A Rapid Screening Method for the Measurement of Neonatal Total Homocysteine in Dried Blood Spots by Liquid Chromatography-Tandem Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Instrumentation
2.3. Dried Blood Spots
2.4. Sample Extraction and Preparation
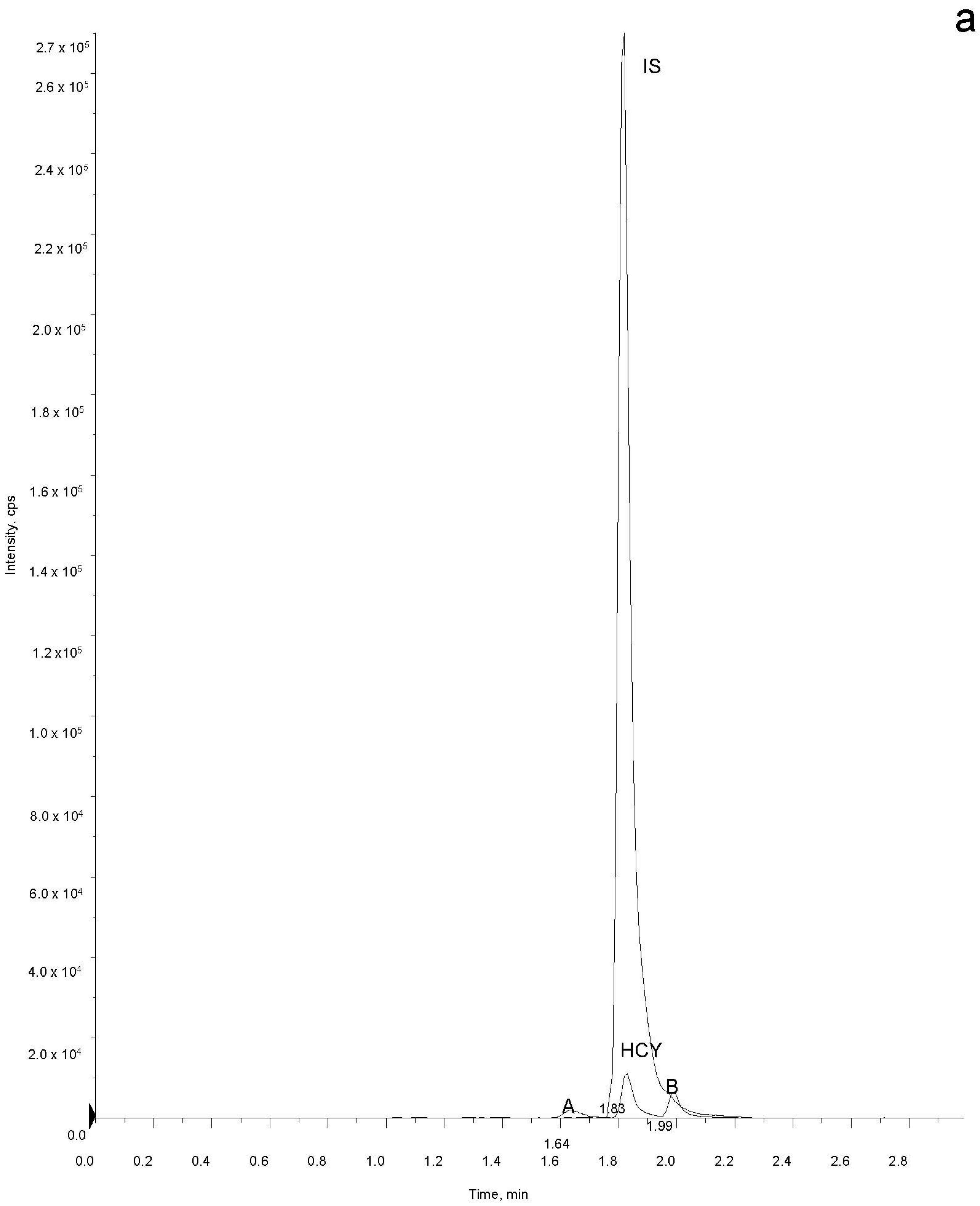
2.5. Sample Analysis
3. Results
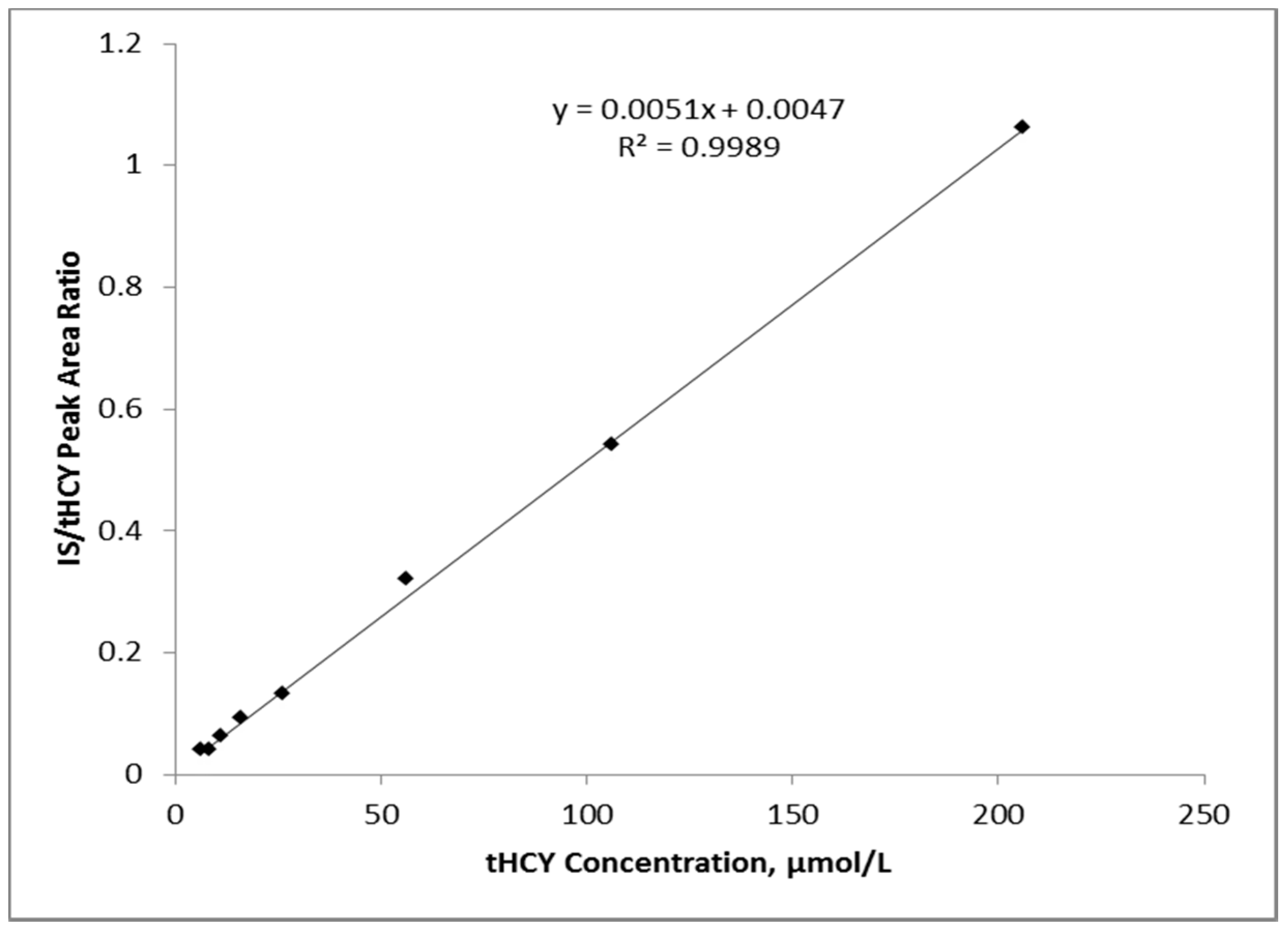
3.1. Linearity and Imprecision
3.2. Sensitivity and Recovery
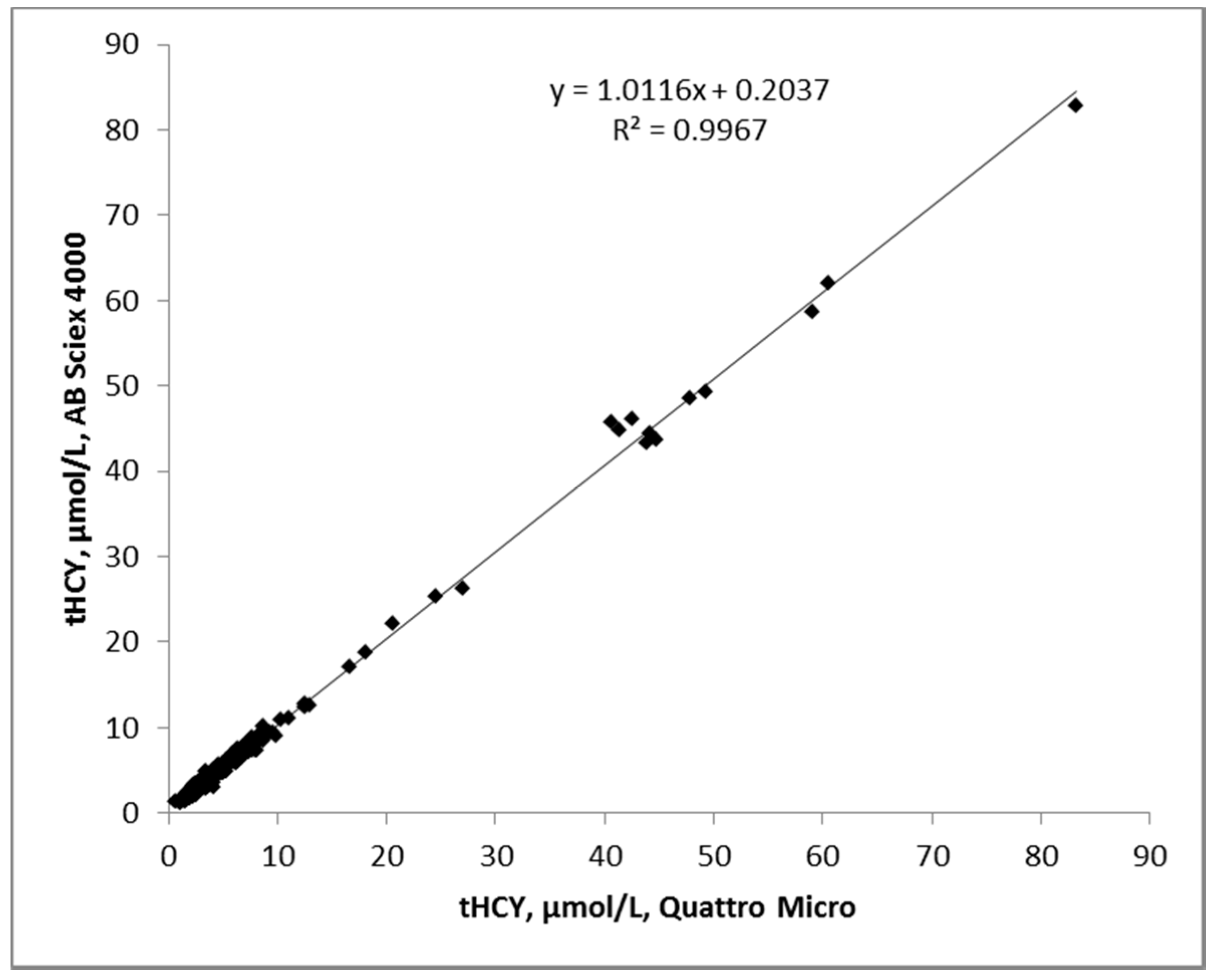
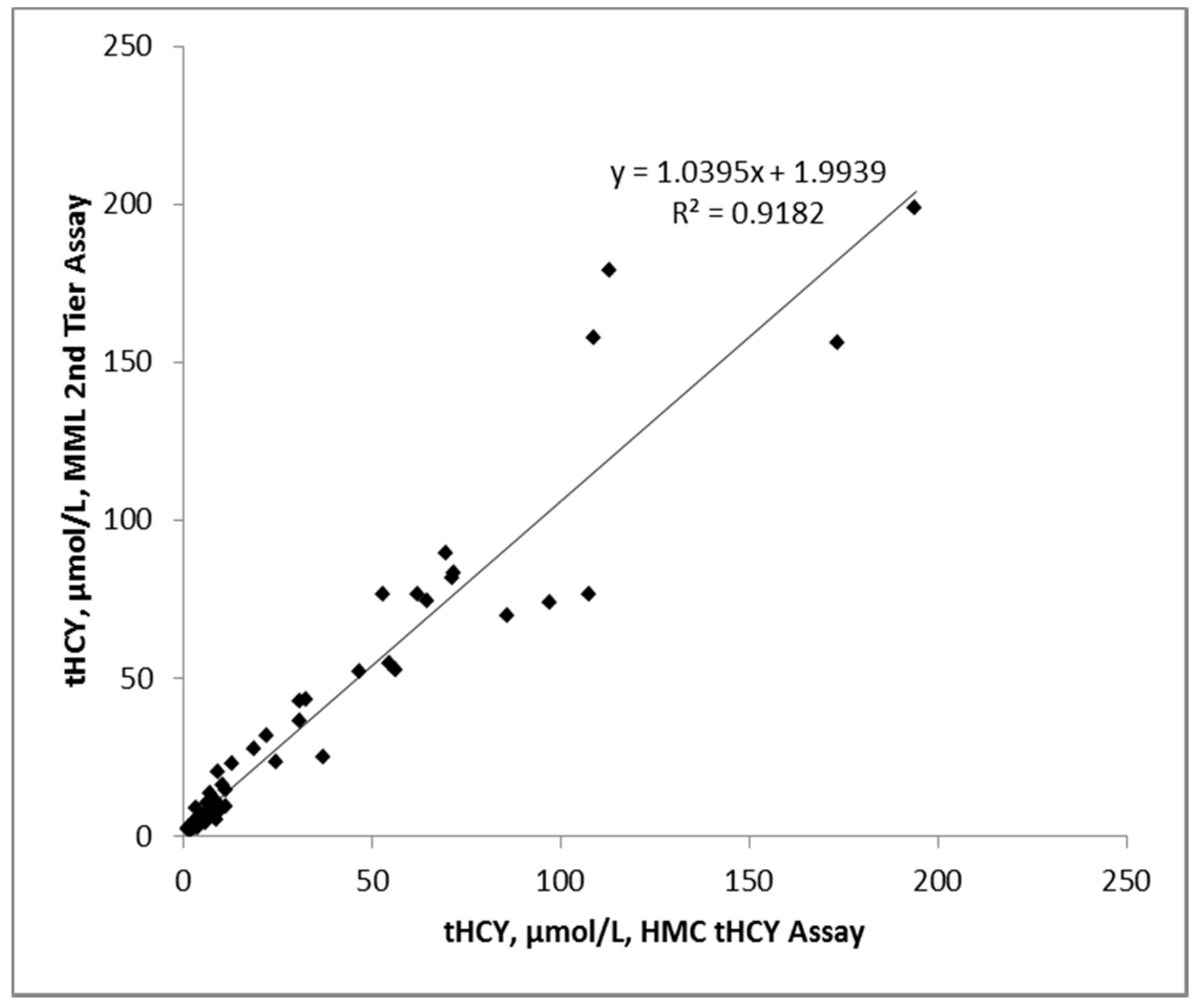
3.3. Accuracy
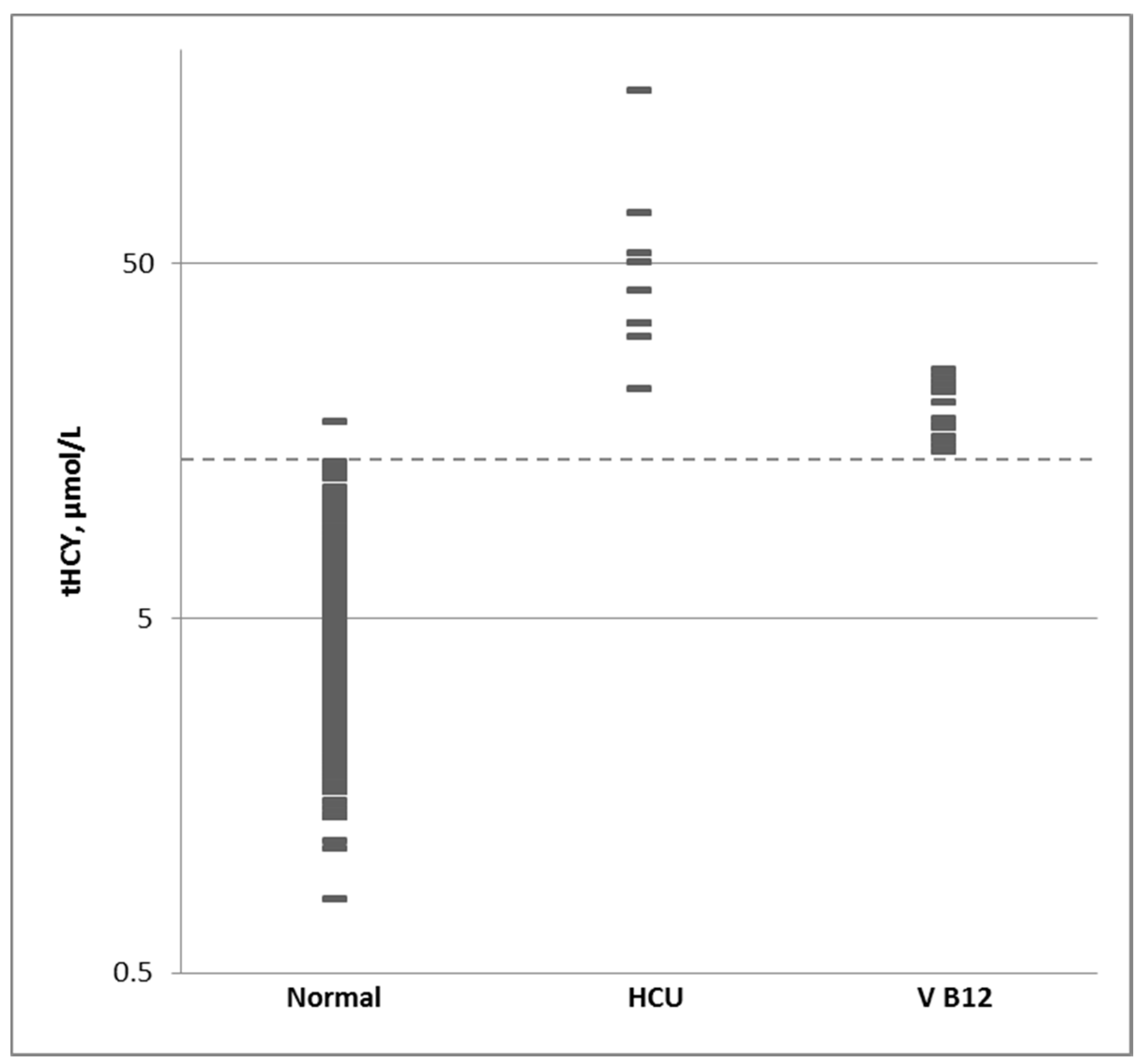
3.4. Normal Population and Cutoff Level
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sacharow, S.J.; Picker, J.D.; Levy, H.L. Homocystinuria Caused by Cystathionine Beta-Synthase Deficiency. In GeneReviews®; University of Washington: Seattle, WA, USA, 1993–2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1524/ (accessed on 10 January 2017).
- Gaustadnes, M.; Ingerslev, J.; Rutiger, N. Prevalence of congenital homocystinuria in Denmark. N. Engl. J. Med. 1999, 340, 1513. [Google Scholar] [CrossRef] [PubMed]
- Refsum, H.; Fredriksen, A.; Meyer, K.; Ueland, P.M.; Kase, B.F. Birth prevalence of homocystinuria. J. Pediatr. 2004, 144, 830–832. [Google Scholar] [PubMed]
- Janosik, M.; Sokolova, J.; Janosikova, B.; Krijt, J.; Klatovska, V.; Kozich, V. Birth prevalence of homocystinuria in Central Europe: Frequency and patogenicity of mutation c.1105 > T (p.R369C) in the cystathionine beta-synthase gene. J. Pediatr. 2009, 154, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Gan-Schreier, H.; Kebbewar, M.; Fang-Hoffmann, J.; Wilrich, J.; Abdoh, G.; Ben-Omran, T.; Shahbek, N.; Bener, A.; Al Rifai, H.; Al Khal, A.L.; et al. Newborn population screening for classic homocystinuria by determination of total homocysteine from Guthrie cards. J. Pediatr. 2010, 156, 427–432. [Google Scholar] [CrossRef] [PubMed]
- El-Said, M.F.; Badii, R.; Bessisso, M.S.; Shahbek, N.; El-Ali, M.G.; El-Marikhie, M.; El-Zyoid, M.; Salem, M.S.Z.; Bener, A.; Hoffmann, G.F.; et al. A common mutation in the CBS gene explains a high incidence of homocystinuria in the Qatari population. Hum. Mutat. 2006, 27, 719. [Google Scholar] [CrossRef] [PubMed]
- Okum, J.G.; Gan-Schreier, H.; Ben-Omran, T.; Schmidt, K.V.; Fang-Hoffmann, J.; Gamer, G.; Abdoh, G.; Shahbeck, N.; Al Rifai, H.; Al Khal, A.L.; et al. Newborn Screening for Vitamin B6 Non-responsive Clinical Homocytinuria: Systematical Evaluation of a Two-Tier Strategy. JIMD Rep. 2017, 32, 87–94. [Google Scholar]
- Skovby, F.; Gaustadnes, M.; Mudd, S.H. A revisit to the natural history of homocystinuria due to cyctathionine beta-synthase deficiency. Mol. Genet. Metab. 2010, 99, 1–3. [Google Scholar] [CrossRef] [PubMed]
- The Human Gene mutaton Database. Available online: http://www.hgmd.cf.ac.uk/ac/gene.php?gene=CBS (accessed on 22 October 2017).
- Adam, S.; Almeida, S.F.; Weber, E.C. Dietary practices in pyridoxine non- responsive homocystinuria: A European survey. Mol. Genet. Metab. 2013, 110, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.H.; Jahnke, N.; Remmington, T. Newborn screening for homocystinuria. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef]
- Yap, S.; Boers, G.H.; Wilcken, B.; Wilcken, D.E.L.; Brenton, D.P.; Lee, P.J.; Walter, J.H.; Howard, P.M.; Naughten, E.R. Vascular outcome in patients with homocystinuria due to cystathionine beta-synthase deficiency treated chronically: A multicentre observational study. Arter. Thromb. Vasc. Biol. 2001, 21, 2080–2085. [Google Scholar] [CrossRef]
- Huemer, M.; Kozich, V.; Rinaldo, P.; Baumgartner, R.; Merinero, B.; Pasquini, E.; Ribes, A.; Blom, H. Newborn screening for homocystinurias and methylation disorders: Systematic review and proposed guidelines. J. Inherit. Metab. Dis. 2015, 38, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Stabler, S.P.; Korson, M.; Jethva, R.; Allen, R.H.; Kraus, J.P.; Spector, E.B.; Wagner, C.; Mudd, H.S. Metabolic profiling of total homocysteine and related compounds in hyperhomocysteinemia: Utility and limitations in diagnosing the cause of puzzling thrombophilia in a family. JIMD Rep. 2013, 11, 149–163. [Google Scholar] [PubMed]
- Bartl, J.; Chrastina, P.; Krijt, J.; Hodik, J.; Peskova, K.; Kozich, V. Simultaneous determination of cystathionine, total homocysteine and methionine in dried blood spots by liquid chromatography/tandem mass spectrometry and its utility for the management of patients with homocystinuria. Clin. Chim. Acta 2014, 437, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Mudd, S.H. Hypermethioninemias of genetic and non-genetic origin: A review. Am. J. Med. Genet. Part C Semin. Med. Genet. 2011, 157C, 3–32. [Google Scholar] [CrossRef] [PubMed]
- Bowron, A.; Barton, A.; Scott, J.; Stansbie, D. Blood spot homocysteine: A feasibility and stability study. Clin. Chem. 2005, 51, 257–258. [Google Scholar] [CrossRef] [PubMed]
- Turgeon, C.T.; Magera, M.J.; Cuthbert, C.D.; Loken, P.R.; Gavrilov, D.K.; Tortorelli, S.; Raymond, K.M.; Oglesbee, D.; Rinaldo, P.; Matern, D. Determination of total homocysteine, methylmalonic acid, and 2-methylcitric acid in dried blood spots by tandem mass spectrometry. Clin. Chem. 2010, 56, 1686–1695. [Google Scholar] [CrossRef] [PubMed]
- Matern, D.; Tortorelli, S.; Oglesbee, D.; Gavrilov, D.; Rinaldo, P. Reduction of the false positive rate in newborn screening by implementation of MS/MS-based second tier tests: The Mayo Clinic experience (2004–2007). J. Inherit. Metab. Dis. 2007, 30, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Oglesbee, D.; Sanders, K.A.; Lacey, J.M.; Magera, M.J.; Casetta, B.; Strauss, K.A.; Tortorelli, S.; Rinaldo, P.; Matern, D. Second tier test for quantification of alloisoleucine and branched-chain amino acids in dried blood spots to improve newborn screening for maple syrup urine disease (MSUD). Clin. Chem. 2008, 54, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Janzen, N.; Steuerwald, U.; Sander, S.; Terhardt, M.; Peter, M.; Sander, J. A UPLC-MS/MS analysis of C5-acylcarnitines in dried blood spots. Clin. Chim. Acta 2013, 421, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Zschocke, J.; Kebbewar, M.; Gan-Schreier, H.; Fischer, C.; Fang-Hoffmann, J.; Wilrich, J.; Abdoh, G.; Ben-Omran, T.; Shahbek, N.; Linder, M.; et al. Molecular neonatal screening for homocystinuria in the Qatari population. Hum. Mutat. 2009, 30, 1021–1022. [Google Scholar] [CrossRef] [PubMed]
- Chace, D.H.; Hillman, S.L.; Millington, D.S.; Kahler, S.G.; Adam, B.W.; Levy, H.L. Rapid diagnosis of homocystinuria and other hypermethioninemias from newborns’ blood spots by tandem mass spectrometry. Clin. Chem. 1996, 42, 349–3522. [Google Scholar] [PubMed]
- Ten Hoedt, A.E.; van Kempen, A.A.; Boelen, A.; Duran, M.; Kemper-Proper, E.A.; Oey-Spauwen, M.J.; Wijburg, F.A.; Bosch, A.M. High incidence of hypermethioninaemeia in a single neonatal intensive care unit detected by a newly introduced neonatal screening programme. J. Inherit. Metab. Dis. 2007, 30, 978. [Google Scholar] [CrossRef] [PubMed]
- Naughten, E.R.; Yapp, S.; Mayne, P.D. Newborn screening for homocystinuria: Irish and world experience. Eur. J. Pediatr. 1998, 157 (Suppl. S2), S84–S97. [Google Scholar] [CrossRef] [PubMed]
- Peterschmitt, M.J.; Simmons, J.R.; Levy, H.L. Reduction of false negative results in screening of newborns for homocystinuria. N. Engl. J. Med. 1999, 341, 1572–1576. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.M.S.; Cameron, C.A.; Abdenur, J.E.; Abdulrahman, M.; Adair, O.; Al Nuaimi, S.A.; Åhlman, H.; Allen, J.J.; Antonozzi, I.; Archer, S.; et al. Clinical validation of cutoff target ranges in newborn screening of metabolic disorders by tandem mass spectrometry: A worldwide collaborative project. Genet. Med. 2011, 13, 230–254. [Google Scholar] [CrossRef] [PubMed]
- Alodaib, A.N.; Carpenter, K.; Wley, V.; Wotton, T.; Chistodoulou, J.; Wilcken, B. Homocysteine measurement n dried blood spot for neonatal detection of homocystinurias. JIMD Rep. 2011, 5, 1–6. [Google Scholar] [PubMed]
- Gempel, K.; Gerbitz, K.-D.; Casetta, B.; Bauer, M.F. Rapid determination of total homocysteine in blood spots by liquid chromatography-electrospray ionisation-tandem mass spectrometry. Clin. Chem. 2000, 46, 122–123. [Google Scholar] [PubMed]
- Minet, J.C.; Bisse, E.; Aebischer, C.P.; Beil, A.; Wieland, H.; Lutschg, J. Assessment of vitamin B12, folate and vitamin B-6 status and relation to sulfur amino acid metabolism in neonates. Am. J. Clin. Nutr. 2000, 72, 751–757. [Google Scholar] [PubMed]
- Monsen, A.B.; Ueland, P.M.; Vollset, S.E.; Guttormsen, A.B.; Markestad, T.; Solheim, E.; Refsum, H. Determinants of cobalamin status in newborns. Pediatrics 2001, 108, 624–630. [Google Scholar] [CrossRef]
- Wong, D.; Tortorelli, S.; Bishop, L.; Sellars, E.A.; Schimmenti, L.A.; Gallant, N.; Prada, C.E.; Hopkin, R.J.; Leslie, N.D.; Berry, S.A.; et al. Outcomes of four patients with homocysteine remethylation disorders detected by newborn screening. Genet. Med. 2016, 18, 162–167. [Google Scholar] [CrossRef] [PubMed]
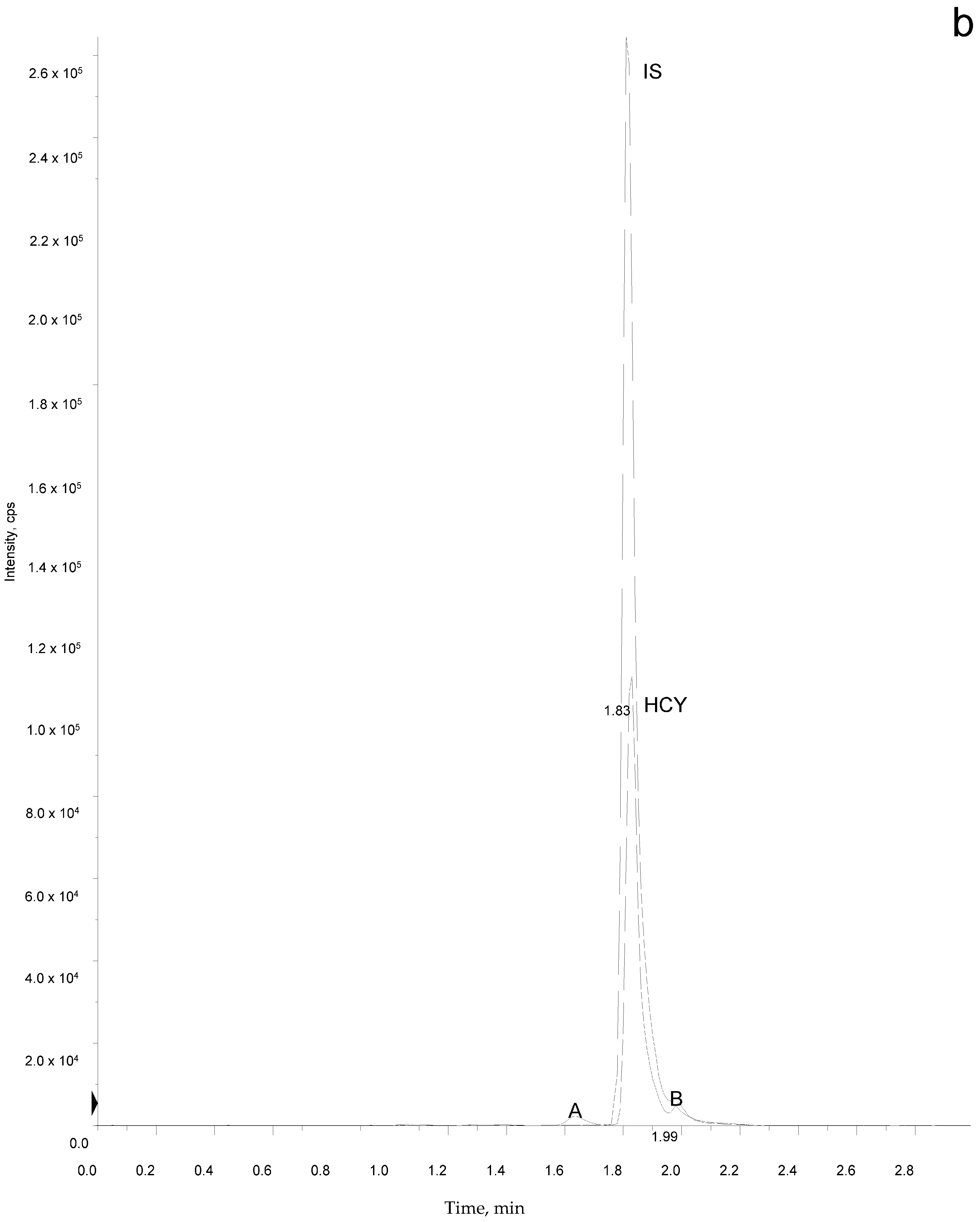
| Concentration | AB Sciex API 4000 | Waters Quattro Micro | ||
|---|---|---|---|---|
| Intra-Assay | Inter-Assay | Intra-Assay | Inter-Assay | |
| 10 µmol/L | 6.6 | 7.2 | 7.8 | 7.9 |
| 45 µmol/L | 7.5 | 6.5 | 6.9 | 6.4 |
| Percentile | 1% | 10% | 50% | 90% | 99% |
|---|---|---|---|---|---|
| Normal | 1.7 | 2.7 | 4.4 | 7.4 | 11.5 |
| HCU | 22.9 | 29.3 | 41.9 | 85.8 | 146.1 |
| B12 Def | 14.8 | 15.5 | 18.3 | 23.8 | 25 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maase, R.; Skrinska, V.; Younes, N.; Hassan, L.; Mitri, R.; Matern, D.; Rinaldo, P.; Turgeon, C. A Rapid Screening Method for the Measurement of Neonatal Total Homocysteine in Dried Blood Spots by Liquid Chromatography-Tandem Mass Spectrometry. Int. J. Neonatal Screen. 2017, 3, 32. https://doi.org/10.3390/ijns3040032
Maase R, Skrinska V, Younes N, Hassan L, Mitri R, Matern D, Rinaldo P, Turgeon C. A Rapid Screening Method for the Measurement of Neonatal Total Homocysteine in Dried Blood Spots by Liquid Chromatography-Tandem Mass Spectrometry. International Journal of Neonatal Screening. 2017; 3(4):32. https://doi.org/10.3390/ijns3040032
Chicago/Turabian StyleMaase, Rose, Victor Skrinska, Noura Younes, Lubna Hassan, Rola Mitri, Dietrich Matern, Piero Rinaldo, and Coleman Turgeon. 2017. "A Rapid Screening Method for the Measurement of Neonatal Total Homocysteine in Dried Blood Spots by Liquid Chromatography-Tandem Mass Spectrometry" International Journal of Neonatal Screening 3, no. 4: 32. https://doi.org/10.3390/ijns3040032




