death induced by ultraviolet-C overexposure. Planta 2007, 227, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.-H.; Shen, S.-C.; Lee, L.-Y.; Lee, S.-H.; Chan, M.-T.; Lin, C.-S. Tape-Arabidopsis Sandwich—A simpler Arabidopsis protoplast isolation method. Plant Methods 2009, 5, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Littlejohn, G.; Love, J. A Simple Method for Imaging Arabidopsis Leaves Using Perfluorodecalin as an Infiltrative Imaging Medium. J. Vis. Exp. 2012, e3394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larkindale, J.; Hall, J.D.; Knight, M.; Vierling, E. Heat Stress Phenotypes of Arabidopsis Mutants Implicate Multiple Signaling Pathways in the Acquisition of Thermotolerance. Plant Physiol. 2005, 138, 882–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larkindale, J.; Mishkind, M.; Vierling, E. Plant Responses to High Temperature. Plant Abiotic Stress 2005, 100–144. [Google Scholar] [CrossRef]
- Wang, Y.; Berkowitz, O.; Selinski, J.; Xu, Y.; Hartmann, A.; Whelan, J. Stress responsive mitochondrial proteins in Arabidopsis thaliana. Free Radic. Biol. Med. 2018, 122, 28–39. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Herrera, C.; Gutiérrez-Mireles, E.R.; Gutiérrez-Aguilar, M. Leaf Mesophyll Mitochondrial Polarization Assessment in Arabidopsis thaliana. Methods Protoc. 2021, 4, 84. https://doi.org/10.3390/mps4040084
Flores-Herrera C, Gutiérrez-Mireles ER, Gutiérrez-Aguilar M. Leaf Mesophyll Mitochondrial Polarization Assessment in Arabidopsis thaliana. Methods and Protocols. 2021; 4(4):84. https://doi.org/10.3390/mps4040084
Chicago/Turabian StyleFlores-Herrera, Cesar, Emilia R. Gutiérrez-Mireles, and Manuel Gutiérrez-Aguilar. 2021. "Leaf Mesophyll Mitochondrial Polarization Assessment in Arabidopsis thaliana" Methods and Protocols 4, no. 4: 84. https://doi.org/10.3390/mps4040084






