High-Performance Anodes Made of Metallic Lithium Layers and Lithiated Silicon Layers Prepared by Vacuum Technologies
Abstract
:1. Introduction
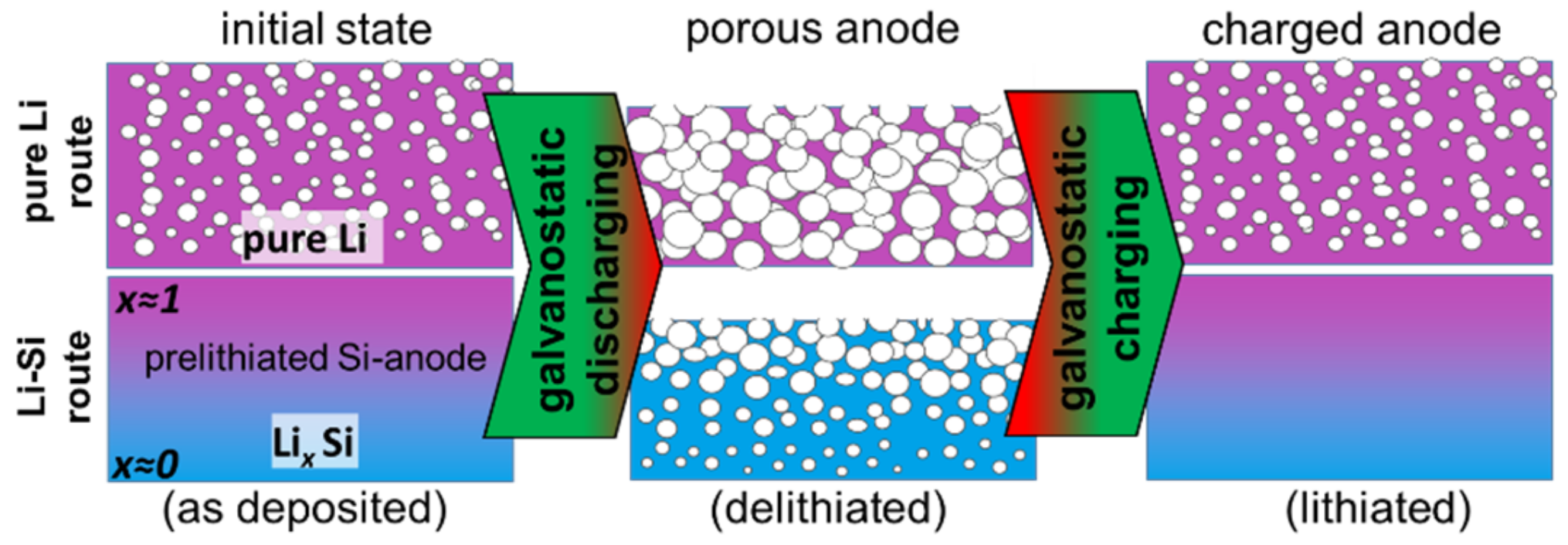
- Pure Li route: Lithium thin films with a porous morphology are deposited in a special manner by applying an adequate plasma pre-treatment prior to the coating process.
- Li-Si route: Lithiated Si layers with varying compositions in the growth direction are prepared.
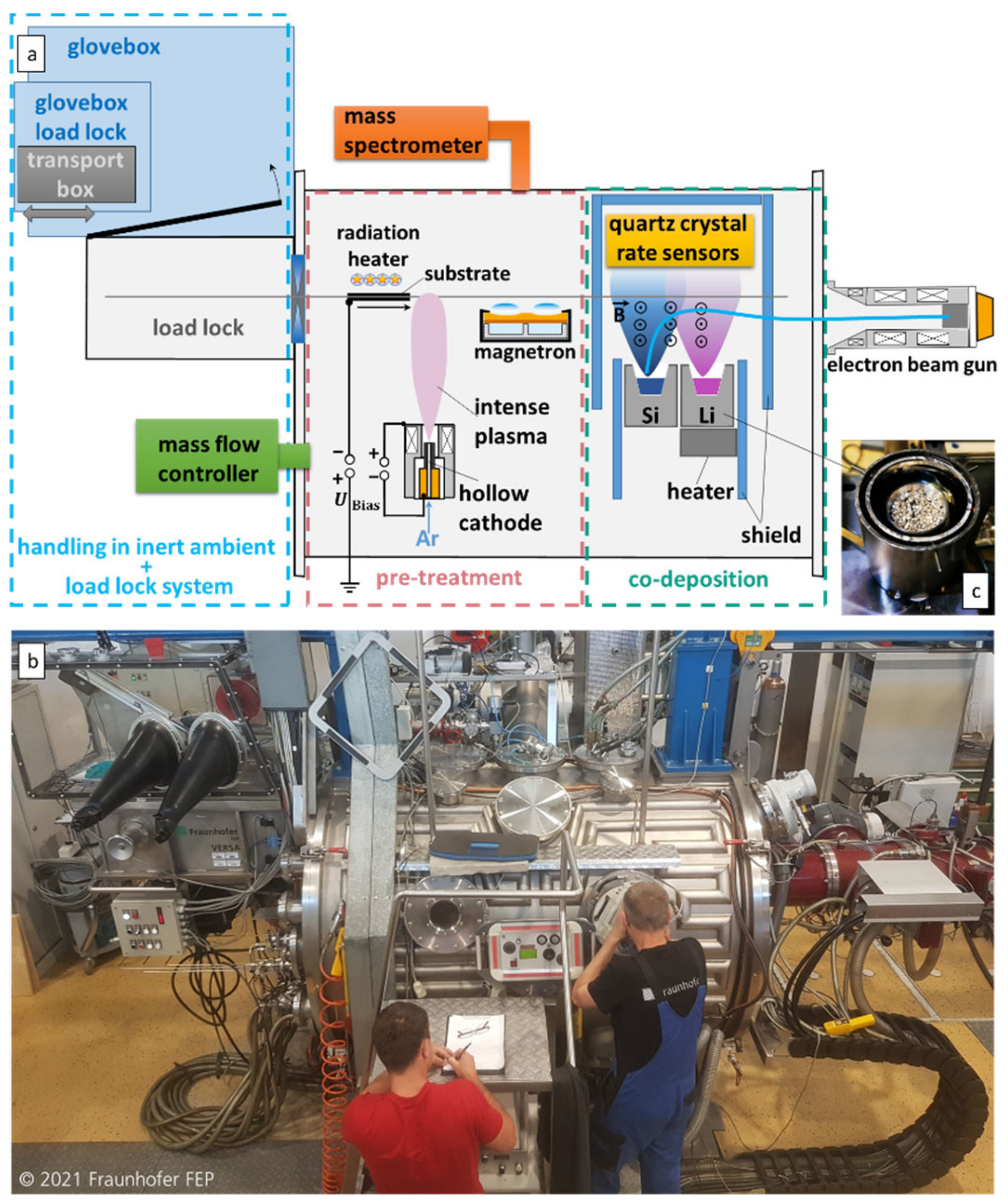
2. Materials and Methods
2.1. Coating Preparation
2.2. Characterization
3. Results
3.1. Substrate Pre-Treatment
3.2. Deposition of Pure Lithium Layers
3.2.1. Lithium Deposition Characteristics
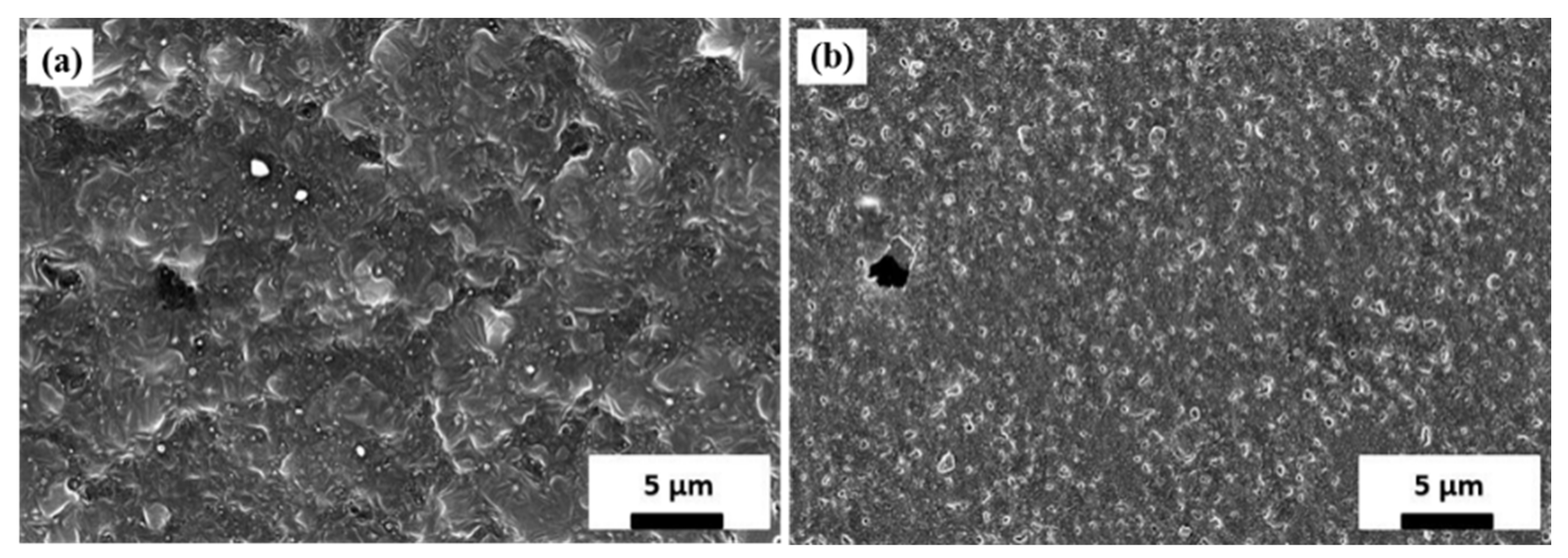
3.2.2. Lithium Layer Morphology
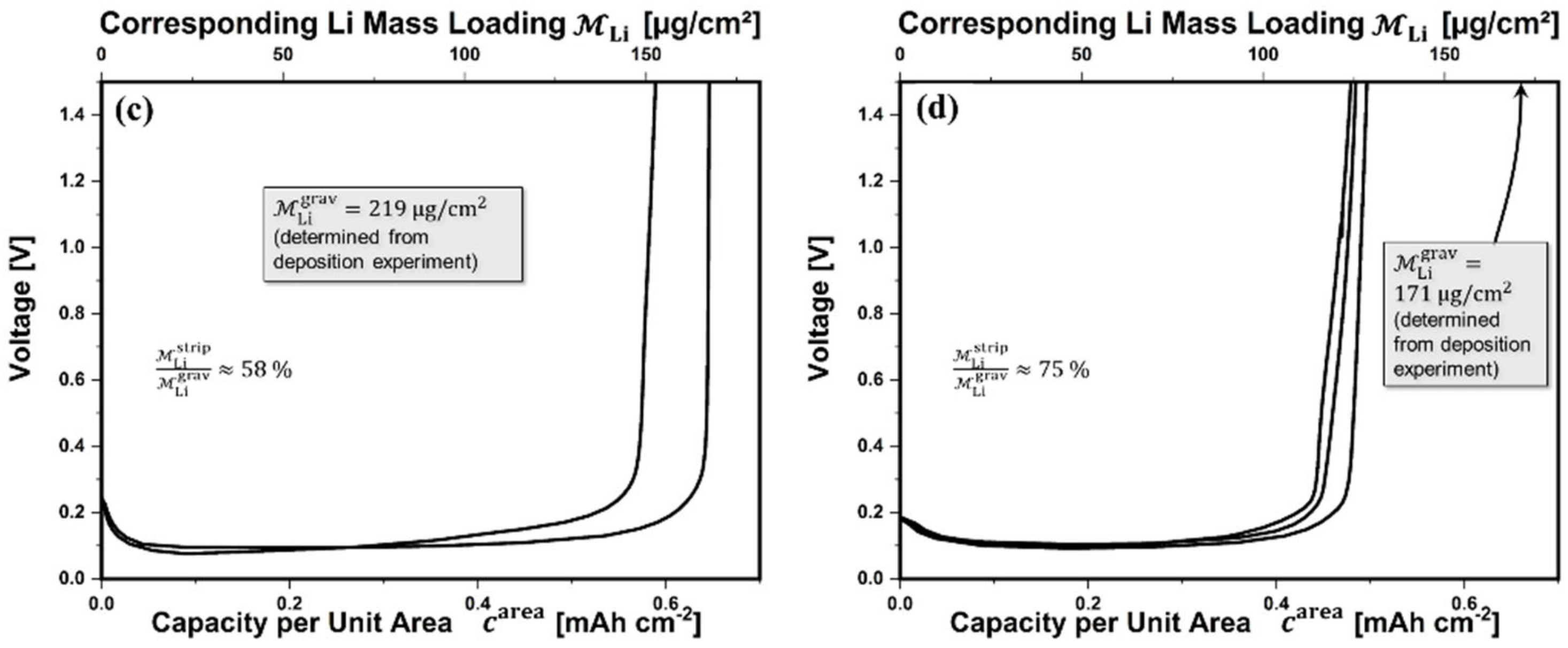
3.2.3. Electrochemical Properties of Pure Lithium Layers
3.3. Deposition of Li-Si Compound Layers
3.3.1. Li-Si Deposition Characteristics
3.3.2. Li-Si Composition
3.3.3. Heat Load to Substrate
4. Discussion
4.1. Substrate Pre-Treatment
4.2. Lithium Coating Process
4.3. Substrate Temperature Regime
4.4. Electrochemical Performance
4.5. General Consideration of Vacuum Processing and Upscaling Potential
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations and Symbols
| Abbreviations | |
| EB-PVD | Electron beam-physical vapor deposition |
| FEP | Fraunhofer Institute for Organic Electronics, Electron Beam and Plasma Technology FEP |
| IWS | Fraunhofer Institute for Material and Beam Technology IWS |
| PET | Polyethylene terephthalate |
| PVD | Physical vapor deposition |
| SEM | Scanning electron microscope |
| Symbols | |
| Vapor-emitting surface at evaporation crucible | |
| Processed substrate area | |
| Parasitic heat ratio | |
| Evaporation coefficient (ideal: | |
| Electrochemical capacity per unit area of the electrode | |
| Theoretical specific gravimetric capacity of lithium | |
| Substrate’s specific heat capacity | |
| Substrate thickness | |
| Specific enthalpy of condensation | |
| Specific enthalpy of fusion | |
| Vertical distance between vapor source and substrate | |
| Angle of vapor propagation | |
| Molecular weight of the evaporant | |
| Mass loading | |
| Mass loading determined by weight difference | |
| Mass loading determined by stripping experiments | |
| Evaporation rate | |
| Substrate mass | |
| Exponent in the characteristic of vapor flux density | |
| Pressure within the vacuum vessel | |
| Saturated vapor pressure at a temperature | |
| Heat flux density of deposition | |
| Heat flux density to the substrate | |
| Universal gas constant | |
| ,, | Deposition rate vertically above vapor source |
| Distribution of deposition rate in lateral direction | |
| Thickness of material removal after pre-treatment | |
| Mass density | |
| Substrate material density | |
| Li volume concentration in layer | |
| Absolute temperature of the evaporant | |
| Substrate temperature | |
| Process time | |
| Vapor transmission coefficient (ideal: | |
| Bias voltage applied to plasma source | |
| Substrate moving velocity during process | |
| Relative substrate position along movement direction | |
| Lateral vapor source positions | |
| Ø | Diameter of copper disc assembled to coin cells |
References
- Koerver, R.; Zhang, W.; Biasi, L.d.; Schweidler, S.; Kondrakov, A.O.; Kolling, S.; Brezesinski, T.; Hartmann, P.; Zeier, W.G.; Janek, J. Chemo-mechanical expansion of lithium electrode materials—On the route to mechanically optimized all-solid-state batteries. Energy Environ. Sci. 2018, 11, 2142–2158. [Google Scholar] [CrossRef]
- Li, J.; Dozier, A.K.; Li, Y.; Yang, F.; Cheng, Y.-T. Crack Pattern Formation in Thin Film Lithium-Ion Battery Electrodes. J. Electrochem. Soc. 2011, 158, A689. [Google Scholar] [CrossRef]
- Sun, L.; Wang, X.; Susantyoko, R.A.; Zhang, Q. Copper–silicon core–shell nanotube arrays for free-standing lithium ion battery anodes. J. Mater. Chem. A 2014, 2, 15294. [Google Scholar] [CrossRef]
- Si, Q.; Hanai, K.; Imanishi, N.; Kubo, M.; Hirano, A.; Takeda, Y.; Yamamoto, O. Highly reversible carbon–nano-silicon composite anodes for lithium rechargeable batteries. J. Power Sources 2009, 189, 761–765. [Google Scholar] [CrossRef]
- Choi, J.W.; Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 2016, 1, 16013. [Google Scholar] [CrossRef] [Green Version]
- Chan, C.K.; Peng, H.; Liu, G.; McIlwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 2008, 3, 31–35. [Google Scholar] [CrossRef]
- Kim, Y.-L.; Sun, Y.-K.; Lee, S.-M. Enhanced electrochemical performance of silicon-based anode material by using current collector with modified surface morphology. Electrochim. Acta 2008, 53, 4500–4504. [Google Scholar] [CrossRef]
- Quiroga-González, E.; Carstensen, J.; Föll, H. Good cycling performance of high-density arrays of Si microwires as anodes for Li ion batteries. Electrochim. Acta 2013, 101, 93–98. [Google Scholar] [CrossRef]
- Saager, S.; Scheffel, B.; Zywitzki, O.; Modes, T.; Piwko, M.; Doerfler, S.; Althues, H.; Metzner, C. Porous silicon thin films as anodes for lithium ion batteries deposited by co-evaporation of silicon and zinc. Surf. Coat. Technol. 2019, 358, 586–593. [Google Scholar] [CrossRef]
- Saager, S.; Scheffel, B.; Modes, T.; Zywitzki, O. Synthesis of porous silicon, nickel and carbon layers by vapor phase dealloying. Surf. Coat. Technol. 2021, 427, 127812. [Google Scholar] [CrossRef]
- Uxa, D.; Hüger, E.; Dörrer, L.; Schmidt, H. Lithium-Silicon Compounds as Electrode Material for Lithium-Ion Batteries. J. Electrochem. Soc. 2020, 167, 130522. [Google Scholar] [CrossRef]
- Strauß, F.; Hüger, E.; Heitjans, P.; Trouillet, V.; Bruns, M.; Schmidt, H. Li–Si thin films for battery applications produced by ion-beam co-sputtering. RSC Adv. 2014, 5, 7192–7195. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y. Overview of the Recent Progress of Suppressing the Dendritic Growth on Lithium Metal Anode for Rechargeable Batteries. J. Phys. Conf. Ser. 2022, 2152, 12060. [Google Scholar] [CrossRef]
- Ren, Y.; Ma, J. Metal Electrodes for Battery Technologies; IOP Publishing: Bristol, UK, 2021. [Google Scholar]
- Seong, I.W.; Hong, C.H.; Kim, B.K.; Yoon, W.Y. The effects of current density and amount of discharge on dendrite formation in the lithium powder anode electrode. J. Power Sources 2008, 178, 769–773. [Google Scholar] [CrossRef]
- Kühnle, H.; Knobbe, E.; Figgemeier, E. In Situ Optical and Electrochemical Investigations of Lithium Depositions as a Function of Current Densities. J. Electrochem. Soc. 2022, 169, 40528. [Google Scholar] [CrossRef]
- Zhang, R.; Li, N.-W.; Cheng, X.-B.; Yin, Y.-X.; Zhang, Q.; Guo, Y.-G. Advanced Micro/Nanostructures for Lithium Metal Anodes. Adv. Sci. 2017, 4, 1600445. [Google Scholar] [CrossRef]
- Cai, Y.; Qin, B.; Lin, J.; Li, C.; Si, X.; Cao, J.; Qi, J. Self-Assembly Lightweight Honeycomb-Like Prussian Blue Analogue on Cu Foam for Lithium Metal Anode. ACS Appl. Mater. Interfaces 2021, 13, 23803–23810. [Google Scholar] [CrossRef]
- Eom, J.-Y.; Choi, S.H.; Kang, J.-H.; Eom, G.H.; Moon, J.; Park, M.-S. Rational Design of a 3D Li-Metal Electrode for High-Energy Lithium Batteries. ACS Appl. Energy Mater. 2021, 4, 1936–1941. [Google Scholar] [CrossRef]
- Yang, T.; Sun, Y.; Qian, T.; Liu, J.; Liu, X.; Rosei, F.; Yan, C. Lithium dendrite inhibition via 3D porous lithium metal anode accompanied by inherent SEI layer. Energy Storage Mater. 2020, 26, 385–390. [Google Scholar] [CrossRef]
- Ho, A.S.; Westover, A.S.; Browning, K.; Maslyn, J.A.; Parkinson, D.Y.; Sahore, R.; Dudney, N.; Balsara, N.P. Comparing the Purity of Rolled versus Evaporated Lithium Metal Films Using X-ray Microtomography. ACS Energy Lett. 2022, 7, 1120–1124. [Google Scholar] [CrossRef]
- Schönherr, K.; Schumm, B.; Hippauf, F.; Lissy, R.; Althues, H.; Leyens, C.; Kaskel, S. Liquid lithium metal processing into ultrathin metal anodes for solid state batteries. Chem. Eng. J. Adv. 2022, 9, 100218. [Google Scholar] [CrossRef]
- Swisher, R.; Yadin, E.; Pipkevich, G. Progress in Vacuum Deposited Lithium Metal Anode Structures. In Proceedings of the 18th International Seminar & Exhibit on Primary and Secondary Batteries, Ft. Landerdale, FL, USA, 5 March 2001. [Google Scholar]
- Swisher, R.; Yadin, E.; Pipkevich, G. Web Coating with Lithium—Technical Solution for Metal Anode Structures in Li Batteries. In Proceedings of the 45th Proceedings of the Annual Technical Conference—Society of Vacuum Coaters, Albuquerque, NM, USA, 23–28 June 2002; pp. 535–538. [Google Scholar]
- Schiller, S.; Goedicke, K.; Metzner, C. Plasma-activated high-rate electron-beam evaporation for coating metal strips. Mater. Sci. Eng. A 1993, 163, 149–156. [Google Scholar] [CrossRef]
- Kersten, H.; Deutsch, H.; Steffen, H.; Kroesen, G.; Hippler, R. The energy balance at substrate surfaces during plasma processing. Vacuum 2001, 63, 385–431. [Google Scholar] [CrossRef]
- Heinß, J.-P.; Klose, L. High-Rate Sputter Etching of Substrates Using Hollow-Cathode Arc Discharge Sources. In Proceedings of the 65th Annual Technical Conference Proceedings—Society of Vacuum Coaters, Los Angeles, CA, USA, 30 April–5 May 2022. [Google Scholar] [CrossRef]
- Holland, L. Vacuum Deposition of Thin Films; Chapman and Hall: London, UK, 1963. [Google Scholar]
- Schiller, S.; Ullrich, H.; Panzer, S. Electron Beam Technology; Verlag Technik GmbH: Berlin, Germany, 1995. [Google Scholar]
- Langmuir, I. The Vapor Pressure of Metallic Tungsten. Phys. Rev. 1913, 2, 329. [Google Scholar] [CrossRef]
- Saager, S.; Scheffel, B.; Heinß, J.-P. High-rate deposition of high-pure silicon thin films for PV-Absorber layers by crucible-free electron beam physical vapor deposition. Surf. Coat. Technol. 2019, 378, 125019. [Google Scholar] [CrossRef]
- Desai, P.D. Thermodynamic Properties of Iron and Silicon. J. Phys. Chem. Ref. Data 1986, 15, 967–983. [Google Scholar] [CrossRef]
- Jeppson, D.W.; Ballif, J.L.; Yuan, W.W.; Chou, B.E. Lithium Literature Review: Lithium’s Properties and Interactions; HEDL-TME-78-15; Hanford Engineering Development Lab.: Richland, WA, USA, 1978. Available online: https://www.osti.gov/biblio/6885395-lithium-literature-review-lithium-properties-interactions (accessed on 14 April 2021).
- Zulehner, W.; Neuer, B.; Rau, G. Silicon. In Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2000. [Google Scholar] [CrossRef]
- Mattox, D.M. Handbook of Physical Vapor Deposition (PVD) Processing, 1st ed.; Noyes Publications: Saddle River, NJ, USA, 1998. [Google Scholar]
- Nix, F.C.; MacNair, D. The Thermal Expansion of Pure Metals: Copper, Gold, Aluminum, Nickel, and Iron. Phys. Rev. 1941, 60, 597–605. [Google Scholar] [CrossRef]
- Winter, M.; University of Sheffield and WebElements Ltd. WebElements Periodic Table Lithium Physical Properties. Available online: https://web.archive.org/web/20220721070335/https://www.webelements.com/lithium/physics.html (accessed on 21 July 2022).
- Heinß, J.-P.; Pfefferling, B.; Saager, S.; Temmler, D. High Purity Deposition of Silicon Layers with Rates ≥300 nm/s. In Proceedings of the 31st European Photovoltaic Solar Energy Conference and Exhibition, Hamburg, Germany, 14–18 September 2015; pp. 1022–1025. [Google Scholar] [CrossRef]
- Huang, Z.; Zhou, G.; Lv, W.; Deng, Y.; Zhang, Y.; Zhang, C.; Kang, F.; Yang, Q.-H. Seeding lithium seeds towards uniform lithium deposition for stable lithium metal anodes. Nano Energy 2019, 61, 47–53. [Google Scholar] [CrossRef]
- Adhitama, E.; Dias Brandao, F.; Dienwiebel, I.; Bela, M.M.; Javed, A.; Haneke, L.; Stan, M.C.; Winter, M.; Gomez-Martin, A.; Placke, T. Pre-Lithiation of Silicon Anodes by Thermal Evaporation of Lithium for Boosting the Energy Density of Lithium Ion Cells. Adv. Funct. Mater. 2022, 32, 2201455. [Google Scholar] [CrossRef]
- Kurt, J.; Lesker Company. Lithium Li Evaporation Process Notes. Available online: https://web.archive.org/web/20220721064742/https://www.lesker.com/newweb/deposition_materials/deposition-materials-notes.cfm?pgid=li1 (accessed on 21 July 2022).
- Vanleeuw, D.; Sapundjiev, D.; Sibbens, G.; Oberstedt, S.; Salvador Castiñeira, P. Physical vapour deposition of metallic lithium. J. Radioanal. Nucl. Chem. 2014, 299, 1113–1120. [Google Scholar] [CrossRef]
- Jahnke, A.; Boger, R.; Götze, T. Method for Refilling an Evaporation Chamber. Google Patents EP 2369033A1, 26 March 2010. [Google Scholar]
- Heinß, J.-P.; Lang, P.; Ruppelt, P. Temperature control of metal strip during high-rate vacuum coating. Surf. Coat. Technol. 2016, 290, 39–42. [Google Scholar] [CrossRef]
- Chen, K.-H.; Wood, K.N.; Kazyak, E.; LePage, W.S.; Davis, A.L.; Sanchez, A.J.; Dasgupta, N.P. Dead lithium: Mass transport effects on voltage, capacity, and failure of lithium metal anodes. J. Mater. Chem. A 2017, 5, 11671–11681. [Google Scholar] [CrossRef]
- Affinito, J.D.; McCann, M.J.; Sheehan, C. Thermal Evaporation of Li: Experiment and a Novel Thermodynamic Model. In Proceedings of the 14th International Conference on Vacuum Web Coating; 2000; pp. 77–94. Available online: https://www.researchgate.net/publication/313139367_Thermal_evaporation_of_Li_experiment_and_a_novel_thermodynamic_model (accessed on 21 July 2022).
- Affinito, J.; McCann, M.; Sheehan, C.; Bullock, S. Web Substrate Heating and a Thermodynamic Calculation Method for Li Film Thickness in a Thermal Evaporation System. In Proceedings of the 44th Annual Technical Conference Proceedings—Society of Vacuum Coaters, Philadelphia, PA, USA, 21–26 April 2001; pp. 492–497. [Google Scholar]





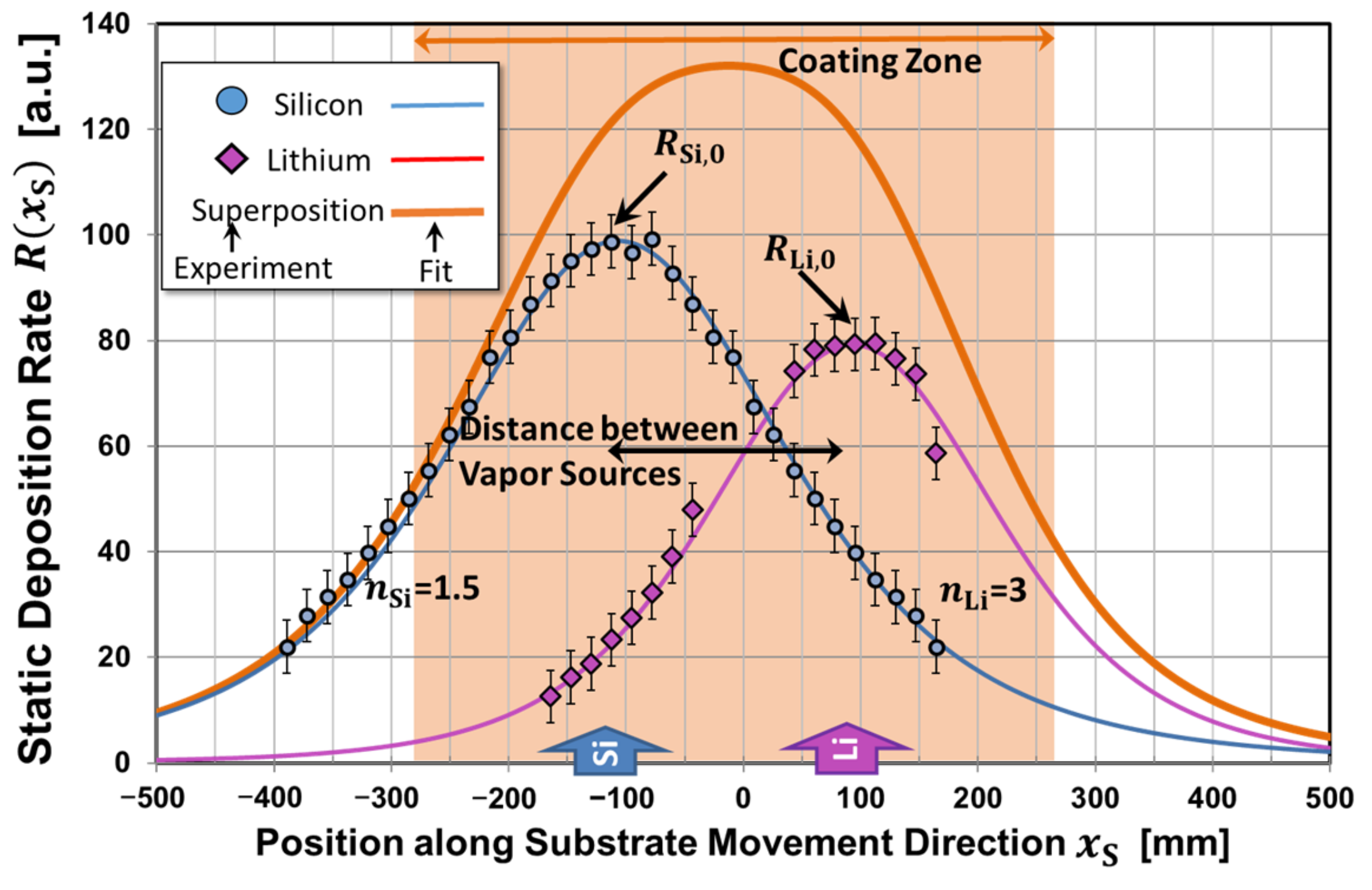
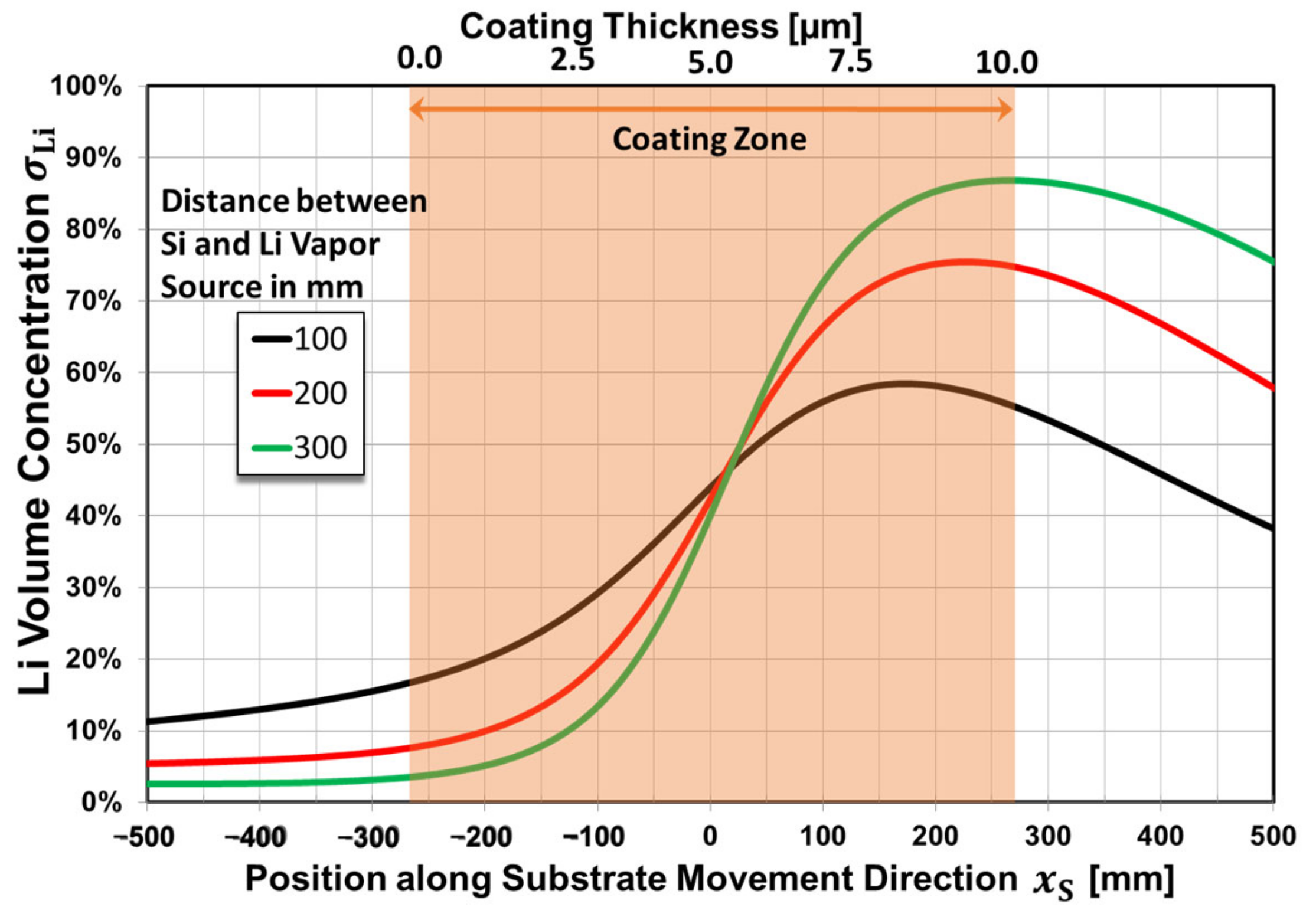
| Substrate Material | Without Bias | |
|---|---|---|
| Stainless steel | ||
| Copper |
| Coating Material | |||
|---|---|---|---|
| lithium | 1.38 | ||
| silicon | 5.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saager, S.; Decker, L.; Kopte, T.; Scheffel, B.; Zimmermann, B. High-Performance Anodes Made of Metallic Lithium Layers and Lithiated Silicon Layers Prepared by Vacuum Technologies. Batteries 2023, 9, 75. https://doi.org/10.3390/batteries9020075
Saager S, Decker L, Kopte T, Scheffel B, Zimmermann B. High-Performance Anodes Made of Metallic Lithium Layers and Lithiated Silicon Layers Prepared by Vacuum Technologies. Batteries. 2023; 9(2):75. https://doi.org/10.3390/batteries9020075
Chicago/Turabian StyleSaager, Stefan, Ludwig Decker, Torsten Kopte, Bert Scheffel, and Burkhard Zimmermann. 2023. "High-Performance Anodes Made of Metallic Lithium Layers and Lithiated Silicon Layers Prepared by Vacuum Technologies" Batteries 9, no. 2: 75. https://doi.org/10.3390/batteries9020075







