Cu(II)/Polydopamine-Modified Glass Fiber Separators for High-Performance Zinc-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of the Cu(II)-PDA/GF Separator
2.2. Characterization
2.3. Electrochemical Measurements
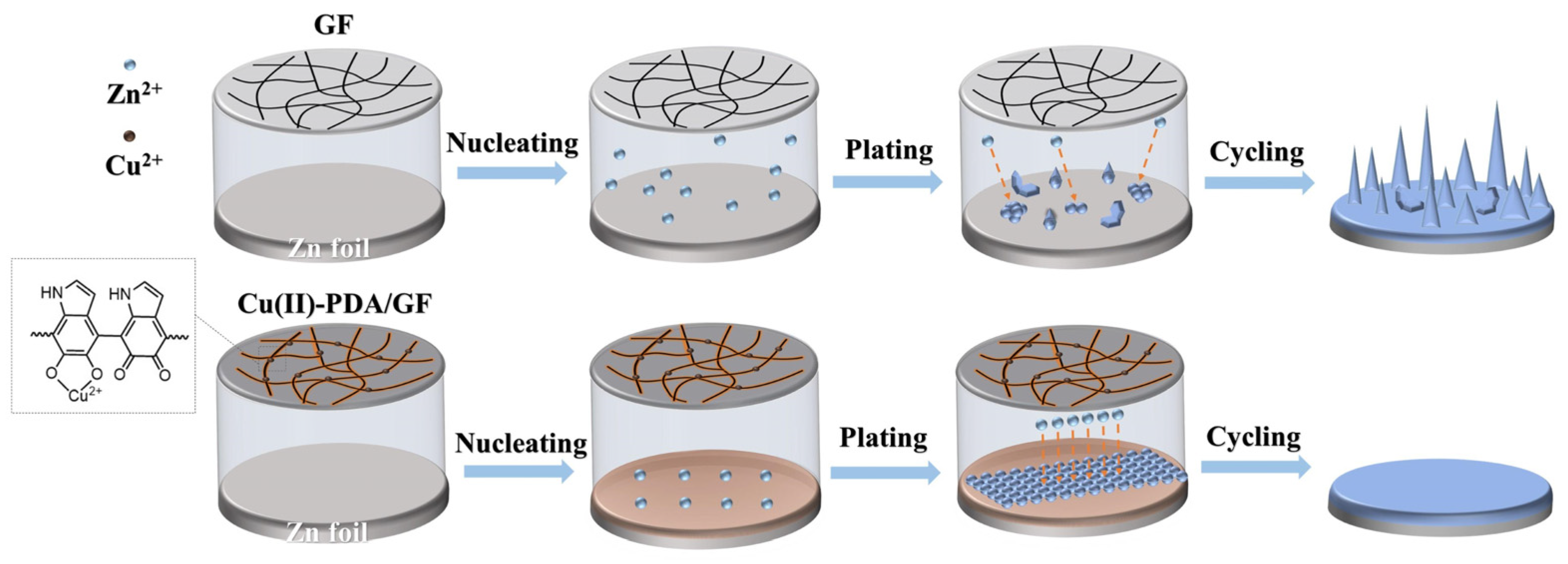
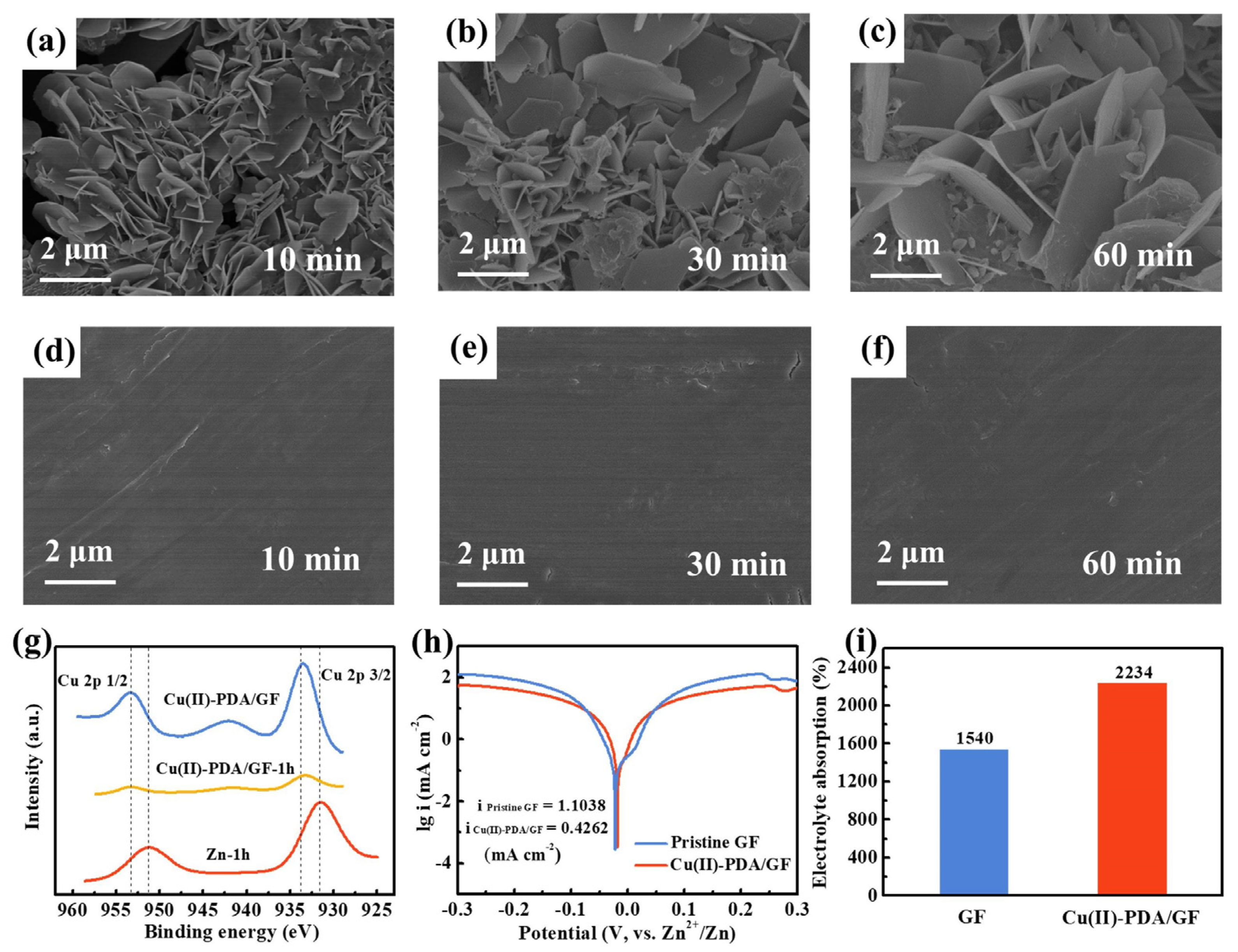
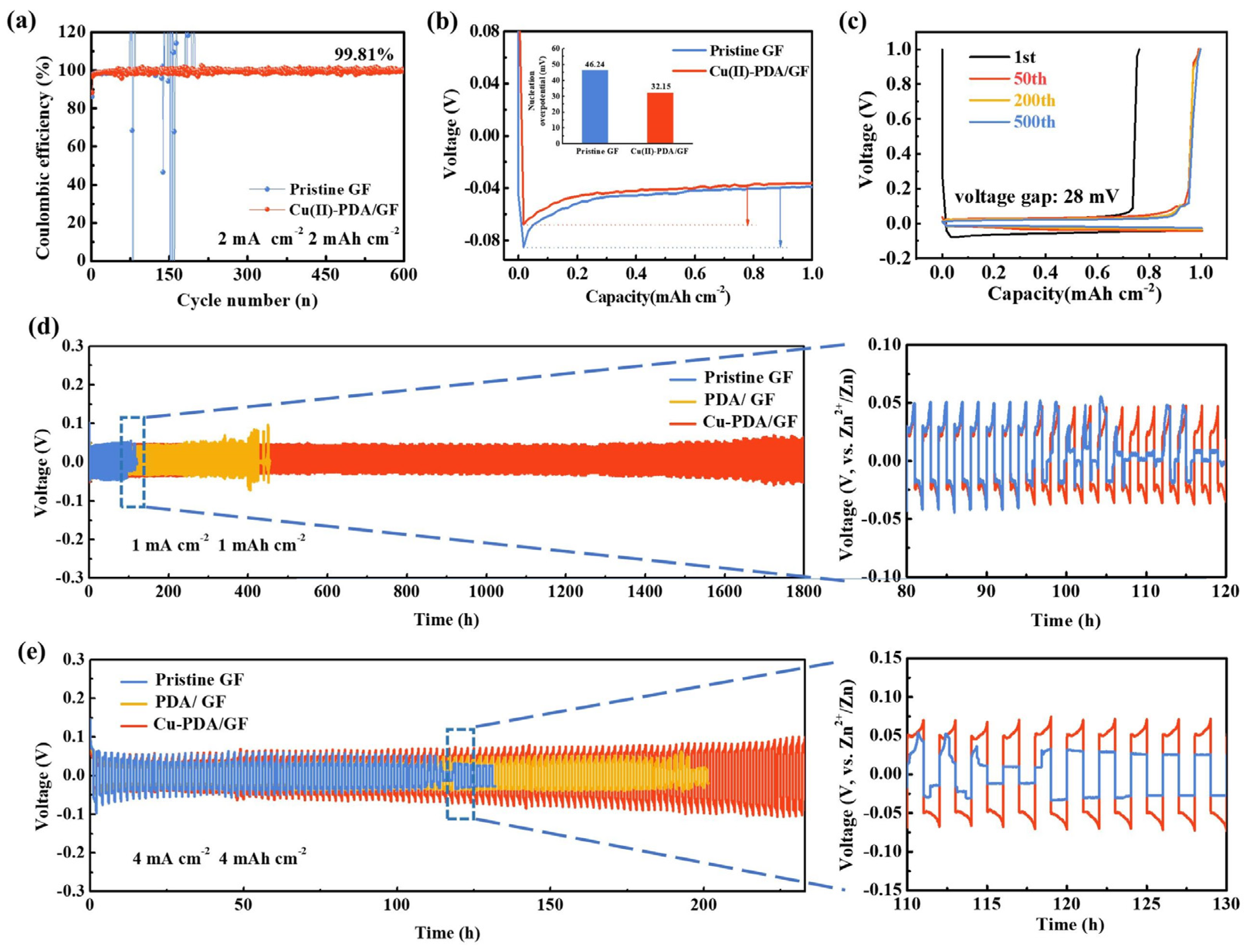
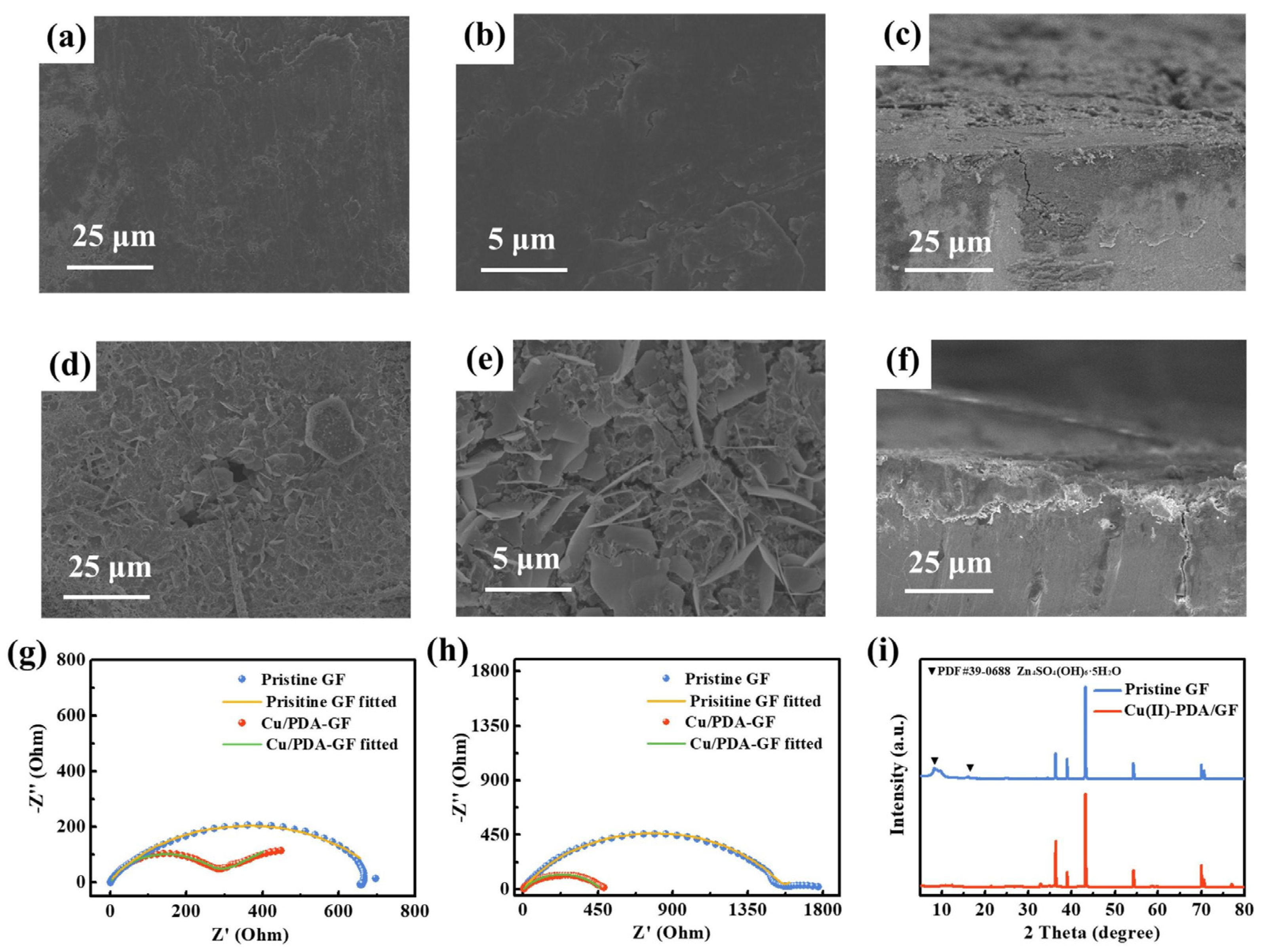
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, Y.; Zhu, M.; Huang, Y.; Pei, Z.; Li, H.; Wang, Z.; Xue, Q.; Zhi, C. Multifunctional Energy Storage and Conversion Devices. Adv. Mater. 2016, 28, 8344–8364. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jin, S.; Archer, L.A.; Nazar, L.F. Toward Practical Aqueous Zinc-Ion Batteries for Electrochemical Energy Storage. Joule 2022, 6, 1733–1738. [Google Scholar] [CrossRef]
- Du, W.; Ang, E.H.; Yang, Y.; Zhang, Y.; Ye, M.; Li, C.C. Challenges in the Material and Structural Design of Zinc Anode towards High-Performance Aqueous Zinc-Ion Batteries. Energy Environ. Sci. 2020, 13, 3330–3360. [Google Scholar] [CrossRef]
- Ray, A.; Saruhan, B. Application of Ionic Liquids for Batteries and Supercapacitors. Materials 2021, 14, 2942. [Google Scholar] [CrossRef]
- Pan, Z.; Liu, X.; Yang, J.; Li, X.; Liu, Z.; Loh, X.; Wang, J. Aqueous Rechargeable Multivalent Metal-Ion Batteries: Advances and Challenges. Adv. Energy Mater. 2021, 11, 2100608. [Google Scholar] [CrossRef]
- Bidhan, P.; Sachin, R.R.; Shoyebmohamad, F.S.; Mohd, U.; Ricardo, A.; Nelson, Y.D.; Emad, S.G.; Abu HS, R.; Harjot, S.G.; Tokeer, A. Regulated Electrochemical Performance of Manganese Oxide Cathode for Potassium-Ion Batteries: A Combined Experimental and First-Principles Density Functional Theory (DFT) Investigation. J. Colloid Interface Sci. 2023, 633, 886–896. [Google Scholar]
- Zong, Y.; He, H.; Wang, Y.; Wu, M.; Ren, X.; Bai, Z.; Wang, N.; Ning, X.; Dou, S.X. Functionalized Separator Strategies toward Advanced Aqueous Zinc-Ion Batteries. Adv. Energy Mater. 2023, 13, 2300403. [Google Scholar] [CrossRef]
- Bidhan, P.; Sachin, R.R.; Nelson, Y.D.; Shoyebmohamad, F.S.; Nitish, K.; Emad, S.G.; Abdullah, A.A.; Rajaram, S.M.; Sanjay, M.; Rahul, R.S. High Stability and Long Cycle Life of Rechargeable Sodium-Ion Battery Using Manganese Oxide Cathode: A Combined Density Functional Theory (DFT) and Experimental Study. ACS Appl. Mater. Interfaces 2021, 13, 11433–11441. [Google Scholar]
- Xu, L.; Meng, T.; Zheng, X.; Li, T.; Brozena, A.H.H.; Mao, Y.; Zhang, Q.; Clifford, B.C.; Rao, J.; Hu, L. Nanocellulose-Carboxymethylcellulose Electrolyte for Stable, High-Rate Zinc-Ion Batteries. Adv. Funct. Mater. 2023, 33, 2302098. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, M.; Tian, Q.; Chen, J.; Xu, X.; Wong, C.-P. Cotton-Derived Cellulose Film as A Dendrite-Inhibiting Separator to Stabilize the Zinc Metal Anode of Aqueous Zinc Ion Batteries. Energy Stor. Mater. 2022, 44, 57–65. [Google Scholar] [CrossRef]
- Hopkins, B.J.; Sassin, M.B.; Chervin, C.N.; Desario, P.A.; Parker, J.F.; Long, J.W.; Rolison, D.R. Fabricating Architected Zinc Electrodes with Unprecedented Volumetric Capacity in Rechargeable Alkaline Cells. Energy Stor. Mater. 2020, 27, 370–376. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Wang, H.; Dou, S.; Gan, W.; Ci, L.; Huang, Y.; Yuan, Q. MOF-Based Ionic Sieve Interphase for Regulated Zn2+ Flux toward Dendrite-Free Aqueous Zinc-Ion Batteries. J. Mater. Chem. A 2022, 10, 366–4375. [Google Scholar] [CrossRef]
- Han, D.; Ma, T.; Sun, T.-J.; Zhang, W.-J.; Tao, Z.-L. Zinc Anode Protection Strategy for Aqueous Zinc-Ion Batteries. Chinese J. Inorg. Chem. 2022, 38, 185–197. [Google Scholar]
- Yu, Y.; Xu, W.; Liu, X.; Lu, X. Challenges and Strategies for Constructing Highly Reversible Zinc Anodes in Aqueous Zinc-Ion Batteries: Recent Progress and Future Perspectives. Adv. Sustain. Syst. 2020, 4, 2000082. [Google Scholar] [CrossRef]
- Zhang, Q.; Luan, J.; Tang, Y.; Ji, X.; Wang, H. Interfacial Design of Dendrite-Free Zinc Anodes for Aqueous Zinc-Ion Batteries. Angew. Chem. Int. Ed. 2020, 59, 13180–13191. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Li, W.; Liu, H.K.; Dou, S.; Wang, J. Principals and Strategies for Constructing A Highly Reversible Zinc Metal Anode in Aqueous Batteries. Nano Energy 2020, 74, 104880. [Google Scholar] [CrossRef]
- Yi, Z.; Chen, G.; Hou, F.; Wang, L.; Liang, J. Strategies for the Stabilization of Zn Metal Anodes for Zn-Ion Batteries. Adv. Energy Mater. 2021, 11, 2003065. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, Z.; Li, J.; Luo, N.; Chai, G.-L.; Miller, T.S.; Lai, F.; Shearing, P.; Brett, D.J.L.; Han, D.; et al. Alleviation of Dendrite Formation on Zinc Anodes via Electrolyte Additives. ACS Energy Lett. 2021, 6, 395–403. [Google Scholar] [CrossRef]
- Du, Y.; Li, Y.; Xu, B.B.; Liu, T.X.; Liu, X.; Ma, F.; Gu, X.; Lai, C. Electrolyte Salts and Additives Regulation Enables High Performance Aqueous Zinc Ion Batteries: A Mini Review. Small 2021, 18, 2104640. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, Y.; Xu, L.; Yang, C.; Hong, M.; Cui, M.; Clifford, B.C.; He, S.; Jing, S.; Yao, Y.; et al. A Sustainable Chitosan-Zinc Electrolyte for High-Rate Zinc-Metal Batteries. Matter 2022, 5, 3402–3416. [Google Scholar] [CrossRef]
- Guo, S.; Qin, L.; Zhang, T.; Zhou, M.; Zhou, J.; Fang, G.; Liang, S. Fundamentals and Perspectives of Electrolyte Additives for Aqueous zinc-Ion Batteries. Energy Stor. Mater. 2021, 34, 545–562. [Google Scholar] [CrossRef]
- Wu, K.; Huang, J.; Yi, J.; Liu, X.; Liu, Y.; Wang, Y.; Zhang, J.; Xia, Y. Recent Advances in Polymer Electrolytes for Zinc Ion Batteries: Mechanisms, Properties, and Perspectives. Adv. Energy Mater. 2020, 10, 1903977. [Google Scholar] [CrossRef]
- Lu, K.; Jiang, T.; Hu, H.; Wu, M. Hydrogel Electrolytes for Quasi-Solid Zinc-Based Batteries. Front. Chem. 2020, 8, 546728. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, D.; Ran, F. Key Approaches and Challenges in Fabricating Advanced Flexible Zinc-Ion Batteries with Functional Hydrogel Electrolytes. Energy Stor. Mater. 2023, 56, 351–393. [Google Scholar] [CrossRef]
- Dong, H.; Li, J.; Guo, J.; Lai, F.; Zhao, F.; Jiao, Y.; Brett, D.J.L.; Liu, T.; He, G.; Parkin, I.P. Insights on Flexible Zinc-Ion Batteries from Lab Research to Commercialization. Adv. Mater. 2021, 33, 2007548. [Google Scholar] [CrossRef]
- Liu, D.; Tang, Z.; Luo, L.; Yang, W.; Liu, Y.; Shen, Z.; Fan, X.-H. Self-Healing Solid Polymer Electrolyte with High Ion Conductivity and Super Stretchability for All-Solid Zinc-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 36320–36329. [Google Scholar] [CrossRef]
- Li, C.; Sun, Z.; Yang, T.; Yu, L.; Wei, N.; Tian, Z.; Cai, J.; Lv, J.; Shao, Y.; Rummeli, M.H.; et al. Directly Grown Vertical Graphene Carpets as Janus Separators toward Stabilized Zn Metal Anodes. Adv. Mater. 2020, 32, 2003425. [Google Scholar] [CrossRef]
- Su, Y.; Liu, B.; Zhang, Q.; Peng, J.; Wei, C.; Li, S.; Li, W.; Xue, Z.; Yang, X.; Sun, J. Printing-Scalable Ti3C2TX MXene-Decorated Janus Separator with Expedited Zn2+ Flux toward Stabilized Zn Anodes. Adv. Funct. Mater. 2022, 32, 2204306. [Google Scholar] [CrossRef]
- Liu, T.; Hong, J.; Wang, J.; Xu, Y.; Wang, Y. Uniform Distribution of Zinc Ions Achieved by Functional Supramolecules for Stable Zinc Metal Anode with Long Cycling Lifespan. Energy Stor. Mater. 2022, 45, 1074–1083. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, D.; Gu, C.; Wang, X.; Wang, S.; Zhang, X.; Qin, J.; Wu, Z.-S. Manipulating Crystallographic Orientation of Zinc Deposition for Dendrite-free Zinc Ion Batteries. Adv. Energy Mater. 2021, 11, 2101299. [Google Scholar] [CrossRef]
- Ryou, M.-H.; Lee, D.J.; Lee, J.-N.; Lee, Y.M.; Park, J.-K.; Choi, J.W. Excellent Cycle Life of Lithium-Metal Anodes in Lithium-Ion Batteries with Mussel-Inspired Polydopamine-Coated Separators. Adv. Energy Mater. 2012, 2, 645–650. [Google Scholar] [CrossRef]
- Dong, Q.; Zhang, X.; Qian, J.; He, S.; Mao, Y.; Brozena, A.H.; Zhang, Y.; Pollard, T.P.; Borodin, O.A.; Wang, Y.; et al. A Cellulose-Derived Supramolecule for Fast Ion Transport. Sci. Adv. 2022, 8, eadd2031. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Meng, C.; He, J.; Dong, X. A Lightweight 3D Zn@Cu Nanosheets@Activated Carbon Cloth as Long-Life Anode with Large Capacity for Flexible Zinc Ion Batteries. J. Power Sources 2020, 480, 228871. [Google Scholar] [CrossRef]
- Li, C.; Shi, X.; Liang, S.; Ma, X.; Han, M.; Wu, X.; Zhou, J. Spatially Homogeneous Copper Foam as Surface Dendrite-Free Host for Zinc Metal Anode. Chem. Eng. J. 2020, 379, 122248. [Google Scholar] [CrossRef]
- Zhang, C.; Ou, Y.; Lei, W.-X.; Wan, L.-S.; Ji, J.; Xu, Z.-K. CuSO4/H2O2-Induced Rapid Deposition of Polydopamine Coatings with High Uniformity and Enhanced Stability. Angew. Chem. Int. Ed. 2016, 55, 3054–3057. [Google Scholar] [CrossRef]
- Yu, F.; Chen, S.; Chen, Y.; Li, H.; Yang, L.; Chen, Y.; Yin, Y. Experimental and Theoretical Analysis of Polymerization Reaction Process on the Polydopamine Membranes and Its Corrosion Protection Properties for 304 Stainless Steel. J. Mol. Struct. 2010, 982, 152–161. [Google Scholar] [CrossRef]
- Zeng, Y.; Sun, P.X.; Pei, Z.; Jin, Q.; Zhang, X.; Yu, L.; Lou, X.W. Nitrogen-Doped Carbon Fibers Embedded with Zincophilic Cu Nanoboxes for Stable Zn-Metal Anodes. Adv. Mater. 2022, 34, 2200342. [Google Scholar] [CrossRef]
- Xie, S.; Li, Y.; Li, X.; Zhou, Y.; Dang, Z.; Rong, J.; Dong, L. Stable Zinc Anodes Enabled by Zincophilic Cu Nanowire Networks. Nano-Micro Lett. 2022, 14, 39. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, X.; Luo, M.; Cao, K.; Lu, Y.; Xu, B.B.; Pan, H.; Tao, K.; Jiang, Y. Amino Acid-Induced Interface Charge Engineering Enables Highly Reversible Zn Anode. Adv. Funct. Mater. 2021, 31, 2103514. [Google Scholar] [CrossRef]
- Wang, T.; Li, S.; Weng, X.; Gao, L.; Yan, Y.; Zhang, N.; Qu, X.; Jiao, L.; Liu, Y. Ultrafast 3D Hybrid-Ion Transport in Porous V2O5 Cathodes For Superior-Rate Rechargeable Aqueous Zinc Batteries. Adv. Energy Mater. 2023, 13, 2204358. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Gong, L.; Wang, X.; Hu, P.; Liu, J. Rational Construction of Ag@MIL-88B(V)-Derived Hierarchical Porous Ag-V2O5 Heterostructures with Enhanced Diffusion Kinetics and Cycling Stability for Aqueous Zinc-Ion Batteries. J. Energy Chem. 2023, 77, 561–571. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Xie, X.; Li, Z.; Wang, P.; Lu, B.; Liang, S.; Tang, Y.; Zhou, J. A Functionalized Separator Enables Dendrite-Free Zn Anode via Metal-Polydopamine Coordination Chemistry. Infomat 2023, 5, e12374. [Google Scholar] [CrossRef]
- Guo, G.; Tan, X.; Wang, K.; Zheng, L.; Zhang, H. Regulating Zinc Deposition Behaviors by Functional Cotton Textiles as Separators for Aqueous Zinc-Metal Batteries. J. Power Sources 2023, 553, 232321. [Google Scholar] [CrossRef]
- Li, L.; Peng, J.; Jia, X.; Zhu, X.; Meng, B.; Yang, K.; Chu, D.; Yang, N.; Yu, J. PBC@Cellulose-Filter Paper Separator Design with Efficient Ion Transport Properties toward Stabilized Zinc-Ion Battery. Electrochim. Acta 2022, 430, 141129. [Google Scholar] [CrossRef]
- Qin, H.; Chen, W.; Kuang, W.; Hu, N.; Zhang, X.; Weng, H.; Tang, H.; Huang, D.; Xu, J.; He, H. A Nature-Inspired Separator with Water-Confined and Kinetics-Boosted Effects for Sustainable and High-Utilization Zn Metal Batteries. Small 2023, 19, 2300130. [Google Scholar] [CrossRef]
- Yang, X.; Li, W.; Lv, J.; Sun, G.; Shi, Z.; Su, Y.; Lian, X.; Shao, Y.; Zhi, A.; Tian, X.; et al. In Situ Separator Modification via CVD-Derived N-doped Carbon for Highly Reversible Zn Metal Anodes. Nano Res. 2022, 15, 9785–9791. [Google Scholar] [CrossRef]
- Ge, X.; Zhang, W.; Song, F.; Xie, B.; Li, J.; Wang, J.; Wang, X.; Zhao, J.; Cui, G. Single-Ion-Functionalized Nanocellulose Membranes Enable Lean-Electrolyte and Deeply Cycled Aqueous Zinc-Metal Batteries. Adv. Funct. Mater. 2022, 32, 2200429. [Google Scholar] [CrossRef]
- Li, Y.; Peng, X.; Li, X.; Duan, H.; Xie, S.; Dong, L.; Kang, F. Functional Ultrathin Separators Proactively Stabilizing Zinc Anodes for Zinc-Based Energy Storage. Adv. Mater. 2023, 35, 2300019. [Google Scholar] [CrossRef]
- Zhu, J.; Bie, Z.; Cai, X.; Jiao, Z.; Wang, Z.; Tao, J.; Song, W.; Fan, H.J. A Molecular-Sieve Electrolyte Membrane enables Separator-Free Zinc Batteries with Ultra long Cycle Life. Adv. Mater. 2022, 34, 2207209. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, T.; Yu, B.; Zhu, M.; Meng, F.; Shi, W.; Zhang, M.; Qi, Z.; Zeng, K.; Xue, J. Manipulating Zn-ion Flux by Two-Dimensional Porous g-C3N4 Nanosheets for Dendrite-Free Zinc Metal Anode. Chem. Eng. J. 2022, 433, 134077. [Google Scholar] [CrossRef]
- Wu, B.; Wu, Y.; Lu, Z.; Zhang, J.; Han, N.; Wang, Y.; Li, X.-M.; Lin, M.; Zeng, L. A Cation Selective Separator Induced Cathode Protective Layer and Regulated Zinc Deposition for Zinc Ion Batteries. J. Mater. Chem. A 2021, 9, 4734–4743. [Google Scholar] [CrossRef]
- Song, Y.; Ruan, P.; Mao, C.; Chang, Y.; Wang, L.; Dai, L.; Zhou, P.; Lu, B.; Zhou, J.; He, Z. Metal-Organic Frameworks Functionalized Separators for Robust Aqueous Zinc-Ion Batteries. Nano-Micro Lett. 2022, 74, 104880. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, F.; Xie, K.; Wu, S.; Zhang, C.; Liao, X.; Wang, Q. Cu(II)/Polydopamine-Modified Glass Fiber Separators for High-Performance Zinc-Ion Batteries. Batteries 2023, 9, 387. https://doi.org/10.3390/batteries9070387
Ma F, Xie K, Wu S, Zhang C, Liao X, Wang Q. Cu(II)/Polydopamine-Modified Glass Fiber Separators for High-Performance Zinc-Ion Batteries. Batteries. 2023; 9(7):387. https://doi.org/10.3390/batteries9070387
Chicago/Turabian StyleMa, Fengcan, Kaixuan Xie, Siheng Wu, Chi Zhang, Xiaodie Liao, and Qinghong Wang. 2023. "Cu(II)/Polydopamine-Modified Glass Fiber Separators for High-Performance Zinc-Ion Batteries" Batteries 9, no. 7: 387. https://doi.org/10.3390/batteries9070387




