Determination of pH Effects on Phosphatidyl-Hydroxytyrosol and Phosphatidyl-Tyrosol Bilayer Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
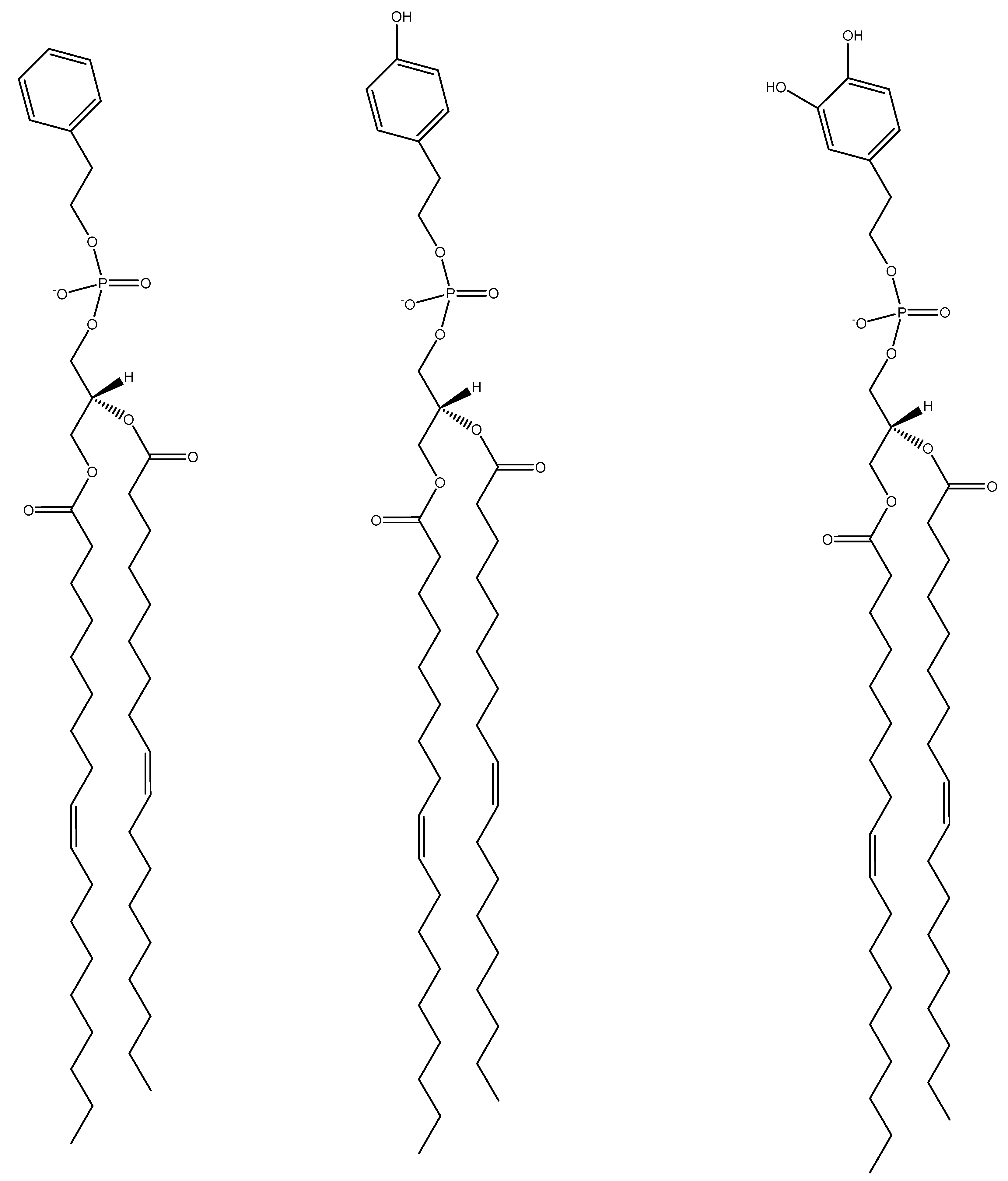
2.2. 1,2-Dioleoylphosphatidyl-tyrosol (DOPT) and 1,2-Dioleoylphosphatidyl-hydroxytyrosol (DOPHT) Lipid Synthesis, Isolation and Verification
2.3. Liposome Preparation
2.4. Dynamic Light Scattering and Zeta Potential
2.5. Quartz Crystal Microbalance with Dissipation Monitoring (QCMD)
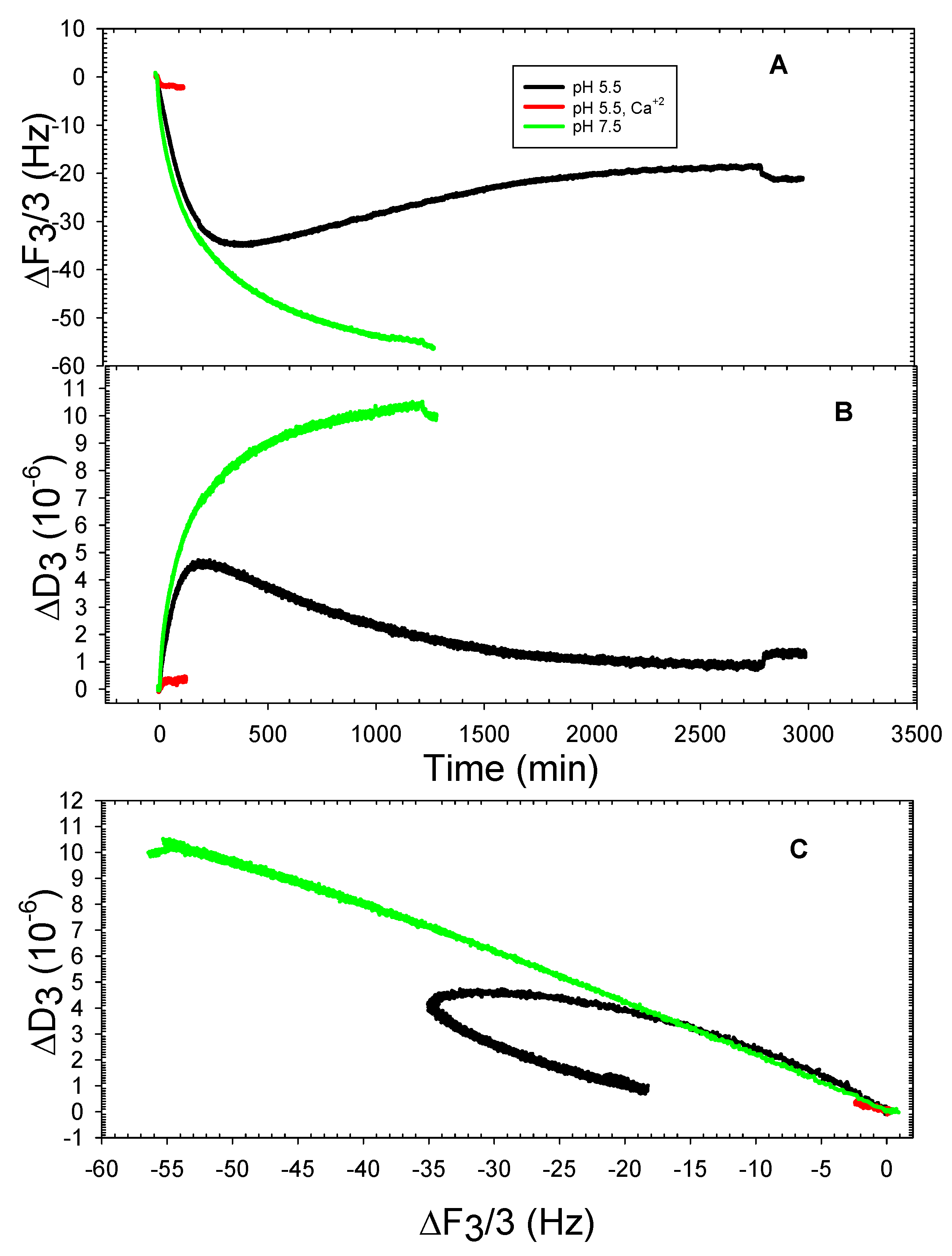
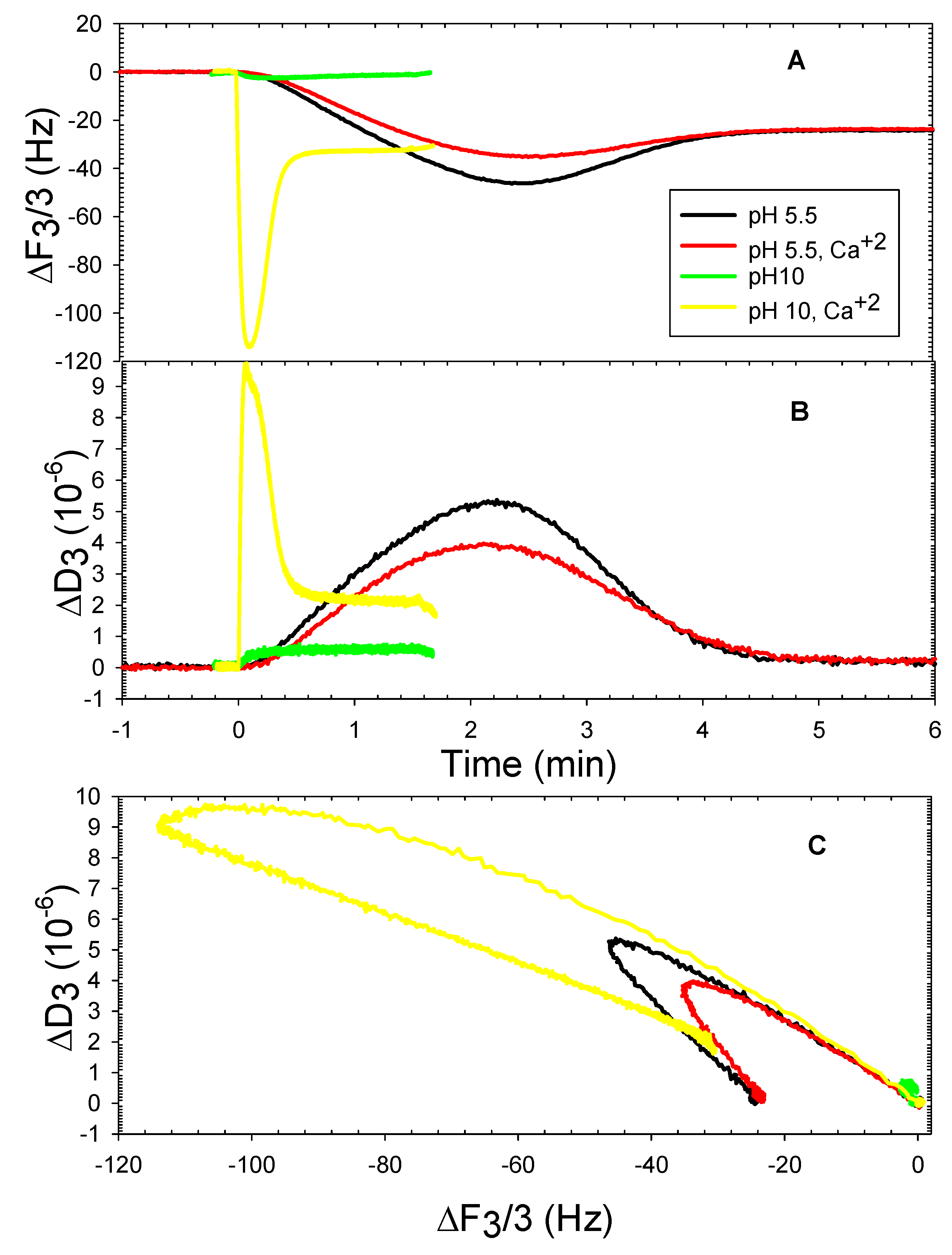
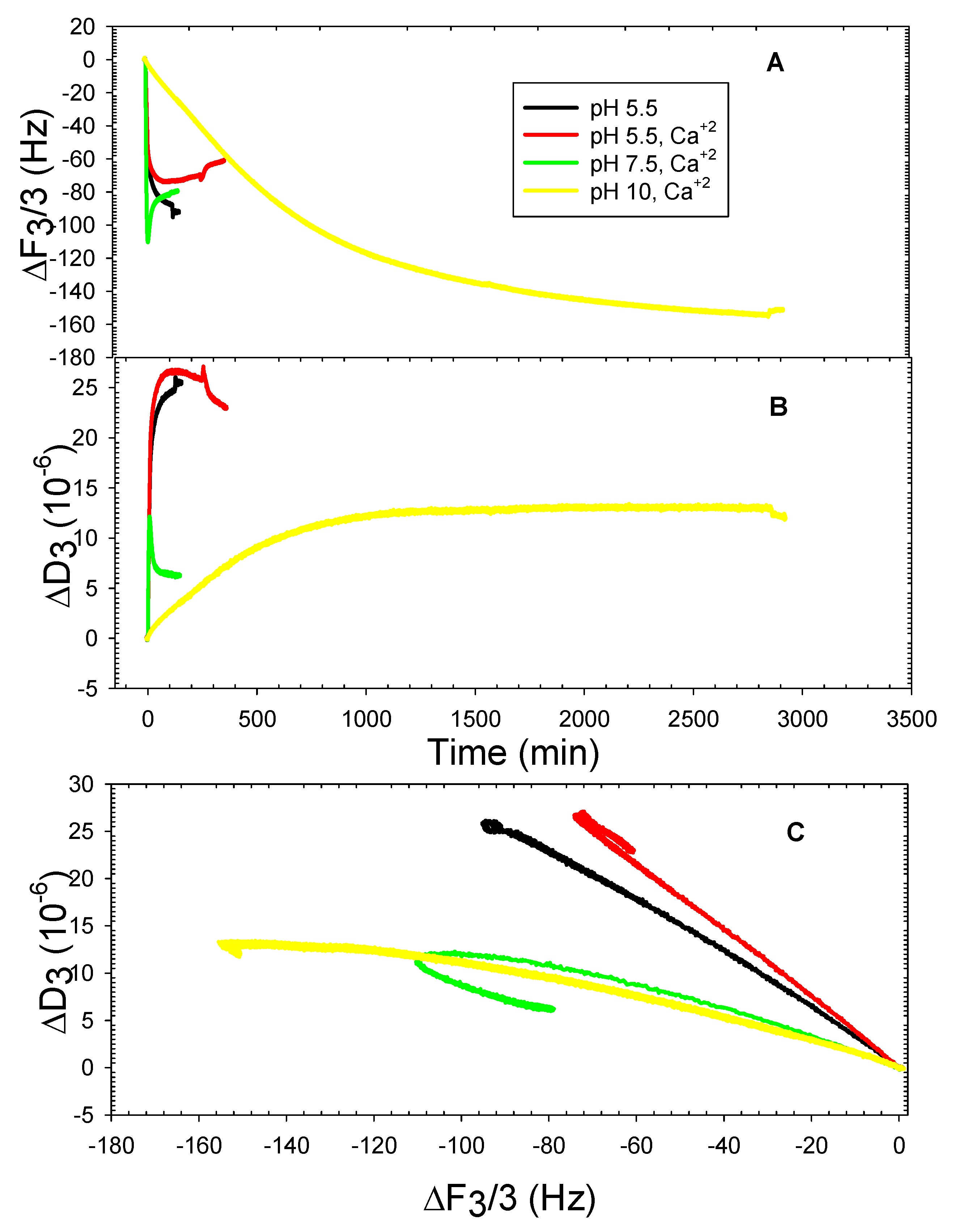
3. Results and Discussion
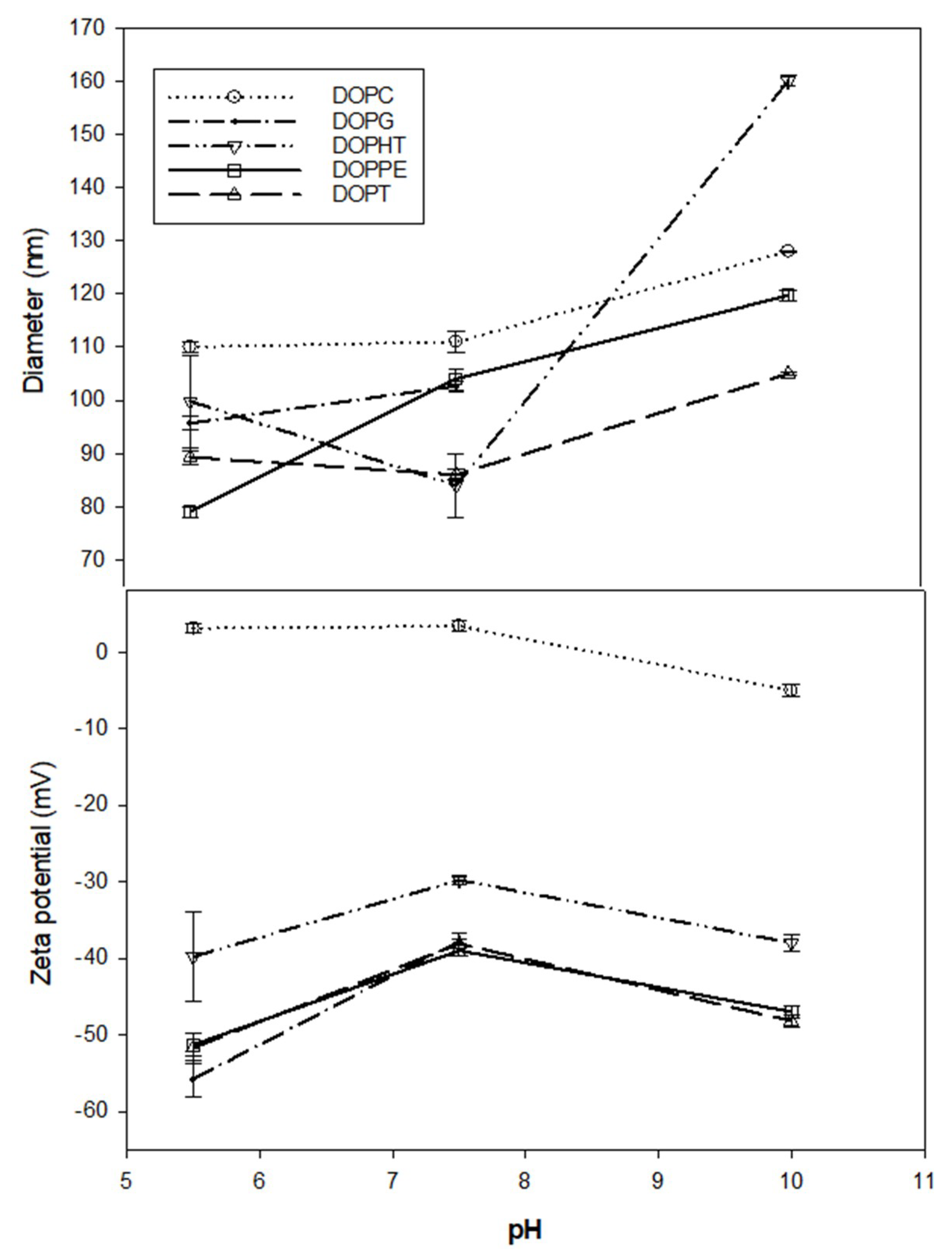
3.1. Liposome Size and Zeta Potential Characterization
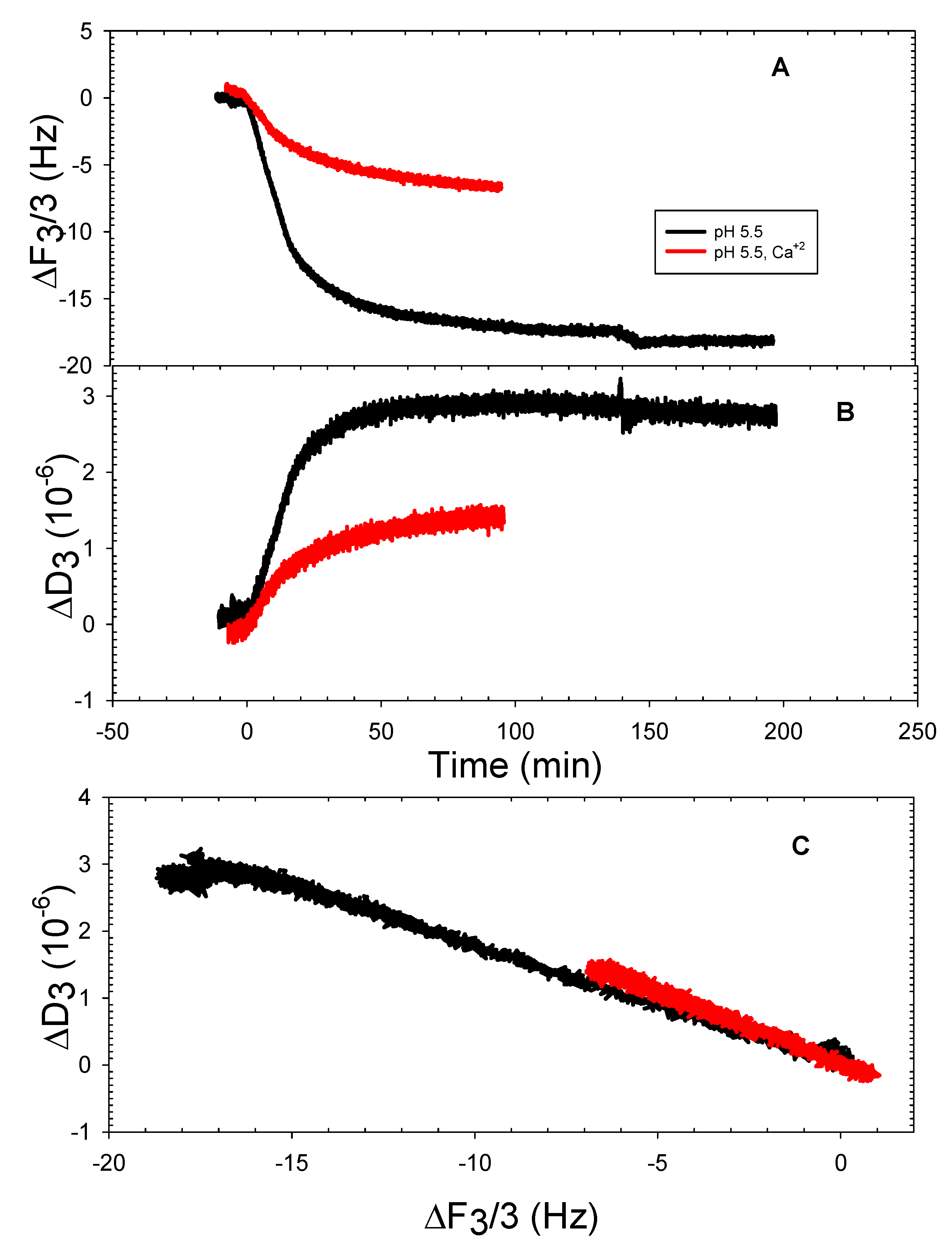
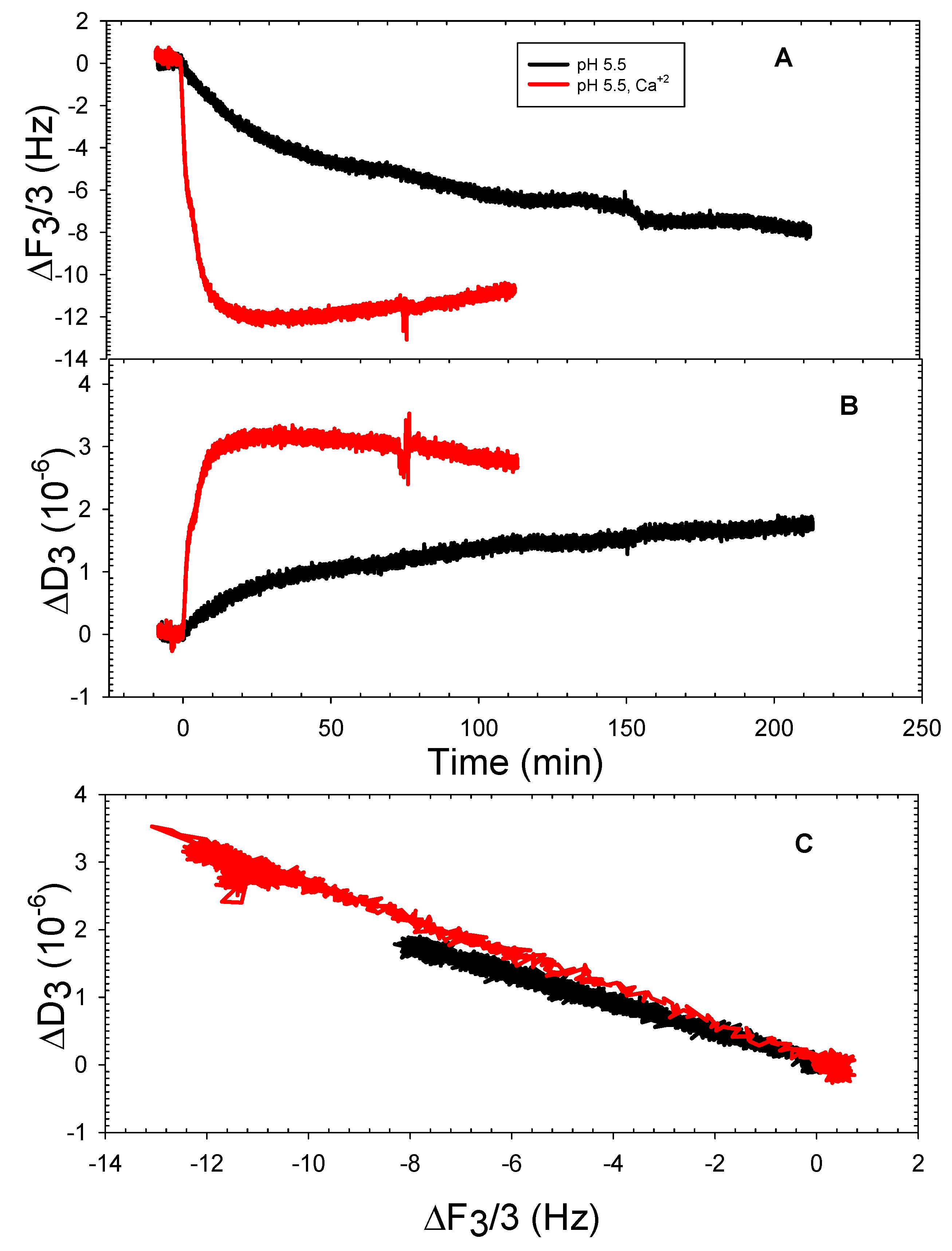
3.2. The DOPHT, DOPPE and DOPT Bilayer Behavior at Various pH Levels
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hu, T.; He, X.-W.; Jiang, J.-G.; Xu, X.-L. Hydroxytyrosol and Its Potential Therapeutic Effects. J. Agric. Food Chem. 2014, 62, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Caramia, G.; Gori, A.; Valli, E.; Cerretani, L. Virgin olive oil in preventive medicine: From legend to epigenetics. Eur. J. Lipid Sci. Technol. 2012, 1114, 375–388. [Google Scholar] [CrossRef]
- Visioli, F. Olive oil phenolics: Where do we stand? Where should we go? J. Agric. Food Chem. 2012, 92, 2017–2019. [Google Scholar]
- Mateos, R.; Trujillo, M.; Pereira-Caro, G.; Madrona, A.S.; Cert, A.; Espartero, J.L. New Lipophilic Tyrosyl Esters. Comparative Antioxidant Evaluation with Hydroxytyrosyl Esters. J. Agric. Food Chem. 2008, 56, 10960–10966. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, M.; Mateos, R.; Collantes de Teran, L.; Espartero, J.L.; Cert, R.; Jover, M.; Alcudia, F.; Bautista, J.; Cert, A.; Parrado, J. Lipophilic Hydroxytyrosyl Esters. Antioxidant Activity in Lipid Matrices and Biological Systems. J. Agric. Food Chem. 2006, 54, 3779–3785. [Google Scholar] [CrossRef] [PubMed]
- Laszlo, J.A.; Cermak, S.C.; Evans, K.O.; Compton, D.L.; Evangelista, R.; Berhow, M.A. Medium-chain alkyl esters of tyrosol and hydroxytyrosol antioxidants by cuphea oil transesterification. Eur. J. Lipid Sci. Technol. 2013, 115, 363–371. [Google Scholar] [CrossRef]
- Evans, K.O.; Laszlo, J.A.; Compton, D.L. Hydroxytyrosol and tyrosol esters partitioning into, location within, and effect on DOPC liposome bilayer behavior. Biochim. Biophys. Acta Biomembr. 2015, 1848, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Evans, K.O.; Compton, D.L. Phosphatidyl-hydroxytyrosol and phosphatidyl-tyrosol bilayer properties. Chem. Phys. Lipids 2017, 202, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Olson, F.; Hunt, C.A.; Szoka, F.C.; Vail, W.J.; Papahadjopoulos, D. Preparation of liposomes of defined size distribution by extrusion through polycarbonate membranes. Biochim. Biophys. Acta Biomembr. 1979, 557, 9–23. [Google Scholar] [CrossRef]
- Richter, R.P.; Berat, R.; Brisson, A.R. Formation of Solid-Supported Lipid Bilayers: An Integrated View. Langmuir 2006, 22, 3497–3505. [Google Scholar] [CrossRef] [PubMed]
- Petelska, A.D.; Figaszewski, Z.A. Effect of pH on the interfacial tension of bilayer lipid membrane formed from phosphatidylcholine or phosphatidylserine. Biochim. Biophys. Acta 2002, 1561, 135–146. [Google Scholar] [CrossRef] [Green Version]
- Stiufiuc, R.; Iacovita, C.; Stiufiuc, G.; Florea, A.; Achim, M.; Lucaciu, C.M. A new class of pegylated plasmonic liposomes: Synthesis and characterization. J. Colloid Interface Sci. 2015, 437, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Jackman, J.A.; Zhao, Z.; Zhdanov, V.P.; Frank, C.W.; Cho, N.-J. Vesicle Adhesion and Rupture on Silicon Oxide: Influence of Freeze–Thaw Pretreatment. Langmuir 2014, 30, 2152–2160. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Trefna, H.; Persson, M.; Kasemo, B.; Svedhem, S. Formation of supported lipid bilayers on silica: Relation to lipid phase transition temperature and liposome size. Soft Matter 2014, 10, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Jackman, J.A.; Yorulmaz, S.; Zhdanov, V.P.; Lee, H.; Cho, N.-J. Contribution of Temperature to Deformation of Adsorbed Vesicles Studied by Nanoplasmonic Biosensing. Langmuir 2015, 31, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Evans, K.O.; Lentz, B.R. Kinetics of lipid rearrangements during poly(ethylene glycol)-mediated fusion of highly curved unilamellar vesicles. Biochemistry 2002, 41, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Reimhult, E.; Höök, F.; Kasemo, B. Vesicla adsorption on SiO2 and TiO2 dependence on vesicle size. J. Chem. Phys. 2002, 117, 7401–7404. [Google Scholar] [CrossRef]
- Richter, R.; Mukhopadhyay, A.; Brisson, A. Pathways of Lipid Vesicle Deposition on Solid Surfaces: A Combined QCM-D and AFM Study. Biophys. J. 2003, 85, 3035–3047. [Google Scholar] [CrossRef] [Green Version]
- Reimhult, E.; Höök, F.; Kasemo, B. Intact Vesicle Adsorption and Supported Biomembrane Formation from Vesicles in Solution: Influence of Surface Chemistry, Vesicle Size, Temperature, and Osmotic Pressure. Langmuir 2003, 19, 1681–1691. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evans, K.O.; Compton, D.L.; Appell, M. Determination of pH Effects on Phosphatidyl-Hydroxytyrosol and Phosphatidyl-Tyrosol Bilayer Behavior. Methods Protoc. 2018, 1, 41. https://doi.org/10.3390/mps1040041
Evans KO, Compton DL, Appell M. Determination of pH Effects on Phosphatidyl-Hydroxytyrosol and Phosphatidyl-Tyrosol Bilayer Behavior. Methods and Protocols. 2018; 1(4):41. https://doi.org/10.3390/mps1040041
Chicago/Turabian StyleEvans, Kervin O., David L. Compton, and Michael Appell. 2018. "Determination of pH Effects on Phosphatidyl-Hydroxytyrosol and Phosphatidyl-Tyrosol Bilayer Behavior" Methods and Protocols 1, no. 4: 41. https://doi.org/10.3390/mps1040041




