Metastasis Model of Cancer Stem Cell-Derived Tumors
Abstract
:1. Introduction
2. Experimental Design
2.1. Materials
- Dulbecco’s Modified Eagle Medium (DMEM; Thermo Fisher Scientific, cat. no. 31966).
- Minimum Essential Media MEM non-essential amino acid solution (100×) (Wako, Osaka, Japan, catalog number: 139-15651).
- Fetal bovine serum (FBS, Gibco, Life Technologies, Massachusetts, MA, USA, Cat. No: 10437-028).
- Penicillin/streptomycin mixed solution (Nacalai Tesque, Kyoto, Japan, Cat. No-26253-84).
- L-glutamine (Nacalai Tesque, Kyoto, Japan, ((catalog number: 16948-04)).
- Trypsin, 0.25% Ethylenediamine tetraacetic acid (EDTA) (Atlanta Biologicals, Flowery Branch, GA, USA; Cat. no.: B81310).
- Phosphate buffered saline (PBS) (Genesee Scientific, El Cajon, CA, USA; Cat. no.: 25-508).
- Hank’s balanced salt solution (HBSS).
- Iodine solution (7.5% (wt/vol); Medline, cat. no. MDS093908).
- Isoflurane (100% (wt/wt); IsoFlo; Abbott, cat. no. B506).
- KnockOutTM Serum Replacement (Gibco, NY, USA; Cat. No.: 10828028).
- Collagenase (Gibco, NY, USA; Cat. No.: 17018029/).
- Mouse induced pluripotent stem cells (miPSCs) (iPS-MEF-Ng-20D-17, Lot No. 012, Riken Cell Bank, Tokyo, Japan), in which puromycin (puro) resistant gene and green fluorescent protein (GFP) gene were cloned under the control of Nanog promoter.
- BALB/c-nu/nu immunodeficient mice, female, four weeks old (Charles River laboratories, kanagawa, Japan).
- 70% ethanol (Sigma-Aldrich; Cat. No.: 459836-2).
- CaCl2 (Sigma-Aldrich; Cat. No.: C5670).
- Leukemia inhibitory factor, 1000 U/mL (LIF, Merk Millipore).
2.2. Equipment
- Sanyo MCO-19AIC(UV) CO2 incubator (Marshall Scientific, Hampton, WV, USA).
- Labculture® Class II, Type A2 Biological Safety Cabinets (E-Series).
- Olympus IX81 microscope (Olympus, Tokyo, Japan).
- Laser scanning confocal microscope, FV-1000, Olympus, Tokyo, Japan.
- Tissue culture-treated plate, 60 mm dish (TPP Techno Plastic Products AG Schaffhausen, Switzerland, Cat. No 93060).
- Tissue culture-treated plate, 100 mm dish (TPP Techno Plastic Products AG Schaffhausen, Switzerland, Cat. No 93100).
- Filter max 250 mL (TPP, Switzerland, Cat. No 99255).
- Falcon® Conical Centrifuge Tubes (15 mL; BD Falcon, New York, NY, USA Cat. No 352095).
- Falcon® Conical Centrifuge Tubes (50 mL; BD Falcon, New York, NY, USA Cat. No 352070).
- Liquid N2 storage tank.
- Inverted microscope with bright field (Nikon, model no. DIAPHOT 200).
- Automated cell counter (TC20; Bio-Rad Laboratories, cat. no. 1450102).
- 1.5-mL microcentrifuge tubes (Eppendorf, cat. no. 0030120086).
- Refrigerated centrifuge (Eppendorf, cat. no. 5810 R).
- 100-mm dishes (tissue culture treated; Corning, cat. no. 353003).
- Anesthesia machine (Vet Tech Solutions).
- Microcentrifuge 1.5-mL tubes (Eppendorf, cat. no. 0030120086).
- 5/10/25-mL plastic disposable pipette.
3. Procedure
- Cancer stem cell induction for in vivo injection.
- II.
- CSCs preparation for injection
- Revive miPS-LLCev cells on gelatin coated dish.
- Change medium every 2 days.
- When cells are 70% confluent, trypsinize the cells.
- Collect cells from culturing flask and transfer it to 15 mL tube.
- Spin down cells at 1000 rpm for 5 min.
- Aspirate supernatant media.
- Wash cells with sterile PBS.
- Add PBS to tube, and again spin down cells at 1000 rpm for 5 min.
- Aspirate supernatant PBS.
- Resuspend cell pellet with 1 mL sterile BSS.
- Count the cells.
- Suspend 106 cells in 100 μL sterile HBSS.
- Keep the Eppendorf in ice.
-
- CRITICAL STEP: draw cells into the syringe without a needle to prevent cell shearing.
- Before injecting, flick or invert the syringe to ensure the cells are in suspension.
- Inject cells slowly subcutaneously.
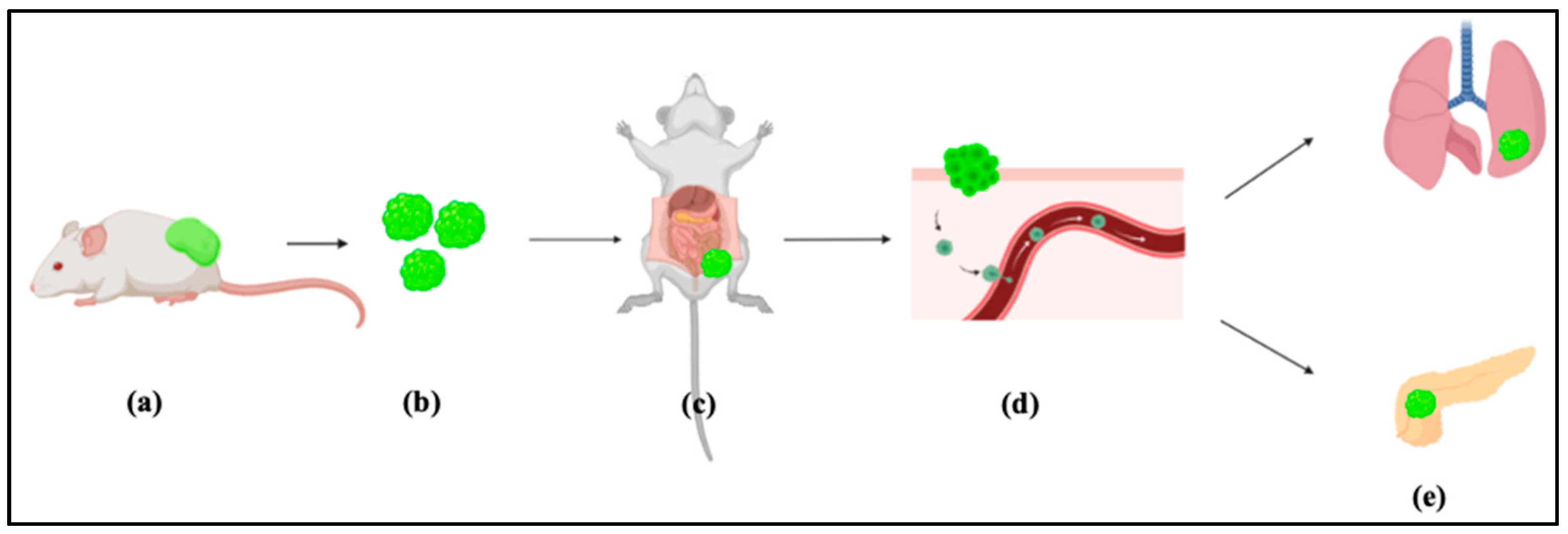
- Four weeks later, malignant tumor should be observed
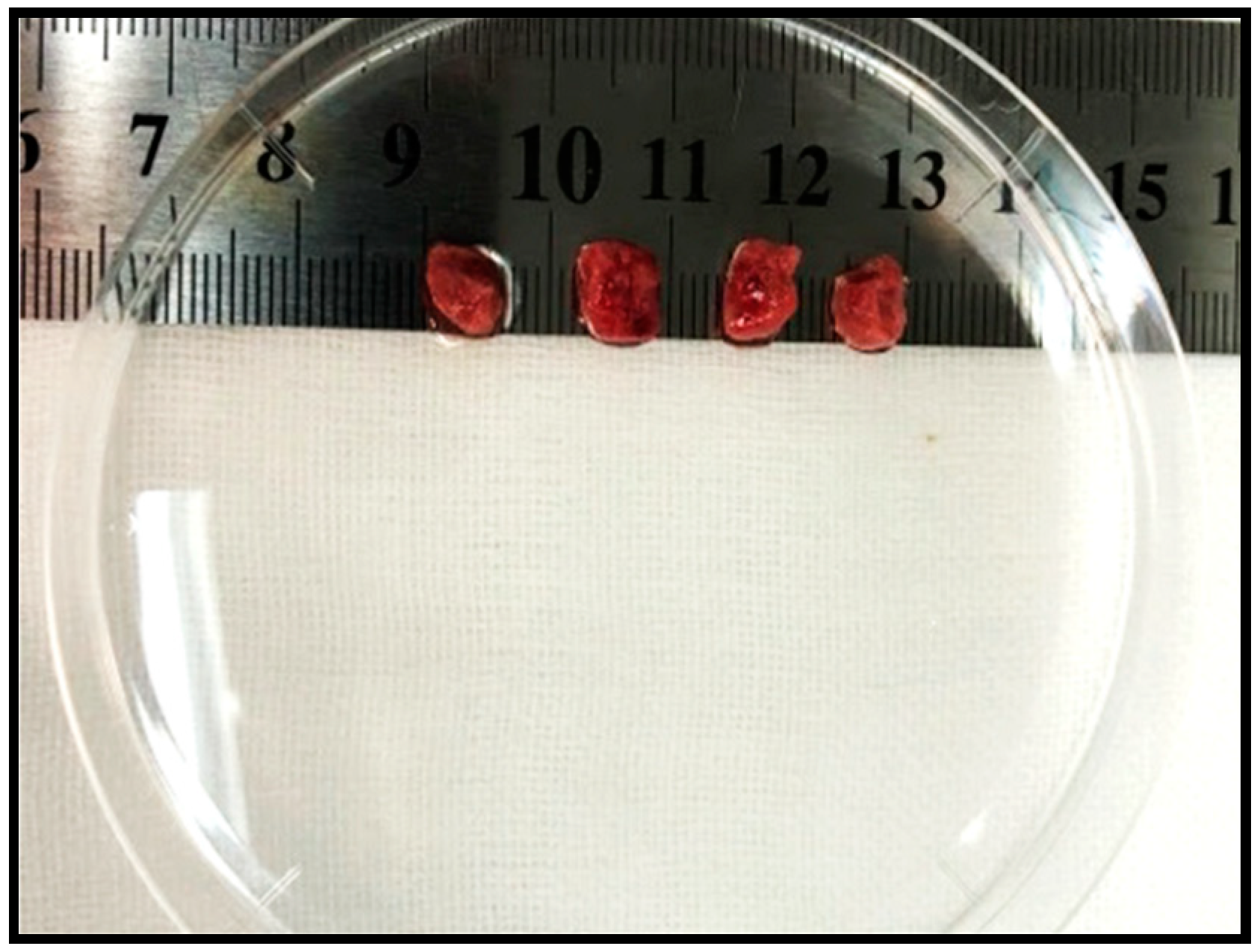
- Excise and minimize the tumor tissue for intraperitoneal transplantation (Figure 2).
- III.
- Intraperitoneal (IP) transplantation
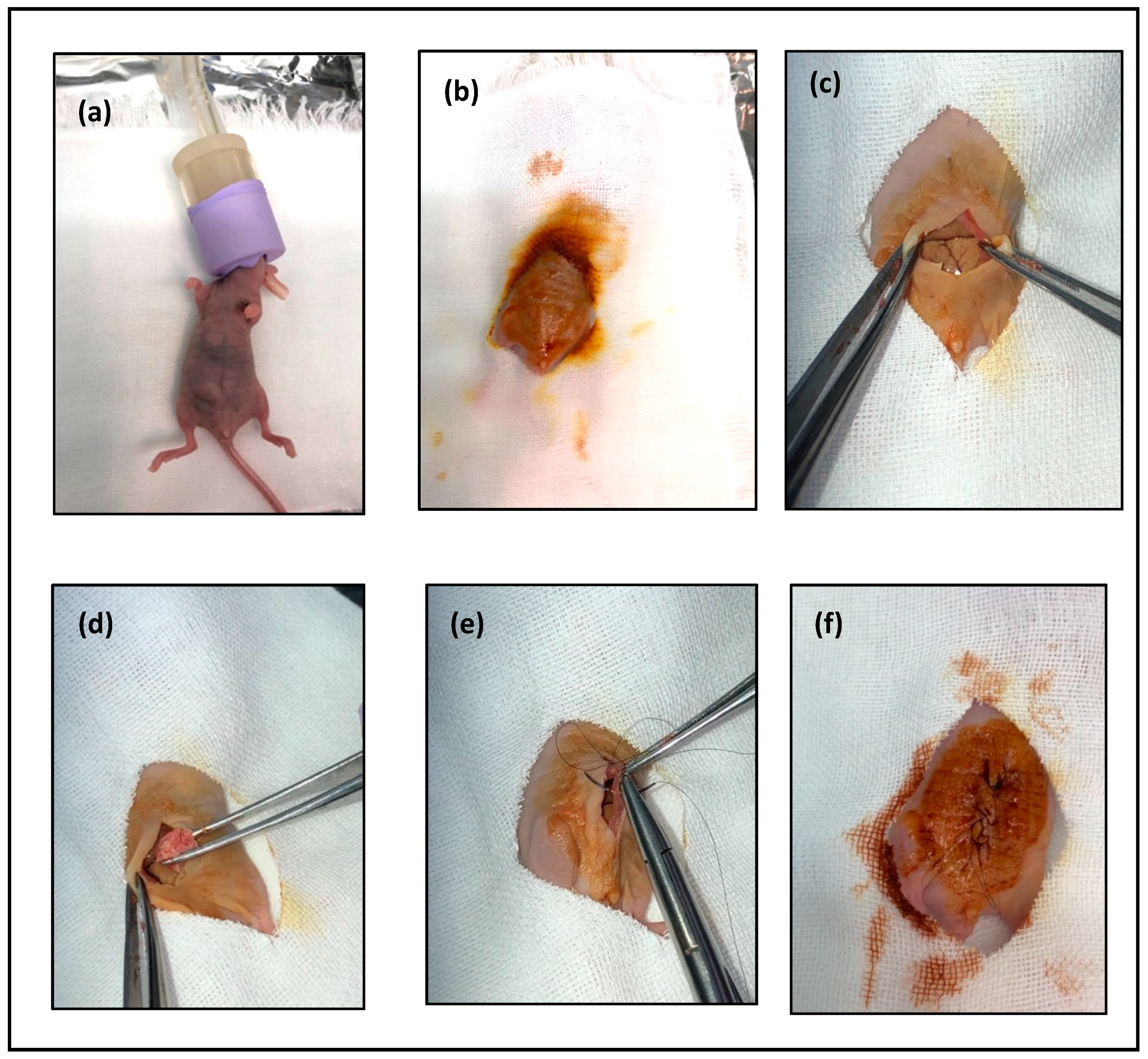
- Anesthetize mouse with 2% isoflurane (Figure 3a).
- After the mouse is anesthetized, prepare the area for transplantation with 70% alcohol (Figure 3b).
- Using the curved iris forceps, hold the skin and make a 15-mm vertical midline incision through the skin using the 24-mm iris scissors (Figure 3c).
- Insert 1 mm of the tumor tissue intraperitoneal (Figure 3d).
-
- CRITICAL STEP: try to avoid damaging any organ in the abdomen.
- Close the abdominal wall and skin opening, performing continuous stitching (Figure 3e,f).
- The day after surgery, check on the animal to make sure that the sutures are still correctly in place.
- After four weeks, euthanatize mice with 5% of isoflurane through inhalation to ensure rapid loss of consciousness and respiratory and cardiac arrest followed by cervical dislocation to ensure the death of mice.
-
- CRITICAL STEP: four weeks are mandatory so that the cells will have enough time to be disseminated from the original tumor to the other organs. Before four weeks, metastasis will not be visible enough.
- The mouse allografts were excised and cut into small pieces (approximately 1 mm3).
- Wash in the PBS for three times.
- Transfer the pieces into a 15-mL tube with 4 mL of dissociation buffer.
- Incubate at 37 °C for 40 min.
- Add 5 mL of DMEM containing 10% FBS to terminate the digestion.
- Transfer suspended cells into new tubes.
- Centrifuge at 1000 rpm for 5 min at 20 °C.
- Resuspend the cell pellet in 5 mL PBS.
- Centrifuge at 1000 rpm for 5 min 20 °C.
- Aspirate PBS.
- Resuspend the cell pellet in 5 mL 5 mL DMEM containing 10% FBS without LIF.
- Seed 5 × 104 cells into a 60 mm dish.
- Treat the cells with puromycin for 24 h to remove the host cells.
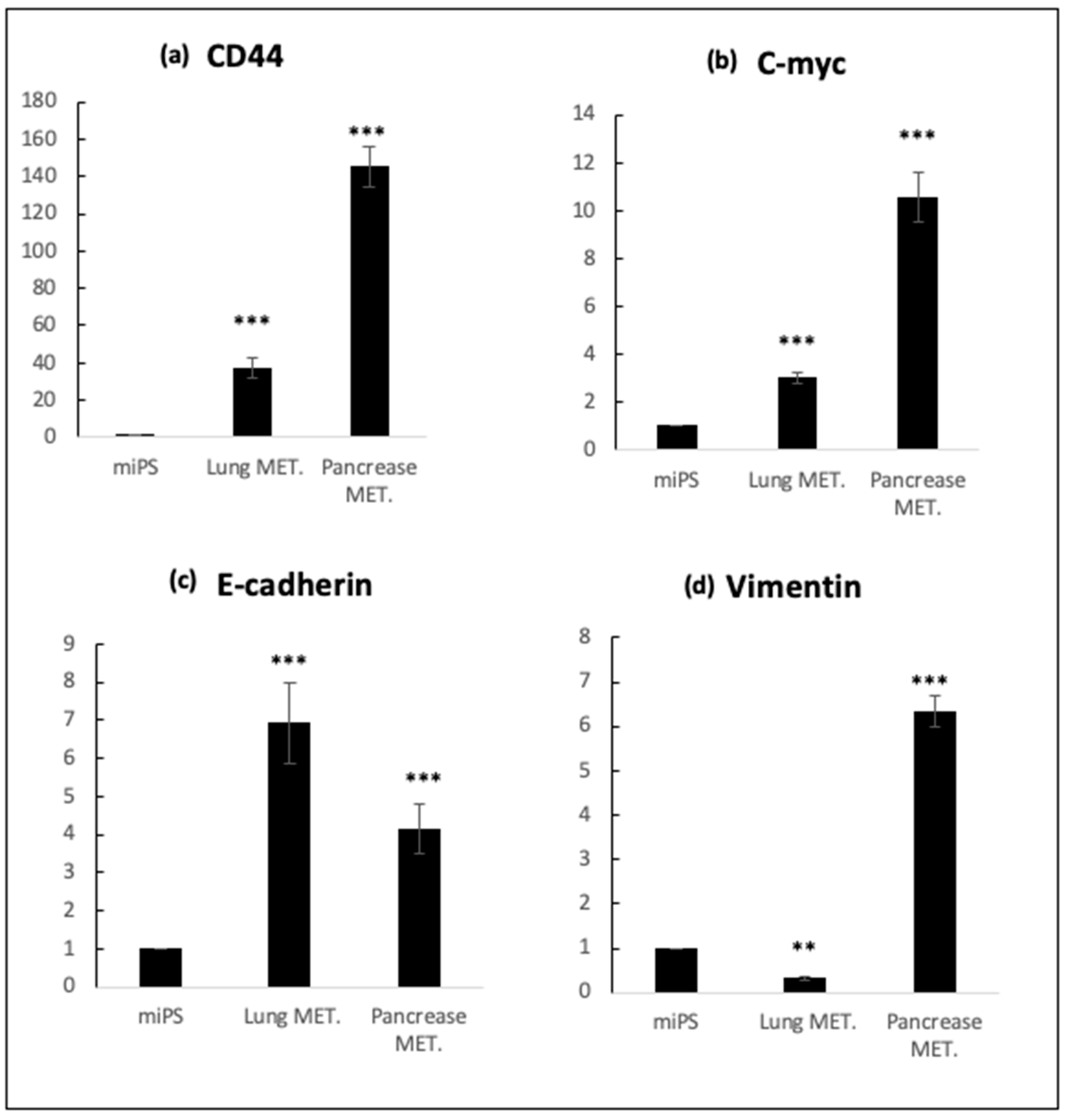
4. Expected Results
5. Reagents Setup
Dissociation Buffer
- 0.25% trypsin
- 0.1% collagenase
- 20% KnockOutTM Serum Replacement (Gibco, NY, USA).
- 1 mM of CaCl2.
Author Contributions
Funding
Conflicts of Interest
References
- Seyfried, T.N.; Huysentruyt, L.C. On the origin of cancer metastasis. Crit. Rev. Oncog. 2013, 18, 43–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fidler, I.J. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer 2003, 3, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Ell, B.; Kang, Y. Transcriptional control of cancer metastasis. Trends Cell Biol. 2013, 23, 603–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, J.C. Cancer stem cells: Role in tumor growth, recurrence, metastasis, and treatment resistance. Medicine (Baltimore) 2016, 95, S20–S25. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Czarnecka, A.M.; Helbrecht, I.; Bartnik, E.; Lian, F.; Szczylik, C. Current approaches in identification and isolation of human renal cell carcinoma cancer stem cells. Stem Cell Res. Ther. 2015, 6, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, Z.; Xu, Y.; Liang, W.; Liang, K.; Hu, X.; Wang, L.; Zou, Z.; Ma, Y. Isolation of cancer progenitor cells from cancer stem cells in gastric cancer. Mol. Med. Rep. 2017, 15, 3637–3643. [Google Scholar] [CrossRef] [PubMed]
- Afify, S.M.; Hassan, G.; Osman, A.; Calle, A.S.; Nawara, H.M.; Zahra, M.H.; El-Ghlban, S.; Mansour, H.; Alam, M.J.; Abu Quora, H.A.; et al. Metastasis of Cancer Stem Cells Developed in the Microenvironment of Hepatocellular Carcinoma. Bioengineering 2019, 6, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calle, A.S.; Nair, N.; Oo, A.K.; Prieto-Vila, M.; Koga, M.; Khayrani, A.C.; Hussein, M.; Hurley, L.; Vaidyanath, A.; Seno, A.; et al. A new PDAC mouse model originated from iPSCs-converted pancreatic cancer stem cells (CSCcm). Am. J. Cancer Res. 2016, 6, 2799–2815. [Google Scholar] [PubMed]
- Chen, L.; Kasai, T.; Li, Y.; Sugii, Y.; Jin, G.; Okada, M.; Vaidyanath, A.; Mizutani, A.; Satoh, A.; Kudoh, T.; et al. A Model of Cancer Stem Cells Derived from Mouse Induced Pluripotent Stem Cells. PLoS ONE 2012, 7, e33544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, G.; Afify, M.S.; Nair, N.; Kumon, K.; Osman, A.; Du, J.; Mansour, H.; Abu Quora, A.H.; Nawara, M.H.; Satoh, A.; et al. Hematopoietic Cells Derived from Cancer Stem Cells Generated from Mouse Induced Pluripotent Stem Cells. Cancers 2019, 12, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seno, A.; Kasai, T.; Ikeda, M.; Vaidyanath, A.; Masuda, J.; Mizutani, A.; Murakami, H.; Ishikawa, T.; Seno, M. Characterization of gene expression patterns among artificially developed cancer stem cells using spherical self-organizing map. Cancer Inform. 2016, 15, CIN-S39839. [Google Scholar] [CrossRef] [PubMed]
- Khanna, C.; Hunter, K. Modeling metastasis in vivo. Carcinogenesis 2005, 26, 513–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suhail, Y.; Cain, M.P.; Vanaja, K.; Kurywchak, P.A.; Levchenko, A.; Kalluri, R.; Kshitiz. Systems Biology of Cancer Metastasis. Cell Syst. 2019, 9, 109–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, T.; Mizutani, A.; Chen, L.; Takaki, M.; Hiramoto, Y.; Matsuda, S. Characterization of cancer stem-like cells derived from mouse induced pluripotent stem cells transformed by tumor-derived extracellular vesicles. J. Cancer 2014, 5, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Afify, S.M.; Seno, M. Conversion of Stem Cells to Cancer Stem Cells: Undercurrent of Cancer Initiation. Cancers 2019, 11, 345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afify, S.M.; Chen, L.; Yan, T.; Sanchez Calle, A.; Nair, N.; Murakami, C.; Seno, M. Method to convert stem cells into cancer stem cells. Methods Protoc. 2019, 3, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansour, H.; Hassan, G.; Afify, S.M.; Yan, T.; Seno, A.; Seno, M. Metastasis Model of Cancer Stem Cell-Derived Tumors. Methods Protoc. 2020, 3, 60. https://doi.org/10.3390/mps3030060
Mansour H, Hassan G, Afify SM, Yan T, Seno A, Seno M. Metastasis Model of Cancer Stem Cell-Derived Tumors. Methods and Protocols. 2020; 3(3):60. https://doi.org/10.3390/mps3030060
Chicago/Turabian StyleMansour, Hager, Ghmkin Hassan, Said M. Afify, Ting Yan, Akimasa Seno, and Masaharu Seno. 2020. "Metastasis Model of Cancer Stem Cell-Derived Tumors" Methods and Protocols 3, no. 3: 60. https://doi.org/10.3390/mps3030060






