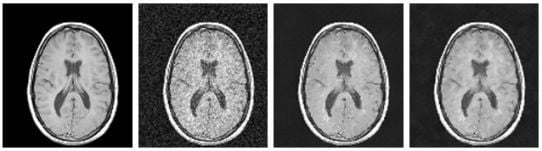
Parameter Optimization for Local Polynomial Approximation based Intersection Confidence Interval Filter Using Genetic Algorithm: An Application for Brain MRI Image De-Noising
Abstract
:1. Introduction
2. Rician Distributed Noise and MR Signals De-Noising
2.1. Rician Noise Mathematical Model
2.2. MR Images De-noising
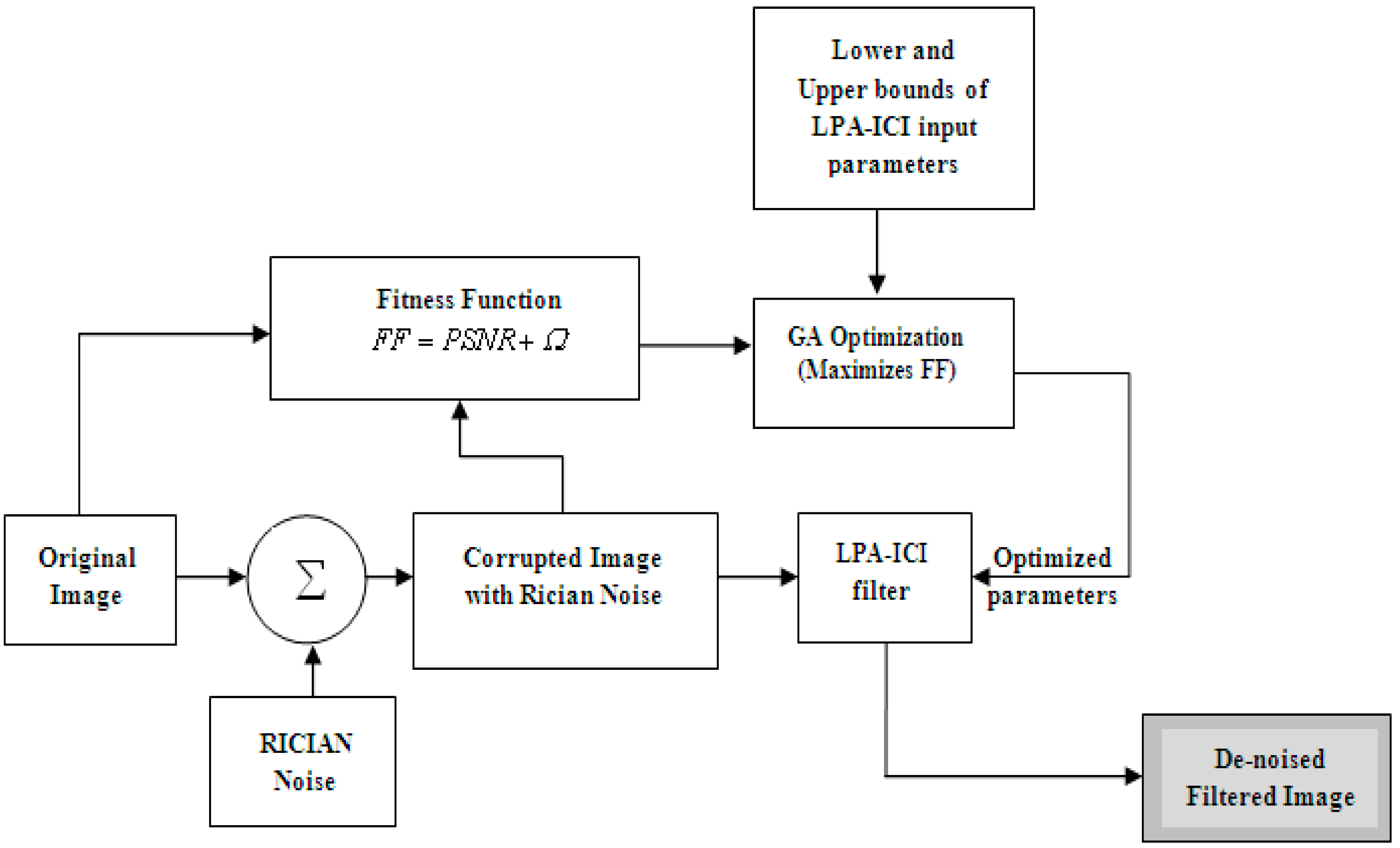
3. Methodology
3.1. Image De-Noising using LPA-ICI
- Step 1:
- Set Γ = and s=1,…,E.
- Step 2:
- Calculates the estimates , the adaptive window size and the estimates for . (Calculate for different scales the estimates and compare them)
- Step 3:
- Repeat steps 1 and 2 for all .
- Step 4:
- Find and select the estimates corresponding to as the final ones. (Find the adaptive scale which is the largest where the estimate does not vary significantly from the estimates corresponding to the smaller scales).
3.2. Optimal Selection of Parameters using Genetic Algorithm
- Step 1:
- (Begin) Generate random population of chromosomes. (Suitable solutions for the problem)
- Step 2:
- (Evaluate population-Fitness) In the population, evaluate the fitness of each chromosome.
- Step 3:
- (New population) Create a new population. (By repeating the following steps until the new population is complete)
- (a)
- Selection: From a population, select two parent chromosomes according to their fitness. (Better fitness, provides bigger chance to be selected to be the parent).
- (b)
- Crossover: With a crossover probability, cross over the parents to form new offspring (children). If no crossover was performed, offspring is the exact copy of parents.
- (c)
- Mutation: With a mutation probability, mutate new offspring at each locus.
- (d)
- Accepting: Place new offspring in the new population.
- Step 4:
- (Replace) Use new generated population for a further algorithm run.
- Step 5:
- (Test) If the end condition is satisfied, stop, and return the best solution in current population.
- Step 6:
- (Loop) Go to step 2.
4. The Proposed LPA-GA System
- Step 1.
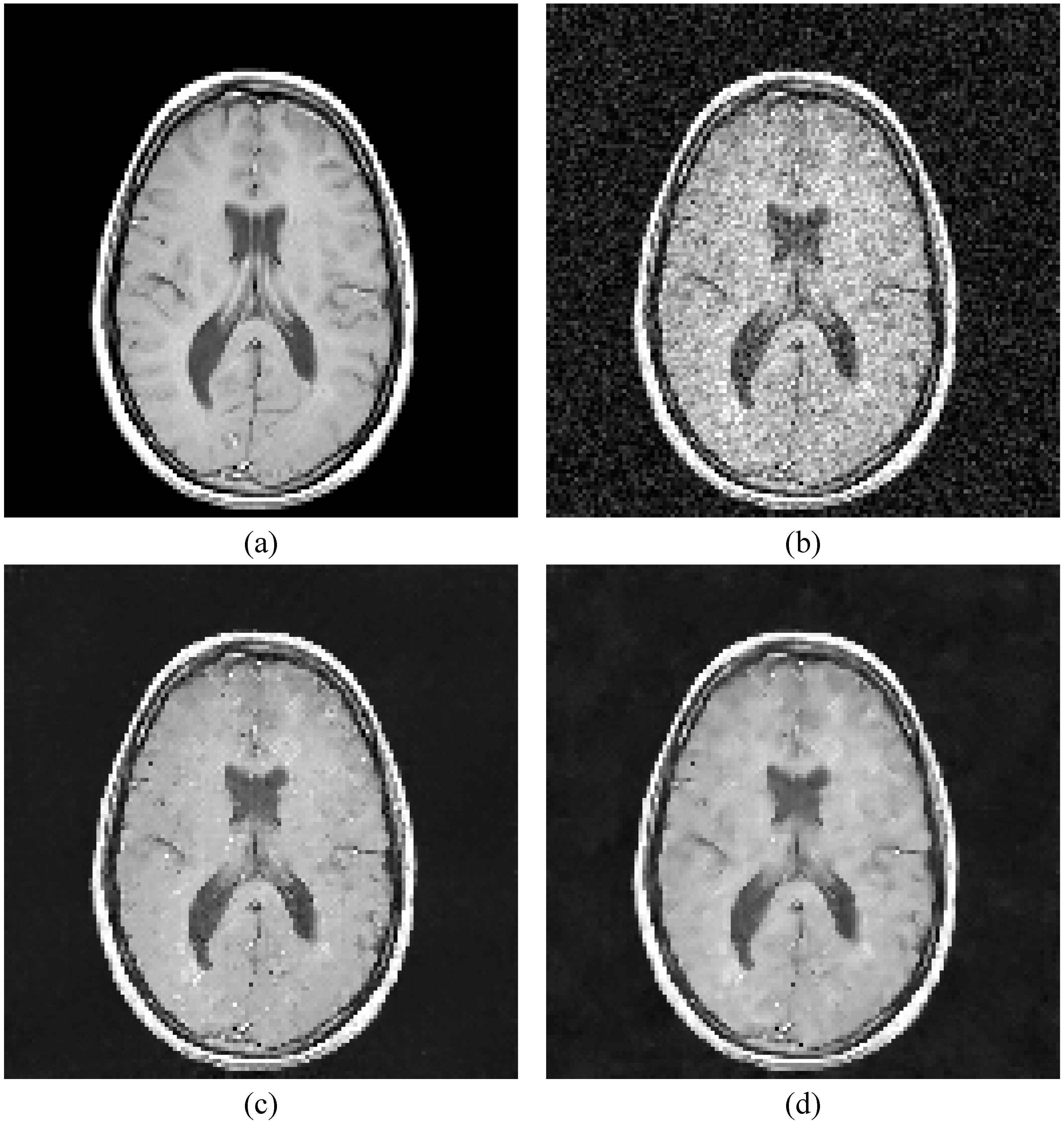
- The original MRI-brain image is added with Rician noise with various values of “s” ranging from 0.1 to 0.6 to produce the corrupted images.
- Step 2.
- The genetic algorithm is used to optimize the input parameters of LPA-ICI.
- Step 3.
- The input parameters include the Gamma ICI parameter Γ, sharpness parameter, number of directions = 4 or 8, and fusion parameter which is classical or piecewise.
- Step 4.
- The upper and lower bounds of the input parameters are set as [−1 to 10] for the Gamma ICI parameter, [0–5] for sharpness parameter, [[4 or 8] for direction parameter, [[1 or 2] for fusion parameter.
- Step 5.
- The fitness function uses the LPA-ICI with optimized parameters given by the GA to produce the restored image.
- Step 6.
- The genetic algorithm is used to maximize the fitness function (by multiplying the result by −1). The number of generations is set to 100 by default.
- Step 7.
- The genetic algorithm stopped around 50 generations for all noise ratios. Where, convergence occurred around 25 generations.
- Step 8.
- The optimized parameters generated by the genetic algorithm are then given to the LPA-ICI filter to de-noise the corrupted image.
- Step 9.
- The fitness function (FF), calculates PSNR+100*MSSIM between the original image and the restored image. Where, the LPA-ICI with parameters selected by GA is used to produce the restored image.
5. Result and Discussion
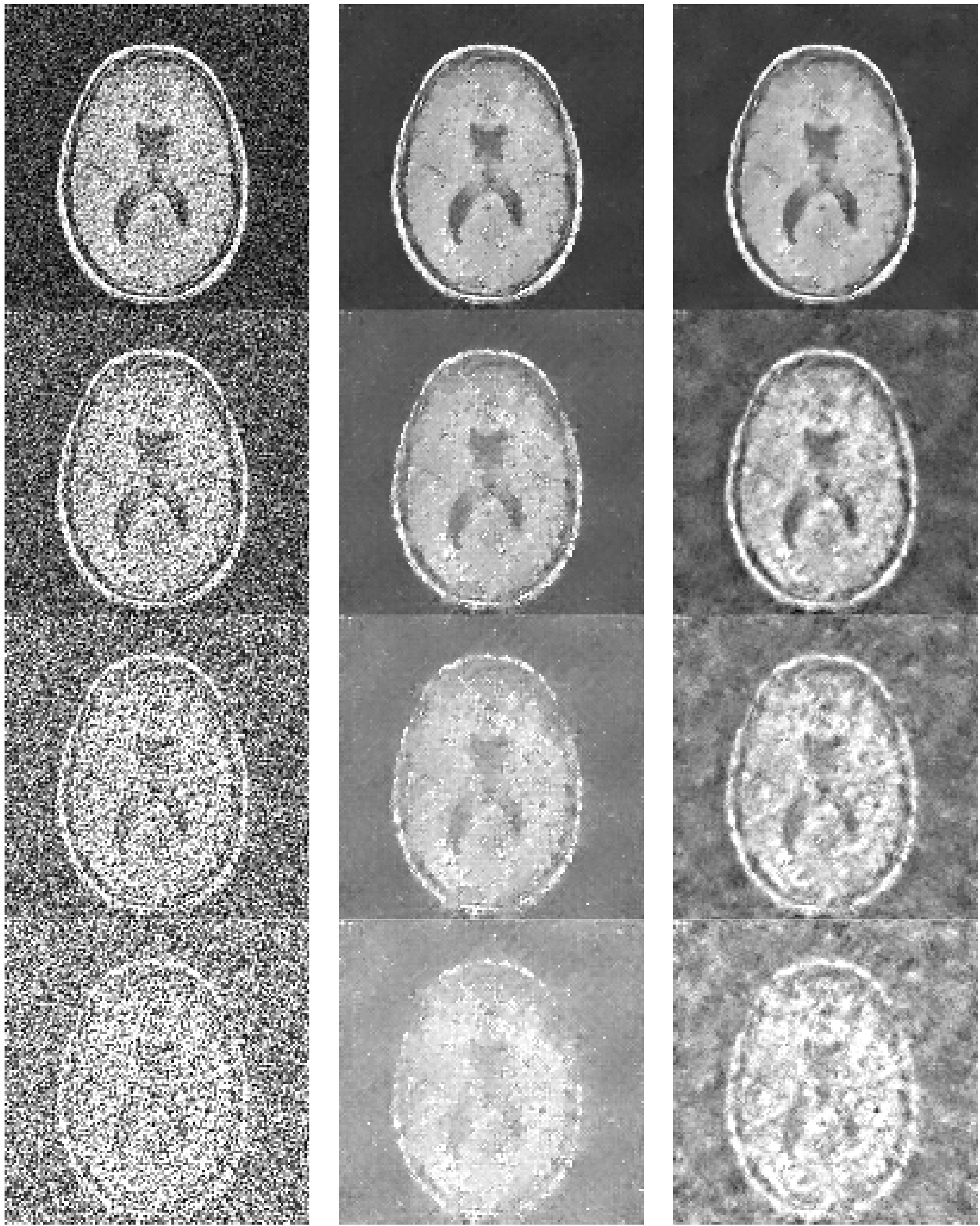
5.1. Comparing the results of LPA-ICI-GA versus LPA-ICI Algorithm
5.2. Proposed System Performance Analysis
- Signal-to-noise ratio (SNR): is defined as the ratio of the power of the original image values and the power of noise values. It is given by:
- The improvement in signal to noise ratio (ISNR) between the original and restored images is given by:
- The peak signal to noise ratio (PSNR) between the original and restored images is given by [68]:
- The mean squared error (MSE): measures the average of the squares of the “errors”, known as the difference between the original image and the restored image. It is given by,
- The mean absolute error (MAE): is the average of the absolute errors between the original image and the restored image. It is given by:
- The maximum absolute difference (MAD) is given by:
- The SSIM index measure between two windows and of the original and restored images is given by:
- The mean structural similarity (MSSIM) index is given by:
| S | ISNR | SNR | PSNR | MSE | RMSE | MAE | MAX | MSSIM |
|---|---|---|---|---|---|---|---|---|
| 0.1 | 1.55 | 12.2267 | 19.5451 | 722.0499 | 26.871 | 23.7222 | 95.379 | 0.44208 |
| 0.2 | 1.6386 | 6.31 | 13.6284 | 2819.9239 | 53.103 | 46.5034 | 190.9031 | 0.31629 |
| 0.3 | 1.6375 | 2.8197 | 10.1381 | 6299.0275 | 79.3664 | 69.6002 | 245.642 | 0.22051 |
| 0.4 | 1.612 | 0.31846 | 7.6369 | 11204.5665 | 105.8516 | 93.8354 | 367.7877 | 0.15175 |
| 0.5 | 1.5691 | -1.6638 | 5.6546 | 17685.5917 | 132.9872 | 119.8152 | 463.6092 | 0.10741 |
| 0.6 | 1.5325 | -3.3066 | 4.0118 | 25816.7765 | 160.676 | 147.2261 | 559.6435 | 0.084966 |
| 0.7 | 1.4968 | -4.718 | 2.6004 | 35730.901 | 89.0262 | 175.6339 | 655.8092 | 0.070399 |
- Sharpness parameter range = [−1, from 0 till 10];
- Γ range = [from 0 till 5];
- Directional resolution range = [[4 or 8];
- Fusing range = [[1 or 2], where Fusing = 1 for classical estimation, and equal 2 for piecewise estimation.
- Step 1.
- According to the noise variance level, the ICI parameter optimal values are changeable. This proves the importance of the GA to obtain the optimized values for these parameters. Also, these parameter values are changeable with any change in the image concerned.
- Step 2.
- The ICI tuned parameters (optimized), are different than the fixed values used in LPA-ICI. Also, as the noise variance increase and equal 0.6 both fusing and the directional resolution switches their values, to be able to overcome the high noise level.
- Step 3.
| S | SharpParam | Γ | Directional Resolution | Fusing |
|---|---|---|---|---|
| 0.1 | −0.4495 | 0.4984 | 8 | 2 |
| 0.2 | −0.7464 | 0.7165 | 8 | 2 |
| 0.3 | 0.0126 | 0.6696 | 8 | 2 |
| 0.4 | −0.1287 | 0.5449 | 8 | 2 |
| 0.5 | −0.0556 | 0.5540 | 8 | 2 |
| 0.6 | −0.2439 | 0.5234 | 8 | 2 |
| 0.7 | −0.8946 | 2.3293 | 4 | 1 |
| S | ISNR | SNR | PSNR | MSE | RMSE | MAE | MAX | MSSIM | Fitness Function = PSNR+100*MSSIM |
|---|---|---|---|---|---|---|---|---|---|
| 0.1 | 1.5982 | 12.275 | 19.5934 | 714.0724 | 26.7221 | 23.4761 | 95.379 | 0.44871 | 64.4645 |
| 0.2 | 1.6994 | 6.3708 | 13.6892 | 2780.722 | 52.7325 | 45.9734 | 190.9031 | 0.32608 | 46.2971 |
| 0.3 | 1.8495 | 3.0317 | 10.3501 | 5998.932 | 77.4528 | 68.2574 | 211.127 | 0.24197 | 34.5472 |
| 0.4 | 1.822 | 0.52845 | 7.8469 | 10,675.67 | 103.3231 | 91.4624 | 238.5945 | 0.18155 | 26.0015 |
| 0.5 | 1.7566 | −1.4763 | 5.8421 | 1,6938.2 | 130.1469 | 116.3874 | 297.4721 | 0.13606 | 19.4483 |
| 0.6 | 1.6849 | −3.1542 | 4.1642 | 2,4926.53 | 157.8814 | 143.7663 | 312.2485 | 0.10795 | 14.9587 |
| 0.7 | 1.4848 | −4.73 | 2.5884 | 3,5829.55 | 189.2869 | 176.1798 | 363.922 | 0.085832 | 11.1716 |
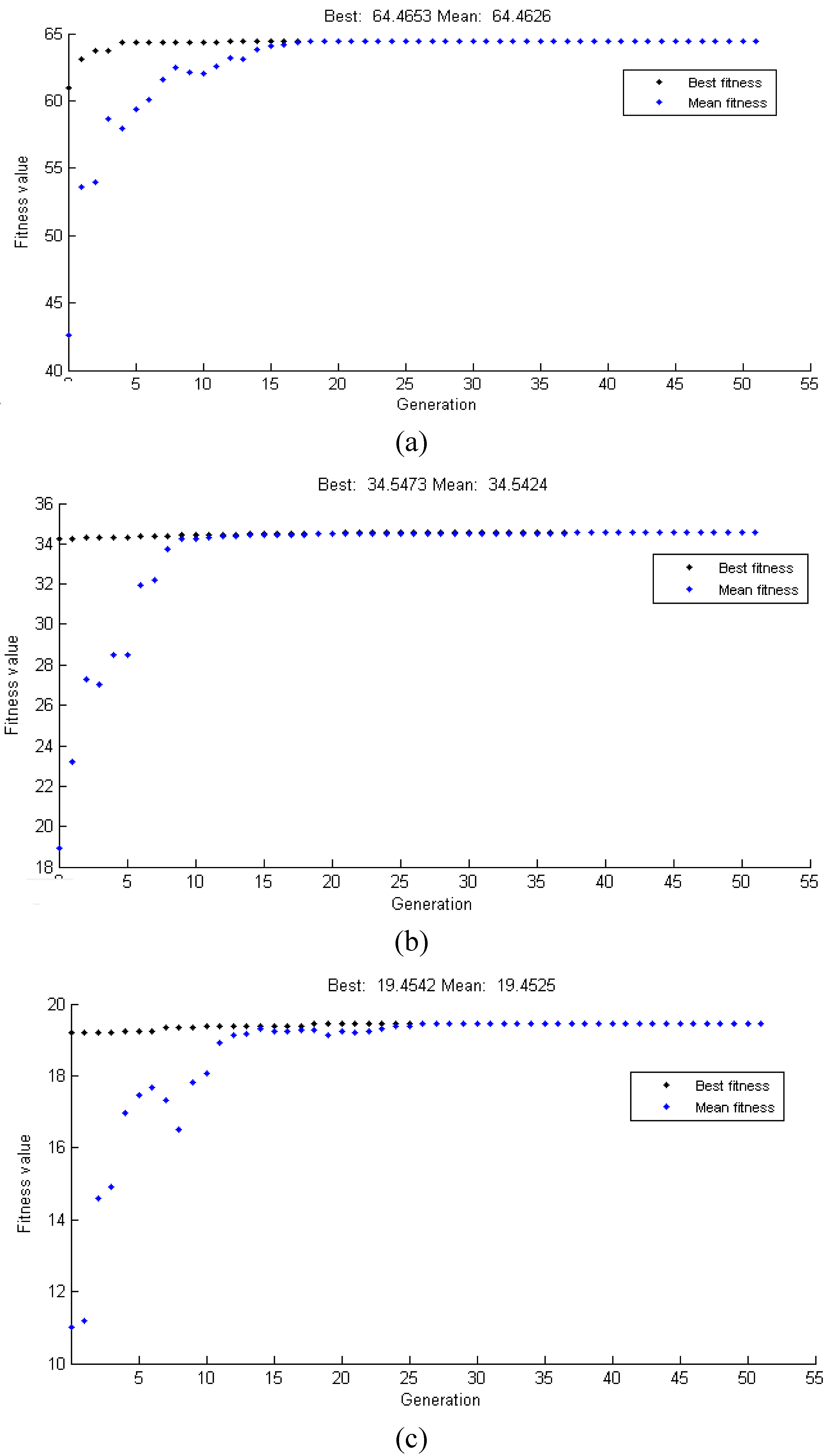
5.3. Proposed System Convergence Graphs
| S. No. | Authors | Year | De-Noising Technique | Comments/Notes |
|---|---|---|---|---|
| 1 | Bao et al. [15] | 2003 | Adaptive wavelet thresholding | To, this method multiplied the adjacent wavelet sub bands, then applied threshold to the multi-scale products to differentiate edge structures in an improved approach from noise and amplify the significant features. Results of MRI images proved that this method achieved high mean-to-standard-deviation ratio (MSR) and contrast to noise ratio (CNR). |
| 2 | Wang et al. [18] | 2006 | Wavelet and the total variation minimization methods | An effective automatic stopping time criterion extensively. |
| 3 | Manjon et al. [19] | 2009 | Novel filter averaging similar patches around the image using a robust multicomponent similarity measure filter, then local Principal Component Analysis decomposition as a post-processing for more noise removal. | This method showed a consistent improvement in the results when the number of images increases in contrast with the erratic behavior of the basic version. |
| 4 | Jaya et al. [23] | 2009 | Median filter, the weighted median and the adaptive filter. | The results proved that weighted median filter was outperforming the other filters with peak signal to noise ratio PSNR = 0.924. |
| 5 | Rajeesh et al. [20] | 2010 | Curvelet shrinkages were superior than using the wavelet method. | The reconstructed MRI data have high Signal to Noise Ratio (SNR) compared to the curvelet and wavelet domain denoising approaches. |
| 6 | Erturk et al. [22] | 2013 | Spectral subtraction method. | The results showed enhanced signal to noise ratio (SNR) up to 40% in the MRI reconstructed signal |
| 7 | Proposed system | 2015 | Local polynomial approximation with intersection confidence interval based genetic algorithm filter. (LPA-ICI-GA) | The results proved that using GA optimization to support the LPA-ICI outperform the classic LPA-ICI. For variance of 0.1, the PSNR using the proposed method is 19.5934 dB, while that obtained by the LPA-ICI was 19.5451. Comparing with respect to the MSE at the same variance value, it was obtained that the LPA-ICI-GA compared to the LPA-ICI achieved 714.0724 and 722.0499; respectively. That established that the proposed method gained les MSE compared to the classical LPA-ICI approach. |
6. Conclusions and Future work
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dey, N.; Das, P.; Roy, A.; Das, A.; Chaudhuri, S. Detection and measurement of Arc of Lumen calcification from intravascular ultrasound using Harris corner detection. In Proceedings of National Conference on Computing and Communication Systems (NCCCS), Durgapur, India, 21–22 November 2012.
- Dey, N.; Roy, A.; Pal, M.; Das, A. FCM based blood vessel segmentation method for retinal images. Int. J. Comput. Sci. Netw. 2012, 1. [Google Scholar]
- Araki, T.; Ikeda, N.; Molinari, F.; Dey, N.; Acharjee, S.; Saba, L.; Nicolaides, A.; Suri, J. Effect of geometric-based coronary calcium volume as a feature along with its shape-based attributes for cardiological risk prediction from low contrast IVUS. J. Med. Imaging Health Inform. 2014, 4, 255–261. [Google Scholar] [CrossRef]
- Dey, N.; Dey, M.; Biswas, D.; Das, P.; Das, A.; Chaudhuri, S. Tamper detection of electrocardiographic signal using watermarked bio-hash code in wireless cardiology. Int. J. Signal Imaging Syst. Eng. 2015, 8, 46–58. [Google Scholar] [CrossRef]
- Bilcu, R.; Vehvilainen, M. A novel decomposition scheme for image de-noising. In Proceedings of the IEEE International Conference on Acoustics, Speech and Signal Processing, Honolulu, HI, USA, 15–20 April 2007; pp. 577–580.
- Alpuente, L.; López, A.; Tur, R. Glioblastoma: Changing expectations? Clin. Trans. Oncol. 2011, 13, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Sijbers, J.; Dekker, A.; Audekerke, J.; Verhoye, M.; Dyck, D. Estimation of the noise in magnitude MR images. Magn. Reson. Imaging 1998, 16, 87–90. [Google Scholar] [CrossRef]
- Rice, S. Mathematical analysis of random noise. Bell Syst. Tech. J. 1944, 23, 282–332. [Google Scholar] [CrossRef]
- Ali, S.; Vathsal, S.; Lalkishore, K. A GA-based window selection methodology to enhance window-based multi-wavelet transformation and thresholding aided CT image denoising technique. Int. J. Comput. Sci. Inf. Secur. 2010, 7. [Google Scholar]
- Ali, S.A.; Vathsal, S.; Kishore, K.L. CT image denoising technique using GA aided window based multiwavelet transformation and thresholding with the incorporation of an effective quality enhancement method. Int. J. Digit. Content Technol. Appl. 2010, 4, 75–87. [Google Scholar]
- Dey, N.; Das, A.; Chaudhuri, S. Wavelet based normal and abnormal heart sound identification using spectrogram analysis. Int. J. Comput. Sci. Eng. Technol. 2012, 3, 186–192. [Google Scholar]
- Dey, N.; Roy, A.; Das, A.; Chaudhuri, S. Stationary wavelet transformation based self-recovery of blind-watermark from electrocardiogram signal in the wireless telecardiology. In Proceedings of the International Workshop on Intelligence and Security Informatics for International Security (IIS’12), Trivandrum, India, 11–12 October 2012.
- Healy, D.; Weaver, J. Two applications of wavelet transforms in magnetic resonance imaging. IEEE Trans. Inf. Theory 1992, 38, 840–860. [Google Scholar] [CrossRef]
- Nowak, R. Wavelet-based Rician noise removal for magnetic resonance imaging. IEEE Trans. Image Process. 1999, 8, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- Bao, P.; Zhang, L. Noise reduction for magnetic resonance images via adaptive multiscale products thresholding. IEEE Trans. Med. Imaging 2003, 22, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yang, W. Adaptive magnetic resonance image denoising using mixture model and wavelet shrinkage. In Proceeding of the VIIth Digital Image Computing: Techniques and Applications, Sydney, Australia, 10–12 December 2003; pp. 831–838.
- Kadah, Y. Adaptive denoising of event-related functional magnetic resonance imaging data using spectral subtraction. IEEE Trans. Biomed. Eng. 2004, 51, 1944–1953. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, H. Total variation wavelet-based medical image denoising. Int. J. Biomed. Imaging 2006, 2006. Article ID 89095. [Google Scholar]
- Manjon, J.; Thacke, N.; Lull, J.; Mar, G.; Bonmat, L.; Robles, M. Research article multicomponent MR image denoising. Int. J. Biomed. Imaging 2009, 2009, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rajeesh, J.; Moni, R.; Palanikumar, S.; Gopalakrishnan, T. Noise reduction in magnetic resonance images using wave atom shrinkage. Int. J. Image Process. 2010, 4, 131–141. [Google Scholar]
- Balafar, M. Review of noise reducing algorithms for brain MRI images. Int. J. Tech. Phys. Probl. Eng. 2012, 4, 54–59. [Google Scholar]
- Erturk, M.; Bottomley, P.; El-Sharkawy, A. Denoising MRI using spectral subtraction. IEEE Trans. Biomed. Eng. 2013, 60, 1556–1562. [Google Scholar] [CrossRef] [PubMed]
- Jaya, J.; Thanushkodi, K.; Karnan, M. Tracking algorithm for de-noising of MR brain images. Int. J. Comput. Sci. Netw. Secur. 2009, 9, 262–267. [Google Scholar]
- Iftikhar, M.A.; Jalil, A.; Rathore, S.; Ali, A.; Hussain, M. Brain MRI denoizing and segmentation based on improved adaptive nonlocal means. Int. J. Imaging Syst. Technol. 2013, 23, 235–248. [Google Scholar] [CrossRef]
- Jalil, A.; Rathore, S.; Hussain, M. Robust brain MRI denoising and segmentation using enhanced non-local means algorithm. Int. J. Imaging Syst. Technol. 2014, 24, 52–66. [Google Scholar]
- Iftikhar, M.A.; Jalil, A.; Rathore, S.; Ali, A.; Hussain, M. An extended non-local means algorithm: Application to brain MRI. Int. J. Imaging Syst. Technol. 2014, 24, 293–305. [Google Scholar] [CrossRef]
- Klepaczko, A. AGPU Accelerated Local Polynomial Approximation Algorithm for Efficient Denoising of MR Images; Burduk, R., Jackowski, K., Kurzynski, M., Wozniak, M., Zolnierek, A., Eds.; Springer International Publishing: Cham, Switzerland, 2013. [Google Scholar]
- Coupé, P.; Manjón, J.; Gedamu, E.; Arnold, D.; Robles, M.; Collins, D.L. Robust Rician noise estimation for MR images. Med. Image Anal. 2010, 14, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Sun, C.; Pham, T.D. Multipoint filtering with local polynomial approximation and range guidance. In Proceeding of the 2014 IEEE Conference in Computer Vision and Pattern Recognition (CVPR), Columbus, OH, USA, 23–28 June 2014; pp. 2941–2948.
- Hu, Y.; Jiang, X.; Xin, F.; Zhang, T.; Yuan, J.; Zhai, L.; Guo, C. An algorithm on processing medical image based on rough-set and genetic algorithm. In Proceedings of the International Conference on Information Technology and Applications in Biomedicine, Shenzhen, China, 30–31 May 2008.
- Samanta, S.; Dey, N.; Das, P.; Acharjee, S.; Chaudhuri, S. Multilevel threshold based gray scale image segmentation using Cuckoo search. In Proceedings of the International Conference on Emerging Trends in Electrical, Communication and Information Technologies (ICECIT), Anantapur, India, 12–23 December 2012.
- Chakraborty, S.; Pal, A.; Dey, N.; Das, D.; Acharjee, S. Foliage area computation using Monarch butterfly algorithm. In Proceedings of the 2014 1st International Conference on Non Conventional Energy, Kalyani, India, 16–17 January 2014.
- Jayashri, P.; Gandhimathi, D. Efficient tumor segmentation in medical images using artificial bee colony optimization algorithm and fuzzy c-means clustering. Int. J. Adv. Res. Comput. Sci. Manag. Stud. 2014, 2, 98–101. [Google Scholar]
- Acharjee, S.; Dey, N.; Samanta, S.; Das, D.; Roy, R.; Chakraborty, S.; Chaudhuri, S. ECG signal compression using ant weight lifting algorithm for tele-monitoring. J. Med. Imaging Health Inform. 2014, 5, 1580–1587. [Google Scholar]
- Day, N.; Samanta, S.; Chakraborty, S.; Das, A.; Chaudhuri, S.; Suri, J. Firefly algorithm for optimization of scaling factors during embedding of manifold medical information: An application in ophthalmology imaging. J. Med. Imaging Health Inform. 2014, 4, 384–394. [Google Scholar] [CrossRef]
- Misra, D.; Sarker, S.; Dhabal, S.; Ganguly, A. Effect of using genetic algorithm to denoise MRI images corrupted with Rician Noise. In Proceedings of the 2013 IEEE International Conference on Emerging Trends in Computing, Communication and Nanotechnology, Tirunelveli, India, 25–26 March 2013; pp. 146–151.
- Liu, Y.; Ma, Y.; Liu, F.; Zhang, X.; Yang, Y. The research based on the genetic algorithm of wavelet image denoising threshold of medicine. J. Chem. Pharm. Res. 2014, 6, 2458–2462. [Google Scholar]
- Tsang, P.; Au, A. A genetic algorithm for projective invariant object recognition. In Proceedings of the 1996 IEEE TENCON: Digital Signal Processing Applications, Perth, WA, USA, 26–29 November 1996; pp. 58–63.
- Yang, J.; Fan, J.; Ai, D.; Zhou, S.; Tang, S.; Wang, Y. Brain MR image denoising for Rician noise using pre-smooth non-local means filter. Biomed. Eng. Online 2015, 14, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Gudbjartsson, H.; Patz, S. The Rician distribution of noisy MRI data. Magn. Reson. Med. 1995, 34, 910–914. [Google Scholar] [CrossRef] [PubMed]
- Jagadeesan, H.; Sasirekha, N. Robust Rician noise estimation and filtering for magnetic resonance imaging. Int. J. Sci. Eng. Res. 2014, 5, 620–624. [Google Scholar]
- Selvathi, D.; Selvi, S.; Malar, C. The SURE-LET approach for MR brain image denoising using different shrinkage rules. Int. J. Healthc. Inf. Syst. Inform. 2010, 5, 73–81. [Google Scholar] [CrossRef]
- Edelstein, W.; Glover, G.; Hardy, C.; Redington, R. The intrinistic signal-to-noise ratio in MR imaging. Magn. Reson. Med. 1986, 3, 604–618. [Google Scholar] [CrossRef] [PubMed]
- Macovski, A. Noise in MRI. Magn. Reson. Med. 1996, 36, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Fletcher, T.; Whitaker, R. Rician noise removal in diffusion tensor MRI. In Medical Image Computing and Computer-Assisted Intervention—MICCAI 2006; Larsen, R., Nielsen, M., Sporring, J., Eds.; Springer: Berlin, Germany, 2006; pp. 117–125. [Google Scholar]
- Papoulis, A. Probability, Random Variables, and Stochastic Processes; McGraw-Hill: New York, NY, USA, 1984. [Google Scholar]
- Nobi, M.; Yousuf, M. A new method to remove noise in magnetic resonance and ultrasound images. J. Sci. Res. 2011, 3, 81–89. [Google Scholar]
- Lee, J.S. Digital image enhancement and noise filtering by use of local statistics. IEEE Trans. Pattern Anal. Mach. Intell. 1980, 2, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Russ, J. The Image Processing Handbook, 6th ed.; CRC Press: Baca Raton, FL, USA, 1999. [Google Scholar]
- Lee, Y.; Rhee, S. Wavelet-based image denoising with optimal filter. Int. J. Inf. Process. Syst. 2005, 1, 32–35. [Google Scholar]
- Gupta, V.; Mahle, R.; Shriwas, R.S. Image denoising using wavelet transform method. In Proceedings of the 2013 Tenth International Conference on Wireless and Optical Communications Networks (WOCN), Bhopal, India, 26–28 July 2013.
- Weaver, D.; Xu, Y.; Driscoll, J. Filtering MR images in the wavelet transform domain. Magn. Reson. Med. 1991, 21, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Hilton, M.; Ogden, T.; Hattery, D.; Eden, G.; Jawerth, B. Wavelet de-noising of functional MRI data. In Wavelets in Biology and Medicine; Aldroubi, A., Unser, M., Eds.; CRC Press: Baca Raton, FL, USA, 1996; pp. 93–112. [Google Scholar]
- Piˇzurica, A.; Philips, W.; Lemahieu, I.; Acheroy, M. A versatile wavelet domain noise filtration technique for medical imaging. IEEE Trans. Med. Imaging 2003, 22, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Katkovnik, V. A new method for varying adaptive bandwidth selection. IEEE Trans. Signal Process. 1999, 47, 2567–2571. [Google Scholar] [CrossRef]
- Ashour, A.; Elkamchouchi, H. Enhancement of moving targets tracking performance using the ICI rule. Alex. Eng. J. 2007, 46, 673–682. [Google Scholar]
- Perona, P.; Malik, J. Scale-space and edge detection using anisotropic diffusion. IEEE Trans. PAMI 1990, 12, 629–639. [Google Scholar] [CrossRef]
- Li, S.; Huang, D. Image denoising using non-negative sparse coding shrinkage algorithm. In Proceedings of the IEEE Computer Society Conference on Computer Vision and Pattern Recognition, San Diego, CA, USA, 20–25 June 2005; pp. 1017–1022.
- Crouse, M.; Nowak, R.; Baraniuk, R. Wavelet-based statistical signal processing using hidden markov models. IEEE Trans. Signal Process. 1998, 46, 886–902. [Google Scholar] [CrossRef]
- Jangra, S.; Yadav, S. A review of Rician noise reduction in MRI images using wave atom transform. Int. J. Comput. Sci. Mob. Comput. 2014, 3, 454–457. [Google Scholar]
- Katkovnik, V.; Egiazarian, K.; Astola, J. Local Approximation Techniques in Signal and Image Processing; SPIE Press: Bellingham, WA, USA, 2006. [Google Scholar]
- Katkovnik, V.; Egiazarian, K.; Astola, J. A spatially adaptive nonparametric regression image deblurring. IEEE Trans. Image Process. 2005, 14, 1469–1478. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Gijbels, I. Local Polynomial Modelling and Its Application; Chapman and Hall: London, UK, 1996. [Google Scholar]
- Katkovnik, V. The Method of Local Approximation; Nauka: Moscow, Russia, 1985. [Google Scholar]
- Katkovnik, V.; Egiazarian, K.; Astola, J. Adaptive window size image de-noising based on Intersection of Confidence Intervals (ICI) rule. J. Math. Imaging Vis. 2002, 16, 223–235. [Google Scholar] [CrossRef]
- Malhotra, R.; Singh, N.; Singh, Y. Genetic algorithms: Concepts, design for optimization of process controllers. Comput. Inf. Sci. 2011, 4. [Google Scholar] [CrossRef]
- Exploring Slices from a 3-Dimensional MRI Data Set. Available online: http://in.mathworks.com/help/images/examples/exploring-slices-from-a-3-dimensional-mri-data-set.html (accessed on 19 August 2015).
- Chen, T.; Wu, H. Space variant median filters for the restoration of impulse noise corrupted images. IEEE Trans. Circuits Syst. II 2011, 48, 784–789. [Google Scholar] [CrossRef]
- Iftikhar, A.; Rathore, S.; Jalil, A. Parameter optimization for non-local de-noising using Elite GA. In Proceedings of the 15th International Multitopic Conference (INMIC), Islamabad, Pakistan, 13–15 December 2012; pp. 194–199.
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dey, N.; Ashour, A.S.; Beagum, S.; Pistola, D.S.; Gospodinov, M.; Gospodinova, Е.P.; Tavares, J.M.R.S. Parameter Optimization for Local Polynomial Approximation based Intersection Confidence Interval Filter Using Genetic Algorithm: An Application for Brain MRI Image De-Noising. J. Imaging 2015, 1, 60-84. https://doi.org/10.3390/jimaging1010060
Dey N, Ashour AS, Beagum S, Pistola DS, Gospodinov M, Gospodinova ЕP, Tavares JMRS. Parameter Optimization for Local Polynomial Approximation based Intersection Confidence Interval Filter Using Genetic Algorithm: An Application for Brain MRI Image De-Noising. Journal of Imaging. 2015; 1(1):60-84. https://doi.org/10.3390/jimaging1010060
Chicago/Turabian StyleDey, Nilanjan, Amira S. Ashour, Samsad Beagum, Dimitra Sifaki Pistola, Mitko Gospodinov, Еvgeniya Peneva Gospodinova, and João Manuel R. S. Tavares. 2015. "Parameter Optimization for Local Polynomial Approximation based Intersection Confidence Interval Filter Using Genetic Algorithm: An Application for Brain MRI Image De-Noising" Journal of Imaging 1, no. 1: 60-84. https://doi.org/10.3390/jimaging1010060