Contrast-Enhanced Tissue Processing of Fibrillin-Rich Elastic Fibres for 3D Visualization by Volume Scanning Electron Microscopy
Abstract
:1. Introduction
2. Experimental Design
2.1. Materials
- 25% EM Grade Glutaraldehyde (Agar Scientific, Stansted, UK; Catalogue No: AGR1010)
- Paraformaldehyde (Agar Scientific, Stansted, UK; Catalogue No: AGR1018)
- Di-sodium hydrogen orthophosphate dihydrate (Fisher Scientific UK Ltd., Loughborough, UK; Catalogue No: S/4480/53)
- Sodium di-hydrogen orthophosphate dihydrate (Fisher Scientific UK Ltd., Loughborough, UK; Catalogue No: S/3760/53)
- 2% Osmium tetroxide (TAAB Laboratories, Aldermaston, UK; Catalogue No. O015/1)
- Tannic acid, low molecular weight: FW 1000–1500 (Electron Microscopy Services, Hatfield, PA, USA; Catalogue No. 21710)
- Uranyl Acetate (TAAB Laboratories, Aldermaston, UK; Catalogue No. U007)
- Lead Acetate (Agar Scientific, Stansted, UK; Catalogue No: AGR1209)
- Absolute ethanol 99%+, extra pure SLR (Fisher Chemical E/0600DF/17)
- Acetone 99.5%, laboratory reagent (Fisher Scientific UK Ltd., Loughborough, UK; Catalogue No: 179973-1L)
- Araldite CY212 epoxy resin (Agar Scientific, Stansted, UK; Catalogue No: AGR1040)
- DDSA (Dodecenyl Succinic anhydride) resin hardener (Agar Scientific, Stansted, UK; Catalogue No: AGR1051)
- BDMA (Benzyl dimethylamine) Accelerator (Agar Scientific, Stansted, UK; Catalogue No: AGR1062)
- Stainless steel razor blades (Agar Scientific, Stansted, UK; Catalogue No: AGT586)
- 7 mL glass specimen vials (Agar Scientific, Stansted, UK; Catalogue No: AGG284)
- Flat silicone embedding mould (Agar Scientific, Stansted, UK; Catalogue No: AGG3549)
- Silver epoxy conductive mounting medium (Agar Scientific, Stansted, UK; Catalogue No: AGG3349)
- Toluidine blue (Agar Scientific, Stansted, UK; Catalogue No: AGR1727)
- Di-sodium tetraborate (Merck KGAA, Darmstadt, Germany; Catalogue No. 106310)
- Luer-lock (optional) syringes 10 mL BD PlastiPak (Fisher Scientific UK Ltd., Loughborough, UK; Catalogue No: 15544835)
- 0.2 µm Syringe filters, Sartorius Minisart™ RC (Fisher Scientific UK Ltd., Loughborough, UK; Catalogue No: 11740966)
- Plastic transfer pipettes, Fisherbrand™ 1 mL (Fisher Scientific UK Ltd., Loughborough, UK; Catalogue No: 13439118)
- Plastic transfer pipettes, Fisherbrand™ 3 mL (Fisher Scientific UK Ltd., Loughborough, UK; Catalogue No: 16405009)
- Glass microscope slides, menzel superfrost, 26 × 76 × 1 mm (Fisher Scientific UK Ltd., Loughborough, UK; Catalogue No: 16252171)
- Gatan 3View SEM aluminum pin stubs (TAAB Laboratories, Aldermaston, UK; Catalogue No. G312/1)
2.2. Equipment
- pH Meter, Mettler Toledo™ S210 Seven Compact (Fisher Scientific UK Ltd., Loughborough, UK; Catalogue No: 15360161)
- Hot Plate Stirrer, Fisherbrand™ (Fisher Scientific UK Ltd., Loughborough, UK; Catalogue No: 15363518)
- Low speed Rotator (Agar Scientific, Stansted, UK; Catalogue No: AG1050)
- Vortex mixer (Starlab, Milton Keynes, UK; N2400-6110)
- Embedding oven, placed inside fume hood (Agar Scientific, Stansted, UK; Catalogue No: AGB7606)
- Diamond knife, Diatome, 3 mm 45 deg (TAAB Laboratories, Aldermaston, UK; Catalogue No. K065/30)
- UC6 ultramicrotome (Leica Microsystems (UK) Ltd., Milton Keynes, UK)
- ACE200 Low vacuum sputter coater (Leica Microsystems (UK) Ltd., Milton Keynes, UK)
- Zeiss Sigma Field Emission Gun Scanning Electron Microscope (Carl Zeiss, Oberkochen, Germany)
- Gatan 3view2XP (Gatan, Pleasanton, CA, USA)
3. Procedure
3.1. Fixation
 CRITICAL STEP Excise tissue from animals immediately after sacrifice and immerse in fixative to prevent autolysis of structures deep within the sample. Samples should be cut into blocks, 1–2 mm3 in size, to ensure the fixative penetrates fully and as quickly as possible.
CRITICAL STEP Excise tissue from animals immediately after sacrifice and immerse in fixative to prevent autolysis of structures deep within the sample. Samples should be cut into blocks, 1–2 mm3 in size, to ensure the fixative penetrates fully and as quickly as possible. CRITICAL STEP Before starting the protocol, make up the following solutions in a fume hood the day before use. Prepare sufficient amounts of each for 4 mL per sample vial.
CRITICAL STEP Before starting the protocol, make up the following solutions in a fume hood the day before use. Prepare sufficient amounts of each for 4 mL per sample vial.- 0.5% aqueous low molecular weight tannic acid solution. Weigh out tannic acid power, transfer into a 50 mL glass conical flask, add distilled water and stir gently until fully dissolved.
- Just prior to use, draw the solution up into a 20 mL Luer-lock syringe and attach a sartorius RC 0.20 µm syringe filter for dispensing into sample vials.
- 2% aqueous uranyl acetate solution. Weigh powder directly into 50 mL polypropylene, solvent-resistant, universal tube with a sealable cap and add distilled water; double-wrap the cap with parafilm to prevent any leakage, and vortex until fully dissolved, then wrap the tube in aluminium foil as the uranyl acetate solution is light sensitive and store at room temperature.
- Just before use, draw the solution up into a 20 mL Luer-lock syringe and attach asartorius RC 0.20 µm syringe filter.
- 1% ethanolic uranyl acetate solution. Weigh powder directly into a 50 mL solvent-resistant polypropylene universal tube and add ethanol. Double-wrap the sealed cap with parafilm to prevent leakage. Vortex the solution until the powder is dissolved, wrap the tube in aluminium foil and store at room temperature in the dark. Prior to use, draw the solution up into a 20 mL Luer-lock syringe and attach a sartorius RC 0.20 µm syringe filter.
3.2. En Bloc Staining
3.3. Dehydration
 CRITICAL STEP Dehydration of the samples is crucial as any residual water would cause failure of resin polymerisation later in the protocol.
CRITICAL STEP Dehydration of the samples is crucial as any residual water would cause failure of resin polymerisation later in the protocol. CRITICAL STEP The addition of 1% alcoholic UA for 2 h further enhances backscatter electron contrast of collagen fibrils improving overall imaging quality for SBF-SEM data acquisition. Note: Staining with 1% ethanolic UA must be kept to 2 h maximum. Prolonged staining can lead to excessive backscatter enhancement of collagen which may prevent visualisation of fibrillin fibres.
CRITICAL STEP The addition of 1% alcoholic UA for 2 h further enhances backscatter electron contrast of collagen fibrils improving overall imaging quality for SBF-SEM data acquisition. Note: Staining with 1% ethanolic UA must be kept to 2 h maximum. Prolonged staining can lead to excessive backscatter enhancement of collagen which may prevent visualisation of fibrillin fibres. CRITICAL STEP During the 2-h en bloc uranyl acetate staining step, prepare lead acetate solution. The solution must be freshly made before use, otherwise the lead acetate will precipitate from solution within 3 h. Lead acetate solution is prepared by adding 1.4 g of lead acetate to a 50 mL plastic universal tube with sealable, screw-top plastic cap; 25 mL of 100% ethanol is added, the cap tightened and parafilm wrapped around the cap to further seal the tube ensuring no leakage. Vortex the solution, and gently shake the tube intermittently for 15 min. After 15 min, add 25 mL of 100% acetone to the ethanolic lead acetate solution. Reseal the cap with parafilm and vortex and shake the solution for a further 15 min. As the solute is in excess, it will appear as a milky white suspension with undissolved lead acetate present. Filter with Whatman 1 filter paper to remove the solid suspension and collect the clear solution into a clean 50 mL universal tube. Finally, draw up the lead solution into a 20 mL Luer-lock syringe and attach a 0.2 µm syringe filter.
CRITICAL STEP During the 2-h en bloc uranyl acetate staining step, prepare lead acetate solution. The solution must be freshly made before use, otherwise the lead acetate will precipitate from solution within 3 h. Lead acetate solution is prepared by adding 1.4 g of lead acetate to a 50 mL plastic universal tube with sealable, screw-top plastic cap; 25 mL of 100% ethanol is added, the cap tightened and parafilm wrapped around the cap to further seal the tube ensuring no leakage. Vortex the solution, and gently shake the tube intermittently for 15 min. After 15 min, add 25 mL of 100% acetone to the ethanolic lead acetate solution. Reseal the cap with parafilm and vortex and shake the solution for a further 15 min. As the solute is in excess, it will appear as a milky white suspension with undissolved lead acetate present. Filter with Whatman 1 filter paper to remove the solid suspension and collect the clear solution into a clean 50 mL universal tube. Finally, draw up the lead solution into a 20 mL Luer-lock syringe and attach a 0.2 µm syringe filter. CRITICAL STEP The 100% acetone transition step is essential to remove any trace of ethanol, which is not miscible with the CY212 resin. Any residual ethanol would lead to unsuccessful polymerisation of resin blocks for SBF SEM 3view.
CRITICAL STEP The 100% acetone transition step is essential to remove any trace of ethanol, which is not miscible with the CY212 resin. Any residual ethanol would lead to unsuccessful polymerisation of resin blocks for SBF SEM 3view.3.4. Resin Infiltration
 CRITICAL STEP Resin infiltration must be performed over a longer duration than normal TEM processing as the heavy metal solutions used in the previous steps make the sample extremely dense. To extend the duration of infiltration, the sample is exposed to the embedding resin in two stages: the first is without BDMA accelerator, and the second is with the complete resin mixture, including the accelerator. BDMA is used to catalyse the polymerisation reaction of the resin, so leaving out BDMA initially allows more time for infiltration.
CRITICAL STEP Resin infiltration must be performed over a longer duration than normal TEM processing as the heavy metal solutions used in the previous steps make the sample extremely dense. To extend the duration of infiltration, the sample is exposed to the embedding resin in two stages: the first is without BDMA accelerator, and the second is with the complete resin mixture, including the accelerator. BDMA is used to catalyse the polymerisation reaction of the resin, so leaving out BDMA initially allows more time for infiltration.| Araldite CY212 monomer | 14 mL |
| DDSA Hardener | 16 mL |
| BDMA Accelerator | 0.6 mL |
 CRITICAL STEP. Embedding the specimens. Place the flask containing residual resin into the embedding oven for 10–15 min before inserting the specimens into wells in the mould. This will make the resin less viscous and assist in applying small amounts by pipette to accurately fill the mould wells. Ensure the samples are orientated in the mould so they can be cut in the correct section plane for 3view imaging. This can be facilitated by manipulating the specimens with fine forceps while observing via a dissecting microscope, placed temporarily into the fume hood. Small labels bearing brief printed details of the specimen can be immersed into the resin and positioned clear of the sample and prospective cutting face. Individual wells are then topped up with sufficient extra resin to form a level surface. Avoid over-filling the wells, as the convex surface so produced makes it difficult later to securely clamp the block in the specimen chuck of the ultramicrotome. The mould, complete with specimens, is then placed into an embedding oven contained within the fume hood. Polymerise the resin at 60 °C for 24 h minimum.
CRITICAL STEP. Embedding the specimens. Place the flask containing residual resin into the embedding oven for 10–15 min before inserting the specimens into wells in the mould. This will make the resin less viscous and assist in applying small amounts by pipette to accurately fill the mould wells. Ensure the samples are orientated in the mould so they can be cut in the correct section plane for 3view imaging. This can be facilitated by manipulating the specimens with fine forceps while observing via a dissecting microscope, placed temporarily into the fume hood. Small labels bearing brief printed details of the specimen can be immersed into the resin and positioned clear of the sample and prospective cutting face. Individual wells are then topped up with sufficient extra resin to form a level surface. Avoid over-filling the wells, as the convex surface so produced makes it difficult later to securely clamp the block in the specimen chuck of the ultramicrotome. The mould, complete with specimens, is then placed into an embedding oven contained within the fume hood. Polymerise the resin at 60 °C for 24 h minimum.3.5. 3View Pin Preparation
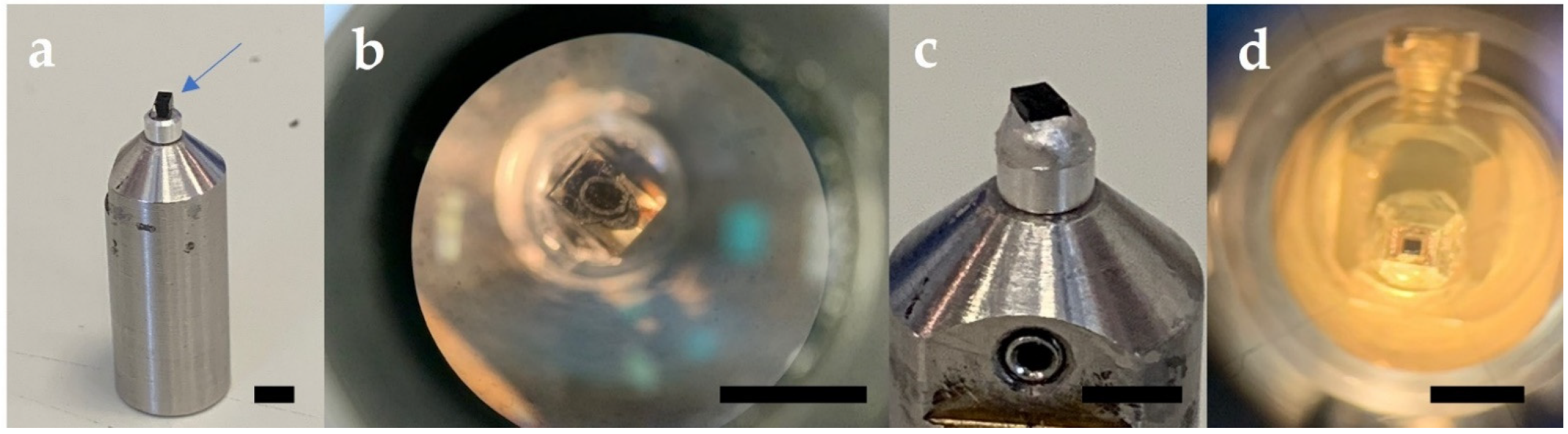
 CRITICAL STEP The small block surface dimensions are required for serial block face cutting in the 3view 2XP system.
CRITICAL STEP The small block surface dimensions are required for serial block face cutting in the 3view 2XP system. CRITICAL STEP Gold coating the specimen is critical for SBF SEM used in variable low-pressure mode, where excessive electrostatic charging from the poorly conductive epoxy resin block can interfere with imaging. The gold coating helps negate this charging effect by conducting the charge away from the block sides and block face.
CRITICAL STEP Gold coating the specimen is critical for SBF SEM used in variable low-pressure mode, where excessive electrostatic charging from the poorly conductive epoxy resin block can interfere with imaging. The gold coating helps negate this charging effect by conducting the charge away from the block sides and block face.4. 3View Imaging, Data Acquisition and 3D Reconstruction
4.1. Imaging and Data Acquisition
4.2. Post-Data Processing
 CRITICAL STEP The serial image data files collected in dm4 Gatan format must first be aligned using Gatan digital micrograph GMS3 software auto alignment tool.
CRITICAL STEP The serial image data files collected in dm4 Gatan format must first be aligned using Gatan digital micrograph GMS3 software auto alignment tool.4.3. 3D Reconstruction
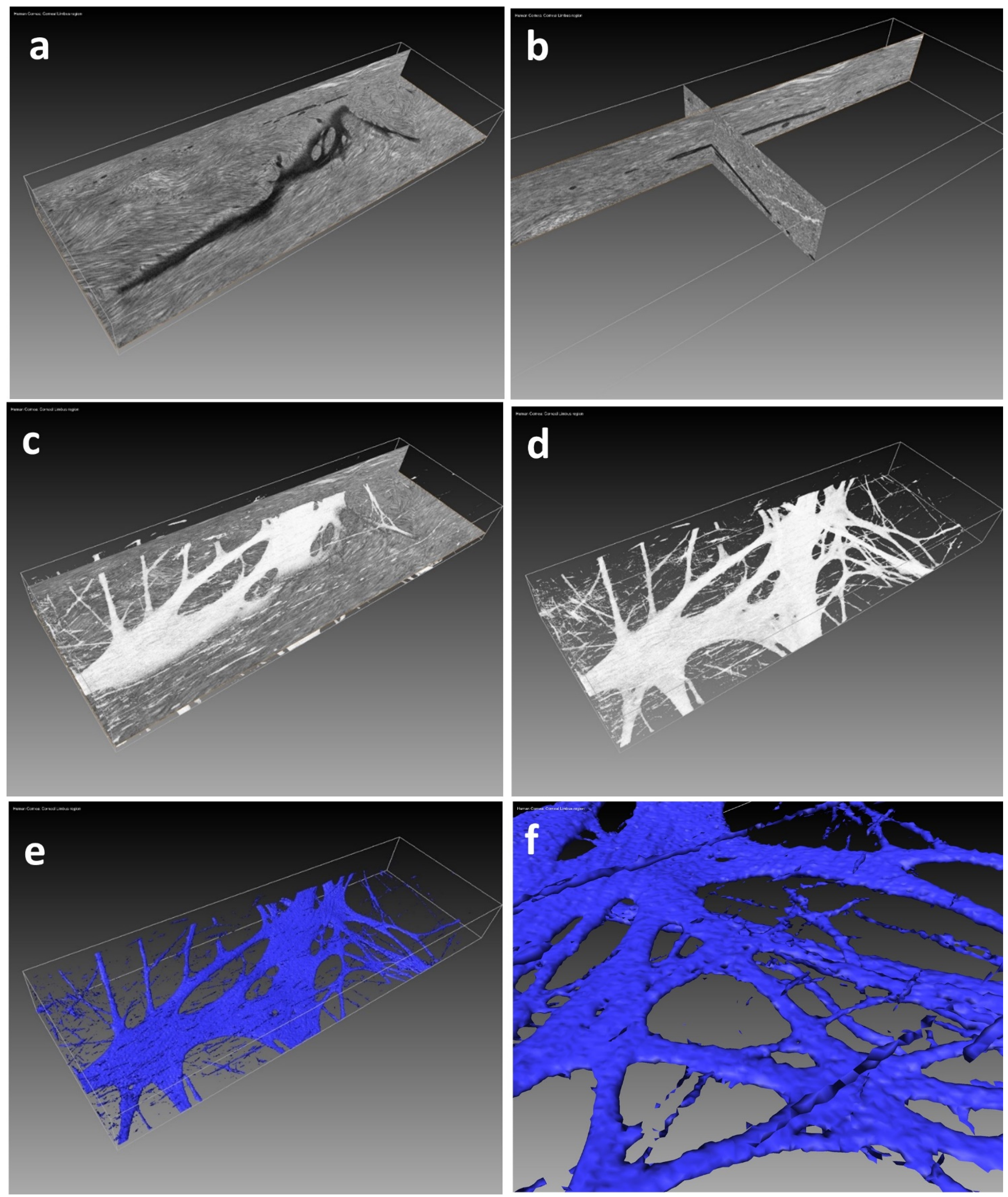
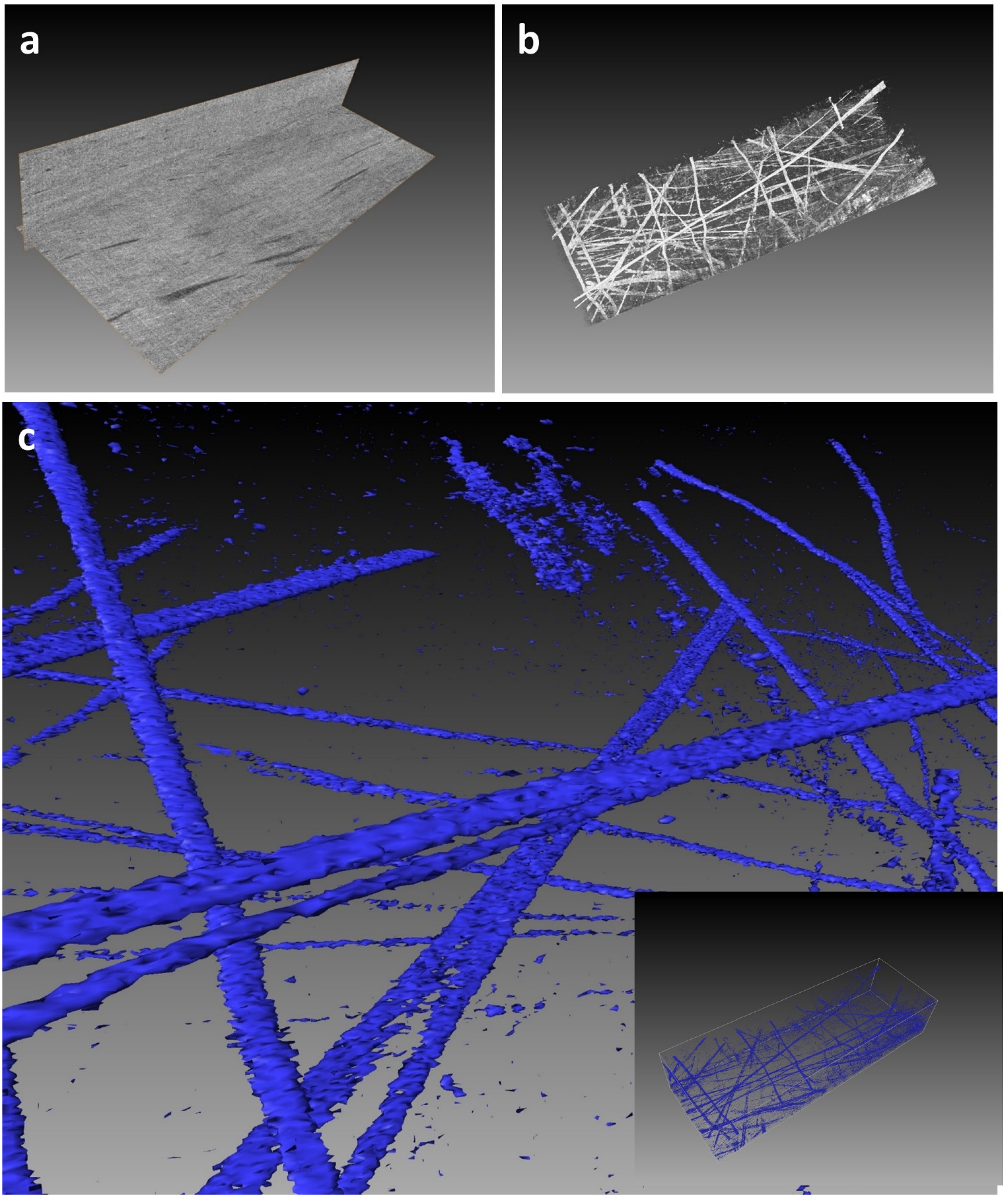
5. Expected Results
6. Discussion: Including Limitations of the Protocol
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosenbloom, J.; Abrams, W.R.; Mecham, R. Extracellular matrix 4: The elastic fibre. FASEB J. 1993, 7, 1208–1218. [Google Scholar] [CrossRef] [Green Version]
- Kielty, C.M.; Sherratt, M.J.; Shuttleworth, C.A. Elastic fibres. J. Cell Sci. 2002, 115, 2817–2828. [Google Scholar] [CrossRef]
- Kielty, C.M. Fell-muir lecture: Fibrillin microfibrils: Structural tensometers of elastic tissues? Int. J. Exp. Path. 2017, 98, 172–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakai, L.Y.; Keene, D.R.; Engvall, E. Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils. J. Cell Biol. 1986, 103, 2499–2509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, R.; Garner, A. Elastic and precursor fibres in the normal human eye. Exp. Eye Res. 1983, 36, 305–315. [Google Scholar] [CrossRef]
- Wheatley, H.M.; Traboulsi, E.I.; Flowers, B.E.; Maumenee, I.H.; Azar, D.; Pyeritz, R.E.; Whittum-Hudson, J.A. Immunohistochemical localization of fibrillin in human ocular tissues: Relevance to the Marfan syndrome. Arch. Ophthalmol. 1995, 113, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, S.D.; Behzad, A.R.; Sakai, L.Y.; Burns, A.R. Corneal stroma microfibrils. Exp Eye Res. 2015, 132, 198–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanagisawa, H.; Davis, E.C. Unraveling the mechanism of elastic fiber assembly: The roles of short fibulins. Int. J. Biochem. Cell Biol. 2010, 42, 1084–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, S.; Robertson, I.B.; Handford, P.A. Dissecting the fibrillin microfibril: Structural insights into organization and function. Structure 2012, 20, 215–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godwin, A.R.; Singh, M.; Lockhart, M.; Alanazi, Y.; Cain, S.A.; Baldock, C. The role of fibrillin and microfibril binding proteins in elastin and elastic fibre assembly. Matrix Biol. 2019, 84, 17–30. [Google Scholar] [CrossRef]
- Lee, B.; Godfrey, M.; Vitale, E.; Hori, H.; Mattei, M.G.; Sarfarazi, M.; Tsipouras, P.; Ramirez, F.; Hollister, D.W. Linkage of Marfan syndrome and a phenotypically related disorder to two different fibrillin genes. Nature 1991, 352, 330–334. [Google Scholar] [CrossRef]
- Denk, W.; Horstmann, H. Serial block-face scanning electron microscopy to reconstruct three-dimensional tissue nanostructure. PLoS Biol. 2004, 2, e329. [Google Scholar] [CrossRef]
- Knott, G.; Marchman, H.; Wall, D.; Lich, B. Serial section scanning electron microscopy of adult brain tissue using focused ion beam milling. J. Neurosci. 2008, 28, 2959–2964. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.N.; White, T.; Young, R.D.; Bell, J.; Winlove, C.P.; Meek, K.M. Three-dimensional arrangement of elastic fibers in the human corneal stroma. Exp. Eye Res. 2015, 146, 43–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, T.; Lewis, P.N.; Young, R.D.; Kitazawa, K.; Inatomi, T.; Kinoshita, S.; Meek, K.M. Elastic microfibril distribution in the cornea: Differences between normal and keratoconic stroma. Exp. Eye Res. 2017, 159, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Feneck, E.M.; Souza, R.B.; Lewis, P.N.; Hayes, S.; Pereira, L.V.; Meek, K.M. Developmental abnormalities in the cornea of a mouse model for Marfan syndrome. Exp. Eye Res. 2020, 194, 108001. [Google Scholar] [CrossRef] [PubMed]
- Deerinck, T.; Bushong, E.; Lev-Ram, V.; Shu, X.; Tsien, R.; Ellisman, M. Enhancing Serial Block-Face Scanning Electron Microscopy to Enable High Resolution 3-D Nanohistology of Cells and Tissues. Microsc. Microanal. 2010, 16, 1138–1139. [Google Scholar] [CrossRef] [Green Version]
- Martell, J.D.; Deerinck, T.J.; Lam, S.S.; Ellisman, M.H.; Ting, A.Y. Electron microscopy using the genetically encoded APEX2 tag in cultured mammalian cells. Nat. Protoc. 2017, 12, 1792–1816. [Google Scholar] [CrossRef] [Green Version]
- Simionescu, N.; Simionescu, M.J. Galloylglucoses of low molecular weight as mordant in electron microscopy. II. The moiety and functional groups possibly involved in the mordanting effect. Cell Biol. 1976, 70, 622–633. [Google Scholar] [CrossRef]
- Kajikawa, K.; Yamaguchi, T.; Katsuda, S.; Miwa, A. An improved electron stain for elastic fibers using tannic acid. J. Electron Microsc. 1975, 24, 287–289. [Google Scholar]
- Kushida, H. Staining of thin sections with lead acetate. J. Electron Microsc. 1966, 15, 93–94. [Google Scholar]
- Starborg, T.; Kalson, N.S.; Lu, Y.; Mironov, A.; Cootes, T.F.; Holmes, D.F.; Kadler, K.E. Using transmission electron microscopy and 3View® to determine collagen fibril size and three-dimensional organization. Nat. Protoc. 2013, 8, 1433–1448. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.; Starborg, T. Serial block face scanning electron microscopy in cell biology: Applications and technology. Tissue Cell 2019, 57, 111–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connell, M.K.; Murthy, S.; Phan, S.; Xu, C.; Buchanan, J.; Spilker, R.; Dalman, R.L.; Zarins, C.K.; Denk, W.; Taylor, C.A. The three-dimensional micro- and nanostructure of the aortic medial lamellar unit measured using 3D confocal & electron microscopy imaging. Matrix Biol. 2008, 27, 171–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezakhaniha, R.; Fonck, E.; Genoud, C.; Stergiopulos, N. Role of elastin anisotropy in structural strain energy functions of arterial tissue. Biomech. Model. Mechanobiol. 2010, 10, 599–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewis, P.N.; Young, R.D.; Souza, R.B.; Quantock, A.J.; Meek, K.M. Contrast-Enhanced Tissue Processing of Fibrillin-Rich Elastic Fibres for 3D Visualization by Volume Scanning Electron Microscopy. Methods Protoc. 2021, 4, 56. https://doi.org/10.3390/mps4030056
Lewis PN, Young RD, Souza RB, Quantock AJ, Meek KM. Contrast-Enhanced Tissue Processing of Fibrillin-Rich Elastic Fibres for 3D Visualization by Volume Scanning Electron Microscopy. Methods and Protocols. 2021; 4(3):56. https://doi.org/10.3390/mps4030056
Chicago/Turabian StyleLewis, Philip N., Robert D. Young, R. B. Souza, Andrew J. Quantock, and Keith M. Meek. 2021. "Contrast-Enhanced Tissue Processing of Fibrillin-Rich Elastic Fibres for 3D Visualization by Volume Scanning Electron Microscopy" Methods and Protocols 4, no. 3: 56. https://doi.org/10.3390/mps4030056





