Improved Detection and Fragmentation of Disulphide-Linked Peptides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. MALDI Matrix Preparation
2.4. Mass Spectrometric Measurements
3. Results and Discussion
3.1. Effect of Different Matrices and Additives on Peptide Calibrant Signal
3.2. Effect of Different Matrices and Additives in Detecting Disulphide Linked Peptides
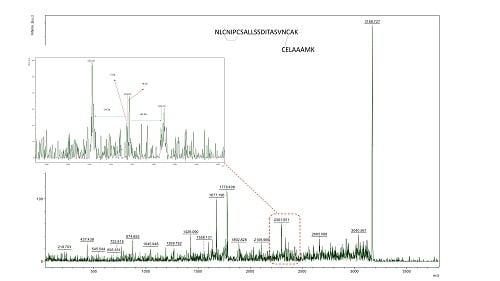
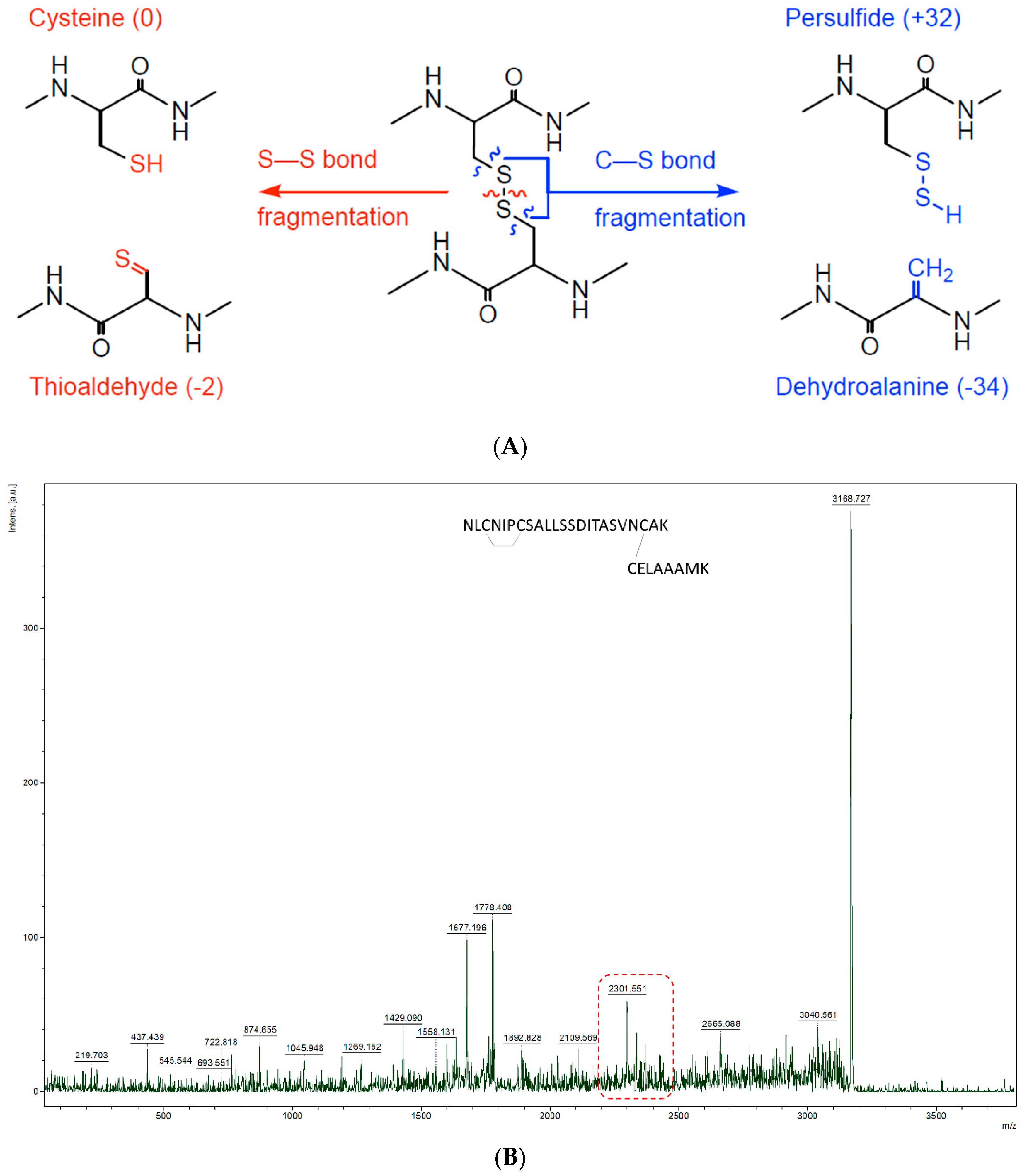
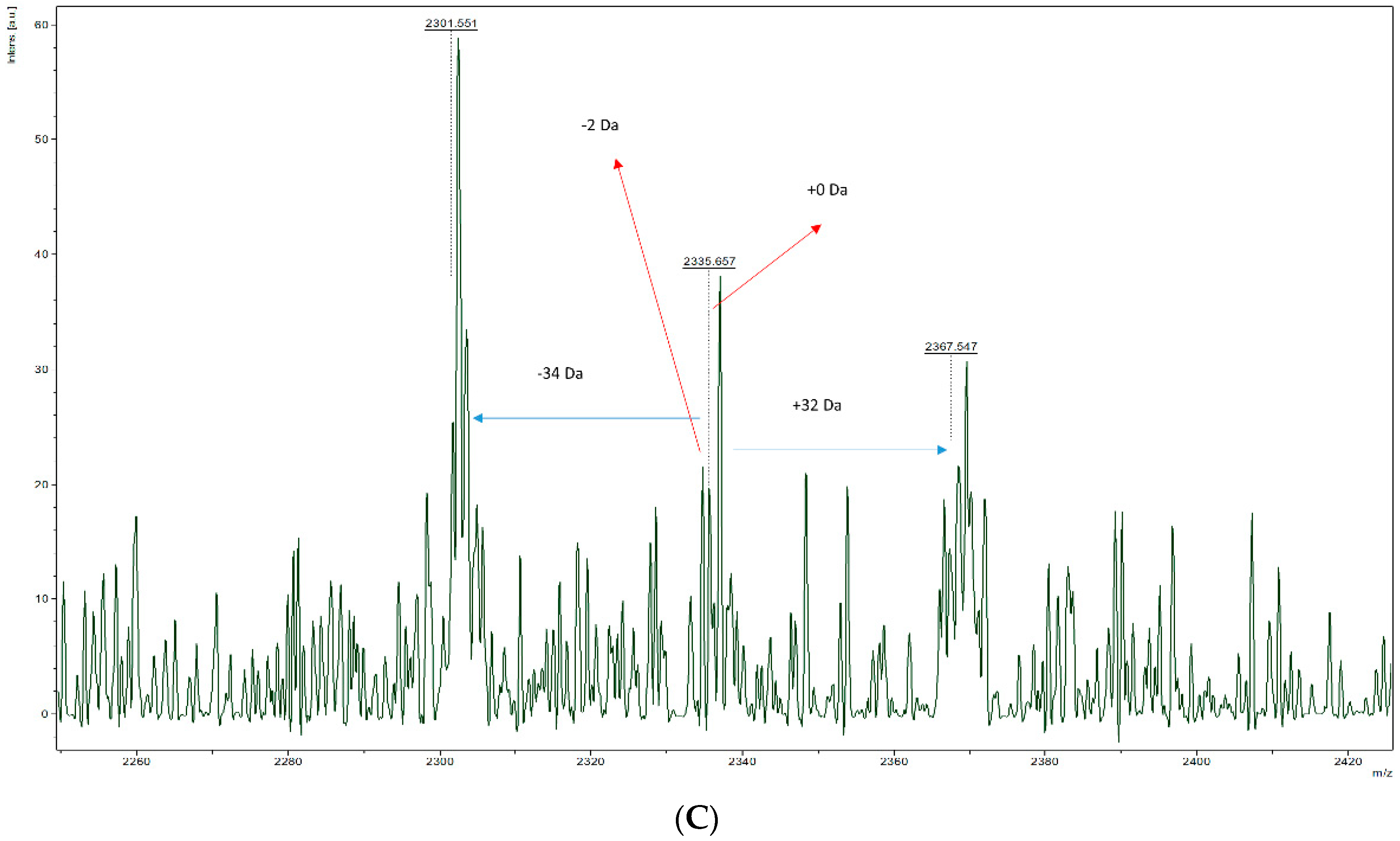
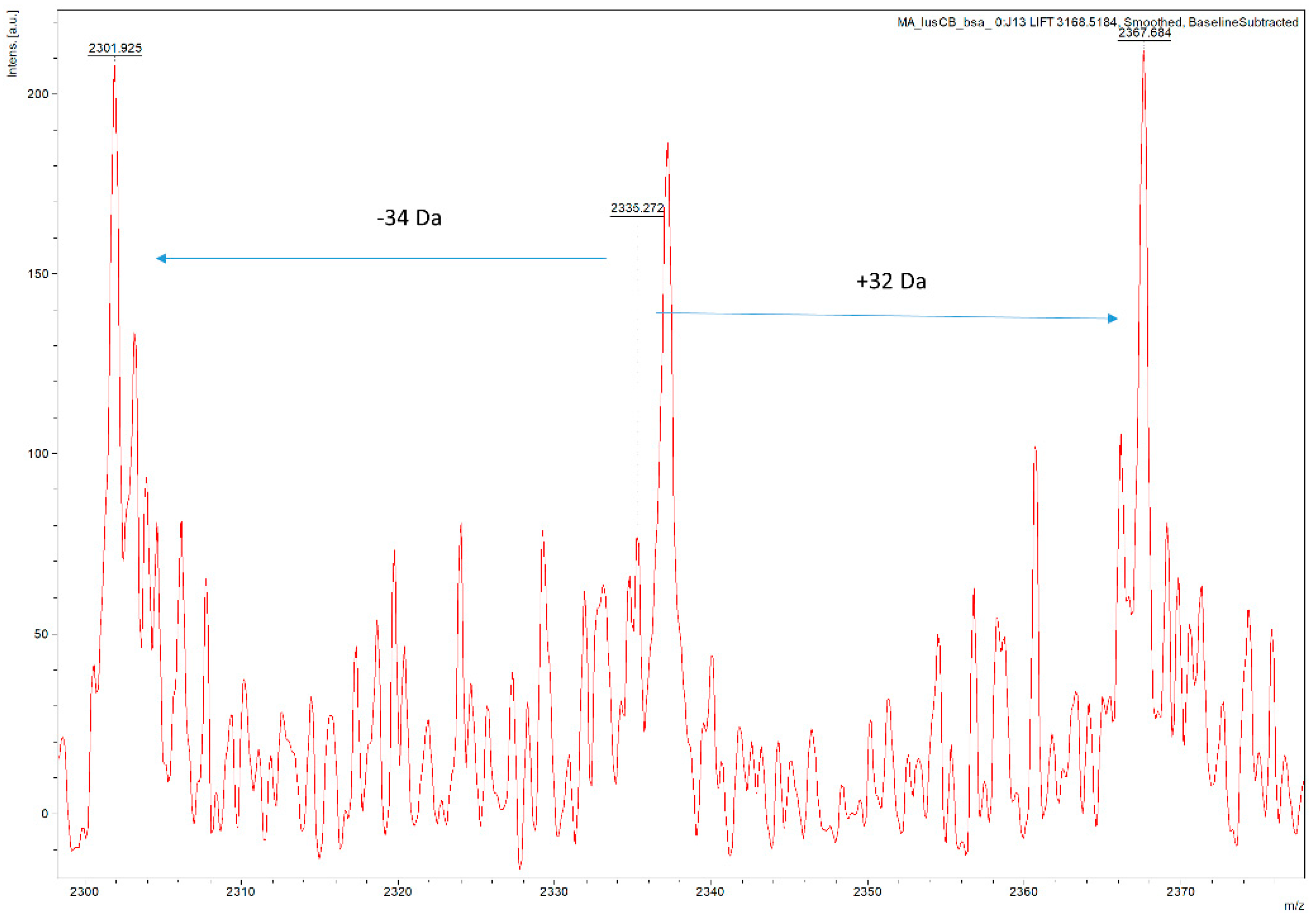
3.3. Ability to Isolate and Fragment Potential Disulphide Bonds
3.4. Optimising the HCCA Matrix:Aniline Ratio for Disulphide Bond Fragmentation
3.5. Characterisation of Inter-Peptide Disulphide Bonds
3.6. Confirmation of the Inter-Peptide Disulphide Bonds Characterisation Results in a More Complex Sample
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goyder, M.S.; Rebeaud, F.; Pfeifer, M.E.; Kalman, F. Strategies in mass spectrometry for the assignment of Cys-Cys disulfide connectivities in proteins. Expert Rev. Proteomics 2013, 10, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, J.; Resemann, A.; Evans, C.; Suckau, D.; Jabs, W. Advanced mass spectrometry workflows for analyzing disulfide bonds in biologics. Expert Rev. Proteomics 2015, 12, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Schnaible, V.; Wefing, S.; Resemann, A.; Suckau, D.; Bücker, A.; Wolf-Kümmeth, S.; Hoffmann, D. Screening for disulfide bonds in proteins by MALDI in-source decay and LIFT-TOF/TOF-MS. Anal. Chem. 2002, 74, 4980–4988. [Google Scholar] [CrossRef] [PubMed]
- Calvano, C.D.; Carulli, S.; Palmisano, F. Aniline/α-cyano-4-hydroxycinnamic acid is a highly versatile ionic liquid for matrix-assisted laser desorption/ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Snovida, S.I.; Chen, V.C.; Perreault, H. Use of a 2,5-dihydroxybenzoic acid/aniline MALDI matrix for improved detection and on-target derivatization of glycans: A preliminary report. Anal. Chem. 2006, 78, 8561–8568. [Google Scholar] [CrossRef] [PubMed]
- Asara, J.M.; Allison, J. Enhanced detection of phosphopeptides in matrix-assisted laser desorption/ionization mass spectrometry using ammonium salts. J. Am. Soc. Mass Spectrom. 1999, 10, 35–44. [Google Scholar] [CrossRef]
- Ota, M.; Ariyoshi, Y. Location of the disulfide bonds of the sweetness-suppressing polypeptide Gurmarin. Biosci. Biotechnol. Biochem. 1995, 59, 1956–1957. [Google Scholar] [CrossRef] [PubMed]
- AlMasoud, N.; Correa, E.; Trivedi, D.K.; Goodacre, R. Fractional factorial design of MALDI-TOF-MS sample preparations for the optimized detection of phospholipids and acylglycerols. Anal. Chem. 2016, 88, 6301–6308. [Google Scholar] [CrossRef] [PubMed]


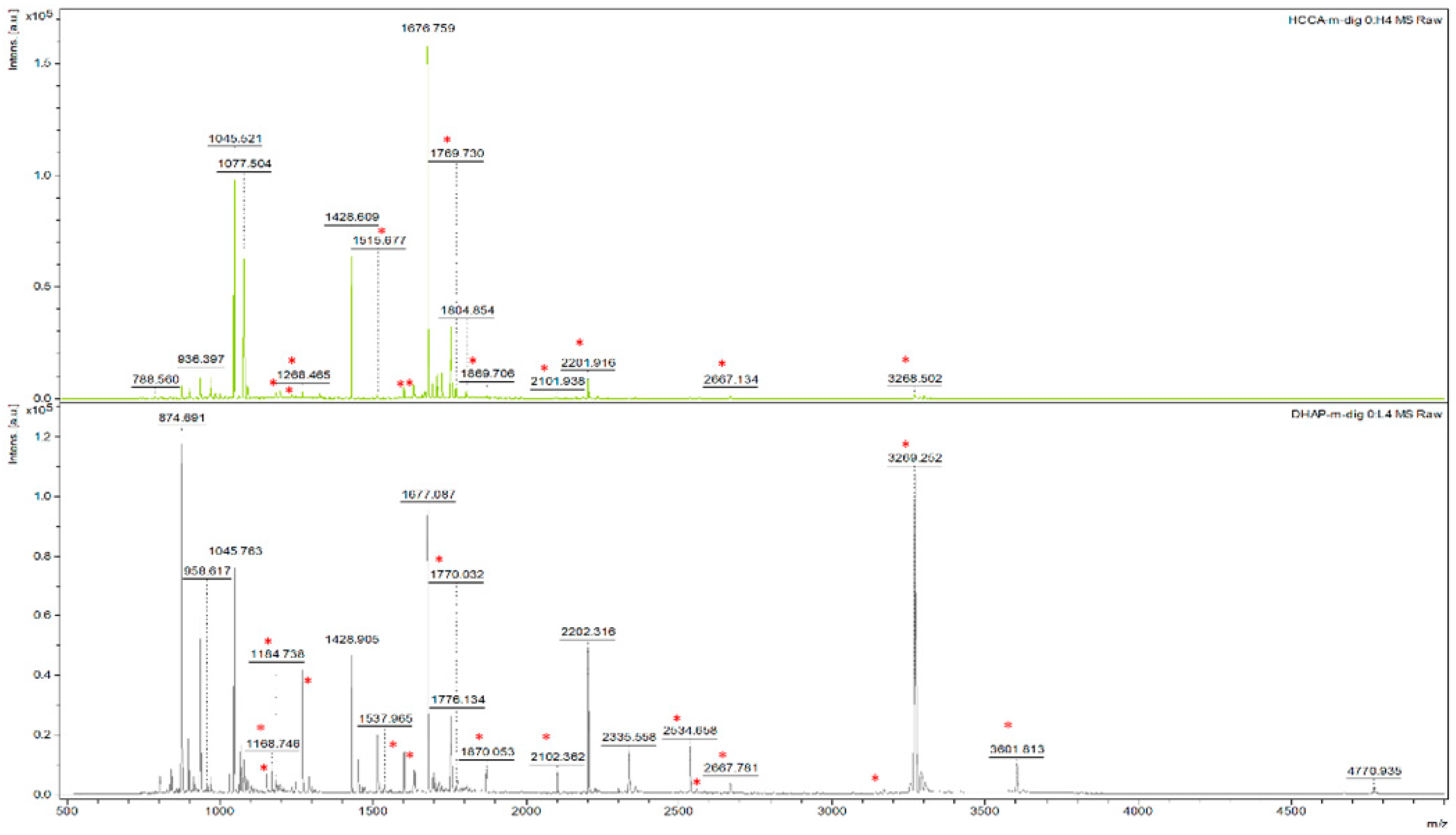

| Matrix-Additive | 757.399 | 1046.542 | 1296.685 | 1347.735 | 1619.822 | 2093.086 | 2465.198 | 3147.471 |
|---|---|---|---|---|---|---|---|---|
| HCCA-m | 3176 | 529,648 | 266,182 | 217,525 | 31,248 | 348,344 | 5511 | 156 |
| HCCA-sc | / | / | / | / | / | / | / | / |
| HCCA-aa | 1881 | 163,495 | 108,986 | 3199 | 9359 | 9359 | 688 | / |
| HCCA-ANI | / | 511,570 | 558,446 | 492,022 | 367,807 | 367,807 | 105,862 | 994 |
| DHAP-m | / | 212,573 | 207,126 | 47,283 | 87,757 | 87,757 | 7605 | 385 |
| DHAP-sc | 5094 | 61,582 | 153,401 | 28,359 | 149,998 | 149,998 | 1450 | 431 |
| DHAP-aa | / | 203,525 | 389,998 | 100,072 | 213,742 | 213,742 | 14,655 | 909 |
| DHAP-ANI | / | 45,705 | 63,629 | 15,356 | 73,780 | 73,780 | 9816 | 212 |
| 497.214 | 582.242 | 667.270 | 1083.49 | 1168.520 * | 1183.470 | 1268.498 | 1515.701 * | 1600.729 | 1669.770 | 1769.748 | 1869.726 | 2101.979 | 2201.957 | 2534.188 | 2582.201 | 2667.229 | 3168.479 | 3268.457 * | 3600.688 | 4667.188 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HCCA-m | / | / | / | / | / | x | x | x | x | / | x | x | x | x | x | / | x | / | x | / | / |
| HCCA-sc | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / |
| HCCA-aa | / | / | / | x | x | x | x | x | x | / | x | x | / | / | / | / | x | / | x | / | / |
| HCCA-ANI | / | / | / | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | / |
| DHAP-m | / | / | / | x | x | x | x | x | x | / | x | x | x | x | x | / | x | / | x | x | / |
| DHAP-sc | / | / | / | / | / | / | x | x | x | / | / | x | x | x | x | / | x | / | x | x | / |
| DHAP-aa | / | / | / | x | x | x | x | x | x | / | / | x | x | x | x | / | x | / | x | x | / |
| DHAP-ANI | / | / | / | x | x | x | x | x | x | / | / | x | x | x | x | / | x | x | x | x | / |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maes, E.; Clerens, S.; Dyer, J.M.; Deb-Choudhury, S. Improved Detection and Fragmentation of Disulphide-Linked Peptides. Methods Protoc. 2018, 1, 33. https://doi.org/10.3390/mps1030033
Maes E, Clerens S, Dyer JM, Deb-Choudhury S. Improved Detection and Fragmentation of Disulphide-Linked Peptides. Methods and Protocols. 2018; 1(3):33. https://doi.org/10.3390/mps1030033
Chicago/Turabian StyleMaes, Evelyne, Stefan Clerens, Jolon M. Dyer, and Santanu Deb-Choudhury. 2018. "Improved Detection and Fragmentation of Disulphide-Linked Peptides" Methods and Protocols 1, no. 3: 33. https://doi.org/10.3390/mps1030033