Public and Healthcare Provider Receptivity toward the Retention of Dried Blood Spot Cards and Their Usage for Extended Genetic Testing in Hong Kong
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Recruitment
2.2. Survey Measures
- (1)
- The source of information, which is evaluated by assessing various sources of knowledge, such as discussions with healthcare professionals, media, leaflets, colleagues, and online forums.
- (2)
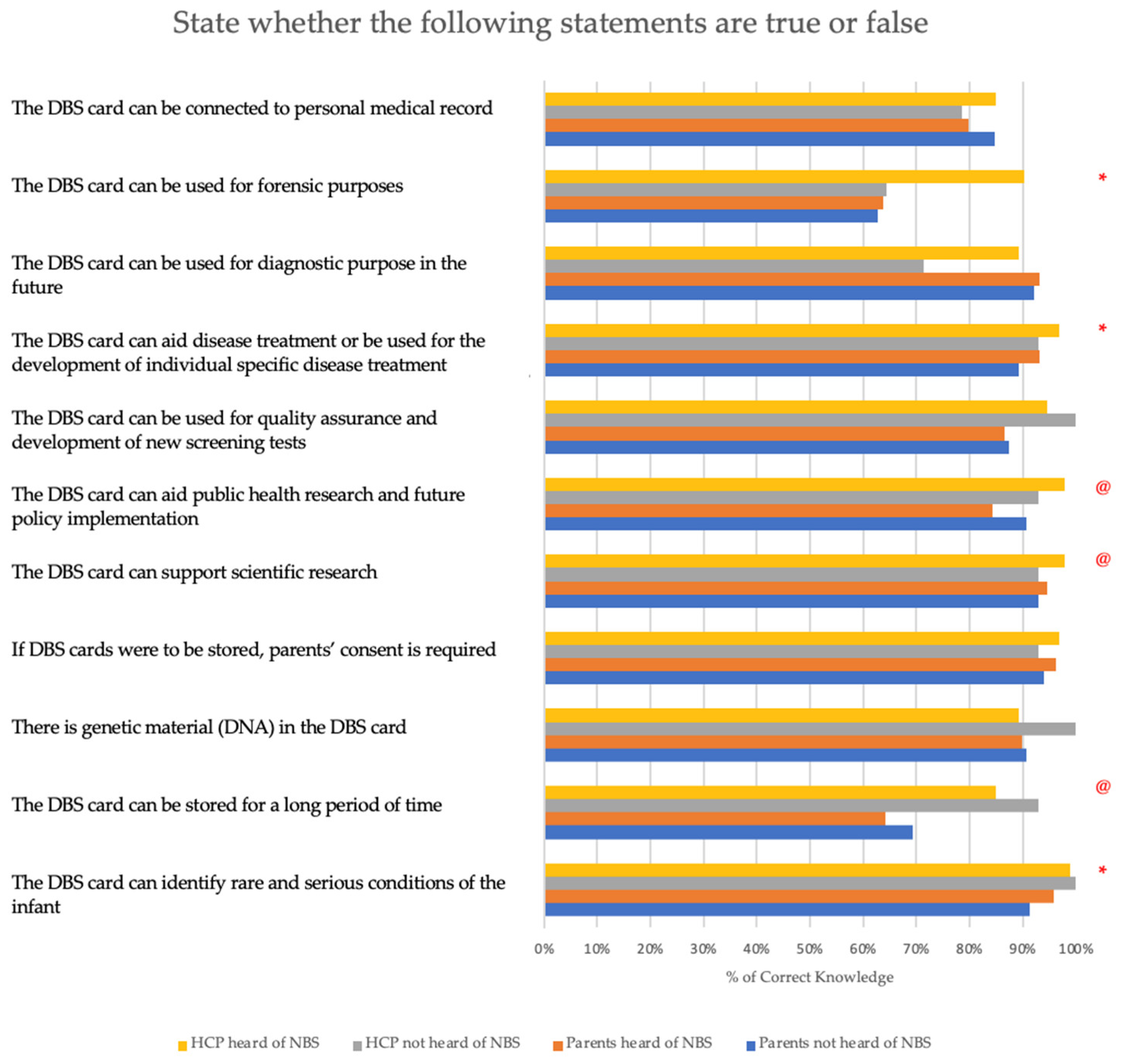
- Knowledge about the potential benefits of storing a DBS card is assessed by presenting participants with 11 true or false statements. These statements have been developed based on a thorough review of the NBS literature and position statements. A higher score indicates a better understanding of the advantages of storing a DBS card.
- (3)
- Attitudes toward DBS card storage are assessed by examining concerns relating to privacy breaches, data sharing among institutions, linking research information to medical records, lack of immediate individual benefits, and serving secondary research purposes. Each item is rated on a 5-point Likert scale, ranging from 0 (not concerned at all) to 5 (extremely concerned).
- (4)
- Practices are assessed by evaluating participants’ interest in storing their child’s sample after the NBS on a 1-to-10 Likert scale (1 indicating not supportive, and 10 indicating extremely supportive). Additionally, participants are asked how long they believe the DBS card should be stored.
2.3. Data Analysis
3. Results
3.1. Demographics Characteristics
3.2. Awareness of Newborn Screening for Uncommon Disorders
3.3. Knowledge about the Potential Benefits of Storing Dried Blood Spot Cards
3.4. Concerns and Views about Storing Dried Blood Spot Cards
3.5. Interest in Opting for Extended Genetic Testing
4. Discussion
5. Conclusions
6. Strengths and Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bombard, Y.; Miller, F.A.; Hayeems, R.Z.; Carroll, J.C.; Avard, D.; Wilson, B.J.; Little, J.; Bytautas, J.P.; Allanson, J.; Axler, R.; et al. Citizens’ values regarding research with stored samples from newborn screening in Canada. Pediatrics 2012, 129, 239–247. [Google Scholar] [PubMed] [Green Version]
- Loeber, J.G.; Platis, D.; Zetterström, R.H.; Almashanu, S.; Boemer, F.; Bonham, J.R.; Borde, P.; Brincat, I.; Cheillan, D.; Dekkers, E.; et al. Neonatal screening in Europe revisited: An ISNS perspective on the current state and developments since 2010. Int. J. Neonatal Screen. 2021, 7, 15. [Google Scholar] [CrossRef]
- Olney, R.S.; Moore, C.A.; Ojodu, J.A.; Lindegren, M.L.; Hannon, W.H. Storage and use of residual dried blood spots from state newborn screening programs. J. Pediatr. 2006, 148, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Lei, K.; Zhao, Y.; Sun, L.; Liang, H.; Luo, R.; Sun, X.; Tao, Y.; Chen, L.; Zhang, L.; Li, A.; et al. A pilot screening of high-risk Gaucher disease children using dried blood spot methods in Shandong province of China. Orphanet J. Rare Dis. 2018, 13, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reuser, A.J.; Verheijen, F.W.; Bali, D.; van Diggelen, O.P.; Germain, D.P.; Hwu, W.-L.; Lukacs, Z.; Mühl, A.; Olivova, P.; Piraud, M.; et al. The use of dried blood spot samples in the diagnosis of lysosomal storage disorders—Current status and perspectives. Mol. Genet. Metab. 2011, 104, 144–148. [Google Scholar] [PubMed]
- Chace, D.H.; DiPerna, J.C.; Mitchell, B.L.; Sgroi, B.; Hofman, L.F.; Naylor, E.W. Electrospray tandem mass spectrometry for analysis of acylcarnitines in dried postmortem blood specimens collected at autopsy from infants with unexplained cause of death. Clin. Chem. 2001, 47, 1166–1182. [Google Scholar] [CrossRef] [PubMed]
- Barbi, M.; Binda, S.; Primache, V.; Tettamanti, A.; Negri, C.; Brambilla, C. Use of Guthrie cards for the early diagnosis of neonatal herpes simplex virus disease. Pediatr. Infect. Dis. J. 1998, 17, 251–252. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, S.N.; Butala, S.J.M.; Ball, R.W.; Braniff, C.T. Pilot study for utilization of dried blood spots for screening of lead, mercury and cadmium in newborns. J. Expo. Sci. Environ. Epidemiol. 2009, 19, 298–316. [Google Scholar] [CrossRef] [PubMed]
- Tuaillon, E.; Kania, D.; Pisoni, A.; Bollore, K.; Taieb, F.; Ngoyi, E.N.O.; Schaub, R.; Plantier, J.-C.; Makinson, A.; Van De Perre, P. Dried blood spot tests for the diagnosis and therapeutic monitoring of HIV and viral hepatitis B and C. Front. Microbiol. 2020, 11, 373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mak, C.; Lam, C.W.; Law, C.Y.; Siu, W.; Kwong, L.; Chan, K.; Chow, K.; Lee, K.; Chan, W.; Chan, A. Parental attitudes on expanded newborn screening in Hong Kong. Public Health 2012, 126, 954–959. [Google Scholar] [PubMed]
- Van Teeffelen, S.R.; Douglas, C.M.W.; van El, C.G.; Weinreich, S.S.; Henneman, L.; Radstake, M.; Cornel, M.C. Mothers’ views on longer storage of neonatal dried blood spots for specific secondary uses. Public Health Genom. 2016, 19, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Tarini, B.A.; Goldenberg, A.; Singer, D.; Clark, S.; Butchart, A.; Davis, M. Not without my permission: Parents’ willingness to permit use of newborn screening samples for research. Public Health Genom. 2010, 13, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Botkin, J.R.; Rothwell, E.; Anderson, R.; Stark, L.; Goldenberg, A.; Lewis, M.; Burbank, M.; Wong, B. Public attitudes regarding the use of residual newborn screening specimens for research. Pediatrics 2012, 129, 231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothwell, E.; Anderson, R.; Goldenberg, A.; Lewis, M.H.; Stark, L.; Burbank, M.; Wong, B.; Botkin, J.R. Assessing public attitudes on the retention and use of residual newborn screening blood samples: A focus group study. Soc. Sci. Med. 2012, 74, 1305–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendrix, K.S.; Meslin, E.M.; Carroll, A.E.; Downs, S.M. Attitudes about the use of newborn dried blood spots for research: A survey of underrepresented parents. Acad. Pediatr. 2013, 13, 451–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hui, L.L.; Nelson, E.A.S.; Deng, H.B.; Leung, T.Y.; Ho, C.H.; Chong, J.S.; Fung, G.P.; Hui, J.; Lam, H.S. The view of Hong Kong parents on secondary use of dried blood spots in newborn screening program. BMC Med. Ethics 2022, 23, 105. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, E.W.; Anderson, R.A.; Burbank, M.J.; Goldenberg, A.J.; Lewis, M.H.; Stark, L.A.; Wong, B.; Botkin, J.R. Concerns of newborn blood screening advisory committee members regarding storage and use of residual newborn screening blood spots. Am. J. Public Health 2011, 101, 2111–2116. [Google Scholar] [CrossRef] [PubMed]
- Benkendorf, J.; Goodspeed, T.; Watson, M.S. Newborn screening residual dried blood spot use for newborn screening quality improvement. Genet. Med. 2010, 12, S269–S272. [Google Scholar] [CrossRef]
- Ngan, O.M.Y.; Li, C.K. Ethical Issues of Dried Blood Spot Storage and Its Secondary Use after Newborn Screening Programme in Hong Kong. Hong Kong J. Paediatr. 2020, 25, 8–15. [Google Scholar]
- Chace, D.H.; Kalas, T.A.; Naylor, E.W. Use of tandem mass spectrometry for multianalyte screening of dried blood specimens from newborns. Clin. Chem. 2003, 49, 1797–1817. [Google Scholar] [PubMed] [Green Version]
- Tluczek, A.; McKechnie, A.C.; Lynam, A. When the cystic fibrosis label does not fit: A modified uncertainty theory. Qual. Health Res. 2010, 20, 209–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pruniski, B.; Lisi, E.; Ali, N. Newborn screening for Pompe disease: Impact on families. J. Inherit. Metab. Dis. 2018, 41, 1189–1203. [Google Scholar] [CrossRef]
- Hong Kong SAR Government, Census and Statistics Department. Population Estimates. Available online: https://www.censtatd.gov.hk/en/scode459.html (accessed on 2 May 2023).
- Population and Household Statistics Analysed by District Council District 2022 Edition. Available online: https://www.censtatd.gov.hk/en/wbr.html?ecode=B11303012022AN22&scode=500 (accessed on 2 May 2023).
- How the Process Works. Available online: https://www.nsu.govt.nz/pregnancy-newborn-screening/newborn-metabolic-screening-programme-heel-prick-test/how-process-works (accessed on 2 May 2023).
- Newborn Screening Materials and Resources: Education Materials and Forms. Available online: https://www.health.state.mn.us/people/newbornscreening/materials/education.html (accessed on 2 May 2023).
- Blood Spots and Test Results: Retention Practices. Available online: https://www.health.state.mn.us/people/newbornscreening/program/retention.html (accessed on 2 May 2023).
- Newborn Bloodspot Screening. Available online: https://www.health.vic.gov.au/sites/default/files/2021-12/newborn-screening-policy-and-guidelines.pdf (accessed on 2 May 2023).
- Nørgaard-Pedersen, B.; Hougaard, D.M. Storage policies and use of the Danish Newborn Screening Biobank. J. Inherit. Metab. Dis. 2007, 30, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Newborn Blood Spot Cards Explained. Available online: https://www.nhs.uk/conditions/baby/newborn-screening/blood-spot-cards-explained/ (accessed on 2 May 2023).
- Davey, A.; French, D.; Dawkins, H.; O’Leary, P. New mothers’ awareness of newborn screening, and their attitudes to the retention and use of screening samples for research purposes. Genom. Soc. Policy 2005, 1, 41. [Google Scholar] [CrossRef] [Green Version]
- Fujii, C.; Sato, Y.; Harada, S.; Kakee, N.; Gu, Y.-H.; Kato, T.; Shintaku, H.; Owada, M.; Hirahara, F.; Umehashi, H.; et al. Attitude to extended use and long-term storage of newborn screening blood spots in Japan. Pediatr. Int. 2010, 52, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Ceyhan-Birsoy, O.; Murry, J.B.; Machini, K.; Lebo, M.S.; Yu, T.W.; Fayer, S.; Genetti, C.A.; Schwartz, T.S.; Agrawal, P.B.; Parad, R.B.; et al. Interpretation of genomic sequencing results in healthy and ill newborns: Results from the BabySeq Project. Am. J. Hum. Genet. 2019, 104, 76–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roman, T.S.; Crowley, S.B.; Roche, M.I.; Foreman, A.K.M.; O’daniel, J.M.; Seifert, B.A.; Lee, K.; Brandt, A.; Gustafson, C.; DeCristo, D.M.; et al. Genomic sequencing for newborn screening: Results of the NC NEXUS project. Am. J. Hum. Genet. 2020, 107, 596–611. [Google Scholar] [PubMed]
- Van Campen, J.C.; Sollars, E.S.A.; Thomas, R.C.; Bartlett, C.M.; Milano, A.; Parker, M.D.; Dawe, J.; Winship, P.R.; Peck, G.; Grafham, D.; et al. Next generation sequencing in newborn screening in the United Kingdom National Health Service. Int. J. Neonatal Screen. 2019, 5, 40. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Wu, D.; Zhu, L.; Wang, W.; Yang, R.; Yang, J.; He, Q.; Zhu, B.; You, Y.; Xiao, R.; et al. Application of a next-generation sequencing (NGS) panel in newborn screening efficiently identifies inborn disorders of neonates. Orphanet J. Rare Dis. 2022, 17, 66. [Google Scholar] [CrossRef]
- Veldman, A.; Kiewiet, M.B.G.; Heiner-Fokkema, M.R.; Nelen, M.R.; Sinke, R.J.; Sikkema-Raddatz, B.; Voorhoeve, E.; Westra, D.; Dollé, M.E.T.; Schielen, P.C.J.I.; et al. Towards next-generation sequencing (NGS)-based newborn screening: A technical study to prepare for the challenges ahead. Int. J. Neonatal Screen. 2022, 8, 17. [Google Scholar] [CrossRef]
- Kingsmore, S.F.; Smith, L.D.; Kunard, C.M.; Bainbridge, M.; Batalov, S.; Benson, W.; Blincow, E.; Caylor, S.; Chambers, C.; Del Angel, G.; et al. A genome sequencing system for universal newborn screening, diagnosis, and precision medicine for severe genetic diseases. Am. J. Hum. Genet. 2022, 109, 1605–1619. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, A.J.; Dodson, D.S.; Davis, M.M.; Tarini, B.A. Parents’ interest in whole-genome sequencing of newborns. Genet. Med. 2014, 16, 78–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, M.A.; Stine, A.; Paquin, R.S.; Mansfield, C.; Wood, D.; Rini, C.; Roche, M.I.; Powell, C.M.; Berg, J.S.; Bailey, D.B. Parental preferences toward genomic sequencing for non-medically actionable conditions in children: A discrete-choice experiment. Genet. Med. 2018, 20, 181–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| All | Healthcare Providers | Parents | Chi/t | p-Value | |
|---|---|---|---|---|---|
| (n = 559) | (n = 107) | (n = 452) | |||
| n (%) | n (%) | n (%) | |||
| Age | |||||
| mean (SD) | 36.8 (6.57) | 39.39 (10.33) | 36.17 (5.18) | 3.06 | <0.001 |
| Gender | ns | ns | |||
| Female | 412 (73.7%) | 76 (71%) | 336 (74.3%) | ||
| Male | 147 (26.3%) | 31 (29%) | 116 (25.7%) | ||
| Ethnicity | 15.6 | <0.001 | |||
| Hong Kong Chinese | 500 (89.5%) | 107 (100%) | 393 (86.9%) | ||
| Mainland Chinese | 32 (5.7%) | - | 32 (7.1%) | ||
| Other | 27 (4.8%) | - | 27 (6%) | ||
| Religion | 31.25 | <0.001 | |||
| None | 370 (66.2%) | 52 (48.6%) | 318 (70.4%) | ||
| Christian | 111 (19.8%) | 39 (36.4%) | 72 (15.9%) | ||
| Catholic | 39 (7.1%) | 12 (11.2%) | 27 (6%) | ||
| Buddhist | 26 (4.6%) | 4 (3.8%) | 22 (4.8%) | ||
| Other | 13 (2.3%) | - | 13 (2.9%) | ||
| Do you work in a health-care related field? | |||||
| No | - | - | 409 (90.5%) | - | - |
| Yes | - | - | 43 (9.5%) | - | - |
| Profession | |||||
| Doctor | - | 34 (31.8%) | - | - | - |
| Nurse | - | 36 (33.6%) | - | - | - |
| Laboratory Technician | - | 5 (4.7%) | - | - | - |
| Others | - | 32 (29.9%) | - | - | - |
| Years of Experience in NBS | - | - | |||
| Mean (SD) | - | 5.49 (7.35) | - | ||
| Highest Education | 118.7 | <0.001 | |||
| Upper Secondary or lower | 174 (31.1%) | 2 (1.9%) | 172 (38.0%) | ||
| Post-Secondary | 78 (14.0%) | 3 (2.8%) | 75 (16.6%) | ||
| Tertiary | 197 (35.2%) | 47 (43.9%) | 150 (33.2%) | ||
| Master or above | 110 (19.7%) | 55 (51.4%) | 55 (12.2%) | ||
| Family Income | 89.55 | <0.001 | |||
| Below HKD 19,999 | 93 (16.7%) | 3 (2.8%) | 90 (19.9%) | ||
| HKD 20,000–29,999 | 81 (14.5%) | 1 (0.9%) | 80 (17.7%) | ||
| HKD 30,000–39,999 | 89 (15.9%) | 6 (5.6%) | 83 (18.4%) | ||
| HKD 40,000–49,999 | 37 (6.6%) | 4 (3.7%) | 33 (7.3%) | ||
| Above HKD 50,000 | 259 (46.3%) | 93 (87.0%) | 166 (36.7%) | ||
| Consanguinity | |||||
| No | - | - | 424 (93.8%) | - | - |
| Yes | - | - | 24 (5.3%) | - | - |
| Not Sure | - | - | 4 (0.9%) | - | - |
| Kid Number | |||||
| 1 | - | - | 198 (43.8%) | - | - |
| 2 | - | - | 202 (44.7%) | - | - |
| 3 or above | - | - | 52 (11.5%) | - | - |
| Diagnosed Disease | |||||
| None | - | - | 291 (64.4%) | - | - |
| Cardiac Diseases | - | - | 40 (8.8%) | - | - |
| Haematology | - | - | 21 (4.7%) | - | - |
| Respiratory | - | - | 16 (3.5%) | - | - |
| Neonatology | - | - | 15 (3.3%) | - | - |
| Endocrine Diseases | - | - | 14 (3.1%) | - | - |
| Neurology | - | - | 9 (2.0%) | - | - |
| Metabolic Diseases | - | - | 8 (1.8%) | - | - |
| Others | - | - | 38 (8.4%) | - | - |
| All | Healthcare Providers | Parents | Chi/t | p-Value | |
|---|---|---|---|---|---|
| (n = 559) | (n = 107) | (n = 452) | |||
| n (%) | n (%) | n (%) | |||
| How long do you think DBS should be stored? | |||||
| Up to 6 months | 155 (27.7%) | 29 (27.2%) | 126 (27.9%) | ns | ns |
| Up to 2 years | 118 (21.1%) | 23 (21.5%) | 95 (21%) | ns | ns |
| Up to 5 years | 89 (15.9%) | 24 (22.4%) | 65 (14.4%) | ns | ns |
| Up to 18 years | 100 (17.9%) | 21 (19.6%) | 79 (17.5%) | ns | ns |
| Indefinitely | 97 (17.4%) | 10 (9.3%) | 87 (19.2%) | ns | ns |
| Do you support DBS storage? (1 as not interested at all; 10 as extremely interested) | |||||
| mean (SD) | 8.15 (1.66) | 8.26 (1.70) | 8.13 (1.65) | ns | ns |
| All | Healthcare Providers | Parents | Chi | p-Value | |
|---|---|---|---|---|---|
| (n = 559) | (n = 107) | (n = 452) | |||
| n (%) | n (%) | n (%) | |||
| If you were the parent, would you wish to receive the following information with regard to your child/If you were a healthcare provider, would you wish to disclose the following information to parents? | |||||
| A childhood-onset disorder is a condition with treatment | 492 (88.0%) | 97 (90.7%) | 395 (87.4%) | ns | ns |
| A condition list on the current medical recommended screening panel | 481 (86.0%) | 94 (87.9%) | 387 (85.6%) | ns | ns |
| Increase risk for an adult-onset disease | 467 (83.5%) | 83 (77.6%) | 384 (85.0%) | ns | ns |
| The newborn is a carrier for the condition but will not develop it | 470 (84.1%) | 93 (86.9%) | 377 (83.4%) | ns | ns |
| An adulthood-onset disorder is a condition with treatment | 466 (83.4%) | 94 (87.9%) | 372 (82.3%) | ns | ns |
| A childhood-onset disorder is a condition with NO available treatment | 456 (81.6%) | 91 (85.0%) | 365 (80.8%) | ns | ns |
| A variant that has unknown clinical implications | 413 (73.9%) | 61 (57.0%) | 352 (77.9%) | 19.5 | <0.001 |
| An adulthood-onset disorder is a condition with NO available treatment | 421 (75.3%) | 81 (75.7%) | 340 (75.2%) | ns | ns |
| What is the factor(s) impacting your interest in extended genetic testing? (1 is not important at all; 5 is extremely important) | |||||
| mean (SD) | mean (SD) | mean (SD) | t | p-value | |
| Shorten the diagnosis time in future | 3.69 (1.17) | 4.20 (0.884) | 3.57 (1.19) | 6.11 | <0.001 |
| Identify new disease genes and diagnose individuals with rare disorders | 3.66 (1.16) | 4.14 (0.976) | 3.54 (1.17) | 5.45 | <0.001 |
| Access to existing treatment for affected individuals | 3.68 (1.15) | 4.31 (0.862) | 3.53 (1.16) | 7.84 | <0.001 |
| Accuracy of the test results/sequencing results | 3.67 (1.19) | 4.32 (0.938) | 3.52 (1.20) | 7.52 | <0.001 |
| Access to specialist follow-up for affected individuals | 3.64 (1.15) | 4.18 (0.92) | 3.51 (1.17) | 6.38 | <0.001 |
| Impinge on the child’s right to an open future | 3.58 (1.19) | 4.06 (0.96) | 3.47 (1.21) | 5.37 | <0.001 |
| Diagnosing susceptibility to adult-onset disease during the newborn period | 3.35 (1.18) | 3.45 (1.27) | 3.32 (1.16) | ns | ns |
| How interested would you be in obtaining your child’s extended genetic testing? (1 as not interested at all; 10 as extremely interested) | |||||
| mean (SD) | 7.83 (1.85) | 7.64 (1.87) | 7.87 (1.85) | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belaramani, K.M.; Fung, C.W.; Kwok, A.M.K.; Lee, S.Y.R.; Yau, E.K.C.; Luk, H.M.; Mak, C.M.; Yeung, M.C.W.; Ngan, O.M.Y. Public and Healthcare Provider Receptivity toward the Retention of Dried Blood Spot Cards and Their Usage for Extended Genetic Testing in Hong Kong. Int. J. Neonatal Screen. 2023, 9, 45. https://doi.org/10.3390/ijns9030045
Belaramani KM, Fung CW, Kwok AMK, Lee SYR, Yau EKC, Luk HM, Mak CM, Yeung MCW, Ngan OMY. Public and Healthcare Provider Receptivity toward the Retention of Dried Blood Spot Cards and Their Usage for Extended Genetic Testing in Hong Kong. International Journal of Neonatal Screening. 2023; 9(3):45. https://doi.org/10.3390/ijns9030045
Chicago/Turabian StyleBelaramani, Kiran Moti, Cheuk Wing Fung, Anne Mei Kwun Kwok, Shing Yan Robert Lee, Eric Kin Cheong Yau, Ho Ming Luk, Chloe Miu Mak, Matthew Chun Wing Yeung, and Olivia Miu Yung Ngan. 2023. "Public and Healthcare Provider Receptivity toward the Retention of Dried Blood Spot Cards and Their Usage for Extended Genetic Testing in Hong Kong" International Journal of Neonatal Screening 9, no. 3: 45. https://doi.org/10.3390/ijns9030045
APA StyleBelaramani, K. M., Fung, C. W., Kwok, A. M. K., Lee, S. Y. R., Yau, E. K. C., Luk, H. M., Mak, C. M., Yeung, M. C. W., & Ngan, O. M. Y. (2023). Public and Healthcare Provider Receptivity toward the Retention of Dried Blood Spot Cards and Their Usage for Extended Genetic Testing in Hong Kong. International Journal of Neonatal Screening, 9(3), 45. https://doi.org/10.3390/ijns9030045







