The Review of Existing Strategies of End-of-Life Graphite Anode Processing Using 3Rs Approach: Recovery, Recycle, Reuse
Abstract
:1. Introduction
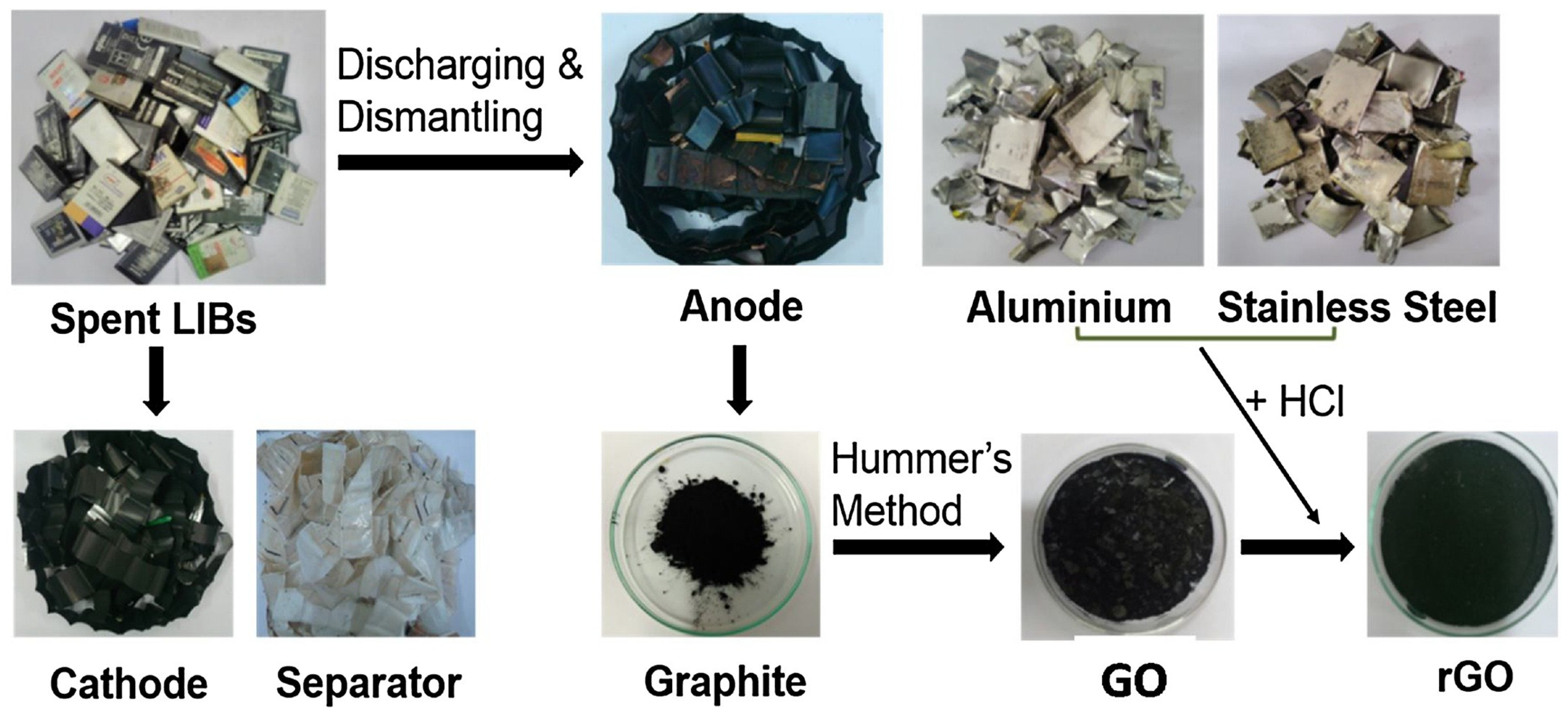
2. Graphite Separation from End-of-Life LIBs
3. Recovery
4. Recycle
5. Reuse
5.1. Adsorbents
5.2. Capacitors
5.3. Catalysts
5.4. Graphene
5.5. Other Types of the Rechargeable Batteries
6. Challenges in the Recovery of Spent Graphite Anodes
- Complex composition—Spent graphite anodes from lithium-ion batteries (LIBs) often have a complex composition, including various impurities, lithium salts, and binders. Removing and separating these components to obtain pure graphite can be a complex and energy-intensive process;
- Environmental impact—Many traditional recovery methods involve the use of strong acids, high-temperature processes, or other chemical treatments. These methods can have negative environmental impacts, including the generation of hazardous waste and emissions;
- Energy consumption—Some recovery methods, such as high-temperature pyrometallurgy, can be energy-intensive. Energy consumption is a concern both in terms of environmental impact and cost-effectiveness;
- Purity requirements—The level of purity required for the recovered graphite depends on the intended application. Meeting high-purity standards, particularly for LIBs, can be challenging and may require more extensive and resource-intensive recovery processes;
- Impurities—Some impurities, such as LiF and ROCO2Li, are challenging to remove using standard recovery methods, leading to the need for more advanced and complex techniques;
- Safety concerns—Handling strong acids and other chemicals in the recovery process can pose safety risks for workers, and the disposal of chemical waste must be managed carefully;
- Energy storage requirements—The recovered graphite’s electrochemical performance, including its capacity and cycling stability, may not match that of newly manufactured graphite. Achieving the same electrochemical properties can be challenging;
- Resource efficiency—Balancing the use of resources (both energy and materials) with the desired level of recovery and purity is an ongoing challenge. Maximizing resource efficiency while achieving acceptable purity levels is critical;
- Recycling infrastructure—Establishing a recycling infrastructure that can efficiently process and recover spent graphite from a growing number of end-of-life batteries is a logistical challenge;
- Cost-effectiveness—Finding cost-effective recovery methods that balance the expenses associated with recovery and recycling against the potential value of the recovered materials is crucial;
- Environmental regulations—Meeting environmental regulations and sustainability goals while recovering and recycling graphite materials is a significant challenge, particularly as regulations may become more stringent.
7. Conclusions and Promising Prospects for Utilizing Spent Graphite-Based Functional Materials
- Energy storage systems—Spent graphite can be processed and modified to create high-performance anode materials for the energy storage systems of the future. Advanced treatments and engineering techniques can enhance the electrochemical properties of recycled graphite, allowing it to store and release energy efficiently. These recycled materials could lead to the development of cost-effective and sustainable energy storage solutions, supporting the growing demand for renewable energy integration and grid stability;
- Supercapacitors—Spent graphite-based materials can find applications in supercapacitors, offering rapid charge–discharge capabilities and extended cycling stability. The unique features of graphite, such as its high surface area and electrical conductivity, make it an ideal candidate for supercapacitor electrodes. Recycling graphite for supercapacitors can enhance their energy storage performance while reducing the need for virgin graphite production;
- Advanced composite materials—The incorporation of spent graphite into composite materials can lead to the development of lightweight and high-strength materials. Graphite’s mechanical stability and electrical conductivity can enhance the properties of composites used in aerospace, automotive, and construction industries, reducing the reliance on virgin graphite and promoting sustainability in material production;
- Environmental remediation—Spent graphite can serve as an effective adsorbent for environmental remediation purposes. Its porous structure and affinity for various pollutants make it suitable for the removal of metals, organic contaminants, and hazardous chemicals from water and air. By repurposing spent graphite in environmental applications, we can contribute to cleaner ecosystems and mitigate pollution;
- Electrochemical sensors—Recycled graphite can be tailored for use in electrochemical sensors and analytical devices. Its electrochemical activity, coupled with surface modification techniques, can enable the sensitive detection of analytes, paving the way for improved sensing technologies in fields such as healthcare, environmental monitoring, and diagnostics;
- Thermal management—The high thermal conductivity of graphite makes it valuable in thermal management applications. Spent graphite-based materials can be incorporated into thermal interface materials, heat sinks, and cooling solutions for electronics and electric vehicle batteries, enhancing heat dissipation and system efficiency;
- Construction and infrastructure—Recycled graphite can be employed in construction materials, such as concrete additives and coatings, to improve durability and reduce carbon emissions. Its inclusion can enhance the overall performance and sustainability of infrastructure projects.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Zhang, R.; Wang, J.; Wang, Y. Current and Future Lithium-Ion Battery Manufacturing. iScience 2021, 24, 102332. [Google Scholar] [CrossRef] [PubMed]
- Heid, B.; Kane, S.; Schaufuss, P. Powering Up Sustainable Energy: Building a More Sustainable Battery Industry. McKinsey Quarterly. 2020. Available online: https://www.mckinsey.com/capabilities/sustainability/our-insights/powering-up-sustainable-energy#section-header-3 (accessed on 15 August 2023).
- Yu, W.; Guo, Y.; Shang, Z.; Zhang, Y.; Xu, S. A Review on Comprehensive Recycling of Spent Power Lithium-Ion Battery in China. eTransportation 2022, 11, 100155. [Google Scholar] [CrossRef]
- Natarajan, S.; Aravindan, V. Recycling Strategies for Spent Li-Ion Battery Mixed Cathodes. ACS Energy Lett. 2018, 3, 2101–2103. [Google Scholar] [CrossRef]
- Wu, X.-L.; Xu, H.-Y. Advances and Challenges on Recycling the Electrode and Electrolyte Materials in Spent Lithium-Ion Batteries. Mater. Lab 2022, 1, 220036. [Google Scholar] [CrossRef]
- Cheng, H.; Shapter, J.G.; Li, Y.; Gao, G. Recent Progress of Advanced Anode Materials of Lithium-Ion Batteries. J. Energy Chem. 2021, 57, 451–468. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Ren, D.; Wang, L.; He, X. Graphite as Anode Materials: Fundamental Mechanism, Recent Progress and Advances. Energy Storage Mater. 2021, 36, 147–170. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhao, H.; Shen, Y.; Li, L.; Rao, Z.; Shao, G.; Lei, Y. Recycling of Graphite Anode from Spent Lithium-ion Batteries: Advances and Perspectives. EcoMat 2023, 5. [Google Scholar] [CrossRef]
- Abdollahifar, M.; Doose, S.; Cavers, H.; Kwade, A. Graphite Recycling from End-of-Life Lithium-Ion Batteries: Processes and Applications. Adv. Mater. Technol. 2023, 8, 2200368. [Google Scholar] [CrossRef]
- Fubao. Available online: http://battery.f139.com/ (accessed on 10 October 2023).
- Arshad, F.; Li, L.; Amin, K.; Fan, E.; Manurkar, N.; Ahmad, A.; Yang, J.; Wu, F.; Chen, R. A Comprehensive Review of the Advancement in Recycling the Anode and Electrolyte from Spent Lithium Ion Batteries. ACS Sustain. Chem. Eng. 2020, 8, 13527–13554. [Google Scholar] [CrossRef]
- Surovtseva, D.; Crossin, E.; Pell, R.; Stamford, L. Toward a Life Cycle Inventory for Graphite Production. J. Ind. Ecol. 2022, 26, 964–979. [Google Scholar] [CrossRef]
- Pinegar, H.; Smith, Y.R. Recycling of End-of-Life Lithium Ion Batteries, Part I: Commercial Processes. J. Sustain. Metall. 2019, 5, 402–416. [Google Scholar] [CrossRef]
- Liu, K.; Yang, S.; Luo, L.; Pan, Q.; Zhang, P.; Huang, Y.; Zheng, F.; Wang, H.; Li, Q. From Spent Graphite to Recycle Graphite Anode for High-Performance Lithium Ion Batteries and Sodium Ion Batteries. Electrochim. Acta 2020, 356, 136856. [Google Scholar] [CrossRef]
- Wang, H.; Huang, Y.; Huang, C.; Wang, X.; Wang, K.; Chen, H.; Liu, S.; Wu, Y.; Xu, K.; Li, W. Reclaiming Graphite from Spent Lithium Ion Batteries Ecologically and Economically. Electrochim. Acta 2019, 313, 423–431. [Google Scholar] [CrossRef]
- Natarajan, S.; Boricha, A.B.; Bajaj, H.C. Recovery of Value-Added Products from Cathode and Anode Material of Spent Lithium-Ion Batteries. Waste Manag. 2018, 77, 455–465. [Google Scholar] [CrossRef]
- Yang, L.; Yang, L.; Xu, G.; Feng, Q.; Li, Y.; Zhao, E.; Ma, J.; Fan, S.; Li, X. Separation and Recovery of Carbon Powder in Anodes from Spent Lithium-Ion Batteries to Synthesize Graphene. Sci. Rep. 2019, 9, 9823. [Google Scholar] [CrossRef]
- Niu, B.; Xiao, J.; Xu, Z. Advances and Challenges in Anode Graphite Recycling from Spent Lithium-Ion Batteries. J. Hazard. Mater. 2022, 439, 129678. [Google Scholar] [CrossRef]
- Wu, M.; Liao, J.; Yu, L.; Lv, R.; Li, P.; Sun, W.; Tan, R.; Duan, X.; Zhang, L.; Li, F.; et al. 2020 Roadmap on Carbon Materials for Energy Storage and Conversion. Chem.—Asian J. 2020, 15, 995–1013. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, N.; Hu, F.; Ye, L.; Xi, Y.; Yang, S. Thermal Treatment and Ammoniacal Leaching for the Recovery of Valuable Metals from Spent Lithium-Ion Batteries. Waste Manag. 2018, 75, 469–476. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, T.; He, Y.; Zhao, Y.; Wang, S.; Zhang, G.; Zhang, Y.; Feng, Y. Recovery of Valuable Materials from Spent Lithium-Ion Batteries by Mechanical Separation and Thermal Treatment. J. Clean. Prod. 2018, 185, 646–652. [Google Scholar] [CrossRef]
- Fu, Y.; He, Y.; Qu, L.; Feng, Y.; Li, J.; Liu, J.; Zhang, G.; Xie, W. Enhancement in Leaching Process of Lithium and Cobalt from Spent Lithium-Ion Batteries Using Benzenesulfonic Acid System. Waste Manag. 2019, 88, 191–199. [Google Scholar] [CrossRef]
- Xiao, J.; Guo, J.; Zhan, L.; Xu, Z. A Cleaner Approach to the Discharge Process of Spent Lithium Ion Batteries in Different Solutions. J. Clean. Prod. 2020, 255, 120064. [Google Scholar] [CrossRef]
- Yao, L.P.; Zeng, Q.; Qi, T.; Li, J. An Environmentally Friendly Discharge Technology to Pretreat Spent Lithium-Ion Batteries. J. Clean. Prod. 2020, 245, 118820. [Google Scholar] [CrossRef]
- Yu, D.; Huang, Z.; Makuza, B.; Guo, X.; Tian, Q. Pretreatment Options for the Recycling of Spent Lithium-Ion Batteries: A Comprehensive Review. Miner. Eng. 2021, 173, 107218. [Google Scholar] [CrossRef]
- Makuza, B.; Tian, Q.; Guo, X.; Chattopadhyay, K.; Yu, D. Pyrometallurgical Options for Recycling Spent Lithium-Ion Batteries: A Comprehensive Review. J. Power Sources 2021, 491, 229622. [Google Scholar] [CrossRef]
- He, Y.; Yuan, X.; Zhang, G.; Wang, H.; Zhang, T.; Xie, W.; Li, L. A Critical Review of Current Technologies for the Liberation of Electrode Materials from Foils in the Recycling Process of Spent Lithium-Ion Batteries. Sci. Total Environ. 2021, 766, 142382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, N.; He, J.; Chen, R.; Li, X. Lithiation-Aided Conversion of End-of-Life Lithium-Ion Battery Anodes to High-Quality Graphene and Graphene Oxide. Nano Lett. 2019, 19, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Zhu, H.; Zu, L.; Bai, Y.; Gao, S.; Gao, Y. A New Model of Trajectory in Eddy Current Separation for Recovering Spent Lithium Iron Phosphate Batteries. Waste Manag. 2019, 100, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Silveira, A.V.M.; Santana, M.P.; Tanabe, E.H.; Bertuol, D.A. Recovery of Valuable Materials from Spent Lithium Ion Batteries Using Electrostatic Separation. Int. J. Miner. Process. 2017, 169, 91–98. [Google Scholar] [CrossRef]
- Widijatmoko, S.D.; Fu, G.; Wang, Z.; Hall, P. Recovering Lithium Cobalt Oxide, Aluminium, and Copper from Spent Lithium-Ion Battery via Attrition Scrubbing. J. Clean. Prod. 2020, 260, 120869. [Google Scholar] [CrossRef]
- Zhang, G.; He, Y.; Wang, H.; Feng, Y.; Xie, W.; Zhu, X. Removal of Organics by Pyrolysis for Enhancing Liberation and Flotation Behavior of Electrode Materials Derived from Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2020, 8, 2205–2214. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, J.; Gan, T.; Lu, D.; Wang, Y.; Zheng, X. High-Intensity Magnetic Separation for Recovery of LiFePO4 and Graphite from Spent Lithium-Ion Batteries. Sep. Purif. Technol. 2022, 297, 121486. [Google Scholar] [CrossRef]
- Mennik, F.; Dinç, N.İ.; Burat, F. Selective Recovery of Metals from Spent Mobile Phone Lithium-Ion Batteries through Froth Flotation Followed by Magnetic Separation Procedure. Results Eng. 2023, 17, 100868. [Google Scholar] [CrossRef]
- Zhan, R.; Yang, Z.; Bloom, I.; Pan, L. Significance of a Solid Electrolyte Interphase on Separation of Anode and Cathode Materials from Spent Li-Ion Batteries by Froth Flotation. ACS Sustain. Chem. Eng. 2021, 9, 531–540. [Google Scholar] [CrossRef]
- He, Y.; Zhang, T.; Wang, F.; Zhang, G.; Zhang, W.; Wang, J. Recovery of LiCoO2 and Graphite from Spent Lithium-Ion Batteries by Fenton Reagent-Assisted Flotation. J. Clean. Prod. 2017, 143, 319–325. [Google Scholar] [CrossRef]
- Yang, Y.; Song, S.; Lei, S.; Sun, W.; Hou, H.; Jiang, F.; Ji, X.; Zhao, W.; Hu, Y. A Process for Combination of Recycling Lithium and Regenerating Graphite from Spent Lithium-Ion Battery. Waste Manag. 2019, 85, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; He, Y.; Wang, H.; Feng, Y.; Xie, W.; Zhu, X. Application of Mechanical Crushing Combined with Pyrolysis-Enhanced Flotation Technology to Recover Graphite and LiCoO2 from Spent Lithium-Ion Batteries. J. Clean. Prod. 2019, 231, 1418–1427. [Google Scholar] [CrossRef]
- Yi, C.; Yang, Y.; Zhang, T.; Wu, X.; Sun, W.; Yi, L. A Green and Facile Approach for Regeneration of Graphite from Spent Lithium Ion Battery. J. Clean. Prod. 2020, 277, 123585. [Google Scholar] [CrossRef]
- Bai, Y.; Li, M.; Jafta, C.J.; Dai, Q.; Essehli, R.; Polzin, B.J.; Belharouak, I. Direct Recycling and Remanufacturing of Anode Scraps. Sustain. Mater. Technol. 2023, 35, e00542. [Google Scholar] [CrossRef]
- Li, P.; Luo, S.; Su, F.; Zhang, L.; Yan, S.; Lei, X.; Mu, W.; Wang, Q.; Zhang, Y.; Liu, X.; et al. Optimization of Synergistic Leaching of Valuable Metals from Spent Lithium-Ion Batteries by the Sulfuric Acid-Malonic Acid System Using Response Surface Methodology. ACS Appl. Mater. Interfaces 2022, 14, 11359–11374. [Google Scholar] [CrossRef]
- Ma, X.; Chen, M.; Chen, B.; Meng, Z.; Wang, Y. High-Performance Graphite Recovered from Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2019, 7, 19732–19738. [Google Scholar] [CrossRef]
- Yang, J.; Fan, E.; Lin, J.; Arshad, F.; Zhang, X.; Wang, H.; Wu, F.; Chen, R.; Li, L. Recovery and Reuse of Anode Graphite from Spent Lithium-Ion Batteries via Citric Acid Leaching. ACS Appl. Energy Mater. 2021, 4, 6261–6268. [Google Scholar] [CrossRef]
- Xin, B.; Zhang, D.; Zhang, X.; Xia, Y.; Wu, F.; Chen, S.; Li, L. Bioleaching Mechanism of Co and Li from Spent Lithium-Ion Battery by the Mixed Culture of Acidophilic Sulfur-Oxidizing and Iron-Oxidizing Bacteria. Bioresour. Technol. 2009, 100, 6163–6169. [Google Scholar] [CrossRef] [PubMed]
- Horeh, N.B.; Mousavi, S.M.; Shojaosadati, S.A. Bioleaching of Valuable Metals from Spent Lithium-Ion Mobile Phone Batteries Using Aspergillus Niger. J. Power Sources 2016, 320, 257–266. [Google Scholar] [CrossRef]
- Bahaloo-Horeh, N.; Mousavi, S.M. Enhanced Recovery of Valuable Metals from Spent Lithium-Ion Batteries through Optimization of Organic Acids Produced by Aspergillus Niger. Waste Manag. 2017, 60, 666–679. [Google Scholar] [CrossRef]
- Yang, K.; Zhao, Z.; Xin, X.; Tian, Z.; Peng, K.; Lai, Y. Graphitic Carbon Materials Extracted from Spent Carbon Cathode of Aluminium Reduction Cell as Anodes for Lithium Ion Batteries: Converting the Hazardous Wastes into Value-Added Materials. J. Taiwan Inst. Chem. Eng. 2019, 104, 201–209. [Google Scholar] [CrossRef]
- Yu, H.; Dai, H.; Zhu, Y.; Hu, H.; Zhao, R.; Wu, B.; Chen, D. Mechanistic Insights into the Lattice Reconfiguration of the Anode Graphite Recycled from Spent High-Power Lithium-Ion Batteries. J. Power Sources 2021, 481, 229159. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, C.; Zhang, J.; Jing, Q.; Ma, B.; Chen, Y.; Zhang, W. Graphite Recycling from the Spent Lithium-Ion Batteries by Sulfuric Acid Curing–Leaching Combined with High-Temperature Calcination. ACS Sustain. Chem. Eng. 2020, 8, 9447–9455. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, J.; Jin, H.; Liang, G.; Ma, L.; Chen, Y.; Wang, C. Regenerating Spent Graphite from Scrapped Lithium-Ion Battery by High-Temperature Treatment. Carbon 2022, 189, 493–502. [Google Scholar] [CrossRef]
- Chen, Q.; Huang, L.; Liu, J.; Luo, Y.; Chen, Y. A New Approach to Regenerate High-Performance Graphite from Spent Lithium-Ion Batteries. Carbon 2022, 189, 293–304. [Google Scholar] [CrossRef]
- Cao, N.; Zhang, Y.; Chen, L.; Chu, W.; Huang, Y.; Jia, Y.; Wang, M. An Innovative Approach to Recover Anode from Spent Lithium-Ion Battery. J. Power Sources 2021, 483, 229163. [Google Scholar] [CrossRef]
- Rothermel, S.; Evertz, M.; Kasnatscheew, J.; Qi, X.; Grützke, M.; Winter, M.; Nowak, S. Graphite Recycling from Spent Lithium-Ion Batteries. ChemSusChem 2016, 9, 3473–3484. [Google Scholar] [CrossRef] [PubMed]
- Yuwen, C.; Liu, B.; Zhang, H.; Tian, S.; Zhang, L.; Guo, S.; Zhou, B. Efficient Recovery and Regeneration of Waste Graphite through Microwave Stripping from Spent Batteries Anode for High-Performance Lithium-Ion Batteries. J. Clean. Prod. 2022, 333, 130197. [Google Scholar] [CrossRef]
- Hou, D.; Guo, Z.; Wang, Y.; Hou, X.; Yi, S.; Zhang, Z.; Hao, S.; Chen, D. Microwave-Assisted Reconstruction of Spent Graphite and Its Enhanced Energy-Storage Performance as LIB Anodes. Surf. Interfaces 2021, 24, 101098. [Google Scholar] [CrossRef]
- Li, Z.; Li, S.; Wang, T.; Yang, K.; Zhou, Y.; Tian, Z. Facile Fabrication of High-Performance Li-Ion Battery Carbonaceous Anode from Li-Ion Battery Waste. J. Electrochem. Soc. 2021, 168, 090513. [Google Scholar] [CrossRef]
- Da, H.; Gan, M.; Jiang, D.; Xing, C.; Zhang, Z.; Fei, L.; Cai, Y.; Zhang, H.; Zhang, S. Epitaxial Regeneration of Spent Graphite Anode Material by an Eco-Friendly In-Depth Purification Route. ACS Sustain. Chem. Eng. 2021, 9, 16192–16202. [Google Scholar] [CrossRef]
- Ruan, D.; Wu, L.; Wang, F.; Du, K.; Zhang, Z.; Zou, K.; Wu, X.; Hu, G. A Low-Cost Silicon-Graphite Anode Made from Recycled Graphite of Spent Lithium-Ion Batteries. J. Electroanal. Chem. 2021, 884, 115073. [Google Scholar] [CrossRef]
- Ma, Z.; Zhuang, Y.; Deng, Y.; Song, X.; Zuo, X.; Xiao, X.; Nan, J. From Spent Graphite to Amorphous Sp2 +sp3 Carbon-Coated Sp2 Graphite for High-Performance Lithium Ion Batteries. J. Power Sources 2018, 376, 91–99. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Song, D.; Miao, Y.; Song, J.; Zhang, L. Effective Regeneration of Anode Material Recycled from Scrapped Li-Ion Batteries. J. Power Sources 2018, 390, 38–44. [Google Scholar] [CrossRef]
- Xu, C.; Ma, G.; Yang, W.; Che, S.; Li, Y.; Jia, Y.; Liu, H.; Chen, F.; Zhang, G.; Liu, H.; et al. One-Step Reconstruction of Acid Treated Spent Graphite for High Capacity and Fast Charging Lithium-Ion Batteries. Electrochim. Acta 2022, 415, 140198. [Google Scholar] [CrossRef]
- Zuo, X.; Zhu, J.; Müller-Buschbaum, P.; Cheng, Y.-J. Silicon Based Lithium-Ion Battery Anodes: A Chronicle Perspective Review. Nano Energy 2017, 31, 113–143. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, Q.; Chen, D.; Zhong, Y.; Wu, Z.; Song, Y.; Wang, G.; Liu, Y.; Zhong, B.; Guo, X. Silicon/Graphite Composite Anode with Constrained Swelling and a Stable Solid Electrolyte Interphase Enabled by Spent Graphite. Green Chem. 2021, 23, 4531–4539. [Google Scholar] [CrossRef]
- Ye, L.; Wang, C.; Cao, L.; Xiao, H.; Zhang, J.; Zhang, B.; Ou, X. Effective Regeneration of High-Performance Anode Material Recycled from the Whole Electrodes in Spent Lithium-Ion Batteries via a Simplified Approach. Green Energy Environ. 2021, 6, 725–733. [Google Scholar] [CrossRef]
- Xiao, Z.; Gao, L.; Su, S.; Li, D.; Cao, L.; Ye, L.; Zhang, B.; Ming, L.; Ou, X. Efficient Fabrication of Metal Sulfides/Graphite Anode Materials Derived from Spent Lithium-Ion Batteries by Gas Sulfidation Process. Mater. Today Energy 2021, 21, 100821. [Google Scholar] [CrossRef]
- Nguyen, T.-V.; Tuong, V.D.; Tran, A.-T.; Truong, T.P.; Lakew, D.S.; Lee, C.; Lee, Y.; Cho, S. User-Preference-Based Proactive Caching in Edge Networks. In Proceedings of the 2021 International Conference on Information Networking (ICOIN), Jeju Island, Republic of Korea, 13–16 January 2021; pp. 755–757. [Google Scholar]
- Zhao, T.; Yao, Y.; Wang, M.; Chen, R.; Yu, Y.; Wu, F.; Zhang, C. Preparation of MnO2-Modified Graphite Sorbents from Spent Li-Ion Batteries for the Treatment of Water Contaminated by Lead, Cadmium, and Silver. ACS Appl. Mater. Interfaces 2017, 9, 25369–25376. [Google Scholar] [CrossRef]
- Hao, J.; Meng, X.; Fang, S.; Cao, H.; Lv, W.; Zheng, X.; Liu, C.; Chen, M.; Sun, Z. MnO2-Functionalized Amorphous Carbon Sorbents from Spent Lithium-Ion Batteries for Highly Efficient Removal of Cadmium from Aqueous Solutions. Ind. Eng. Chem. Res. 2020, 59, 10210–10220. [Google Scholar] [CrossRef]
- Divya, M.L.; Natarajan, S.; Lee, Y.-S.; Aravindan, V. Achieving High-Energy Dual Carbon Li-Ion Capacitors with Unique Low- and High-Temperature Performance from Spent Li-Ion Batteries. J. Mater. Chem. A 2020, 8, 4950–4959. [Google Scholar] [CrossRef]
- Divya, M.L.; Lee, Y.; Aravindan, V. Li-ion Capacitor via Solvent-Co-Intercalation Process from Spent Li-ion Batteries. Batter. Supercaps 2021, 4, 671–679. [Google Scholar] [CrossRef]
- Divya, M.L.; Natarajan, S.; Lee, Y.; Aravindan, V. Highly Reversible Na-Intercalation into Graphite Recovered from Spent Li–Ion Batteries for High-Energy Na-Ion Capacitor. ChemSusChem 2020, 13, 5654–5663. [Google Scholar] [CrossRef] [PubMed]
- Schiavi, P.G.; Altimari, P.; Zanoni, R.; Pagnanelli, F. Full Recycling of Spent Lithium Ion Batteries with Production of Core-Shell Nanowires//Exfoliated Graphite Asymmetric Supercapacitor. J. Energy Chem. 2021, 58, 336–344. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, Y.; Shi, X.; Zhong, Y.; Wu, Z.; Song, Y.; Wang, G.; Liu, Y.; Zhong, B.; Guo, X. The Direct Application of Spent Graphite as a Functional Interlayer with Enhanced Polysulfide Trapping and Catalytic Performance for Li–S Batteries. Green Chem. 2021, 23, 942–950. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, H.; Chen, L.; Chen, C.; Duan, X.; Xie, Y.; Song, W.; Sun, H.; Wang, S. Tailored Synthesis of Active Reduced Graphene Oxides from Waste Graphite: Structural Defects and Pollutant-Dependent Reactive Radicals in Aqueous Organics Decontamination. Appl. Catal. B Environ. 2018, 229, 71–80. [Google Scholar] [CrossRef]
- Guan, J.; Li, Z.; Chen, S.; Gu, W. Zero-Valent Iron Supported on Expanded Graphite from Spent Lithium-Ion Battery Anodes and Ferric Chloride for the Degradation of 4-Chlorophenol in Water. Chemosphere 2022, 290, 133381. [Google Scholar] [CrossRef]
- Chen, S.; Long, F.; Gao, G.; Belver, C.; Li, Z.; Li, Z.; Guan, J.; Guo, Y.; Bedia, J. Zero-Valent Iron-Copper Bimetallic Catalyst Supported on Graphite from Spent Lithium-Ion Battery Anodes and Mill Scale Waste for the Degradation of 4-Chlorophenol in Aqueous Phase. Sep. Purif. Technol. 2022, 286, 120466. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Z.; Xu, C.; He, W.; Li, G.; Huang, J.; Zhu, H. Preparing Graphene Oxide–Copper Composite Material from Spent Lithium Ion Batteries and Catalytic Performance Analysis. Res. Chem. Intermed. 2018, 44, 5075–5089. [Google Scholar] [CrossRef]
- Sun, X.; Huang, C.; Wang, L.; Liang, L.; Cheng, Y.; Fei, W.; Li, Y. Recent Progress in Graphene/Polymer Nanocomposites. Adv. Mater. 2021, 33, 2001105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Zhou, C. Review of Chemical Vapor Deposition of Graphene and Related Applications. Acc. Chem. Res. 2013, 46, 2329–2339. [Google Scholar] [CrossRef] [PubMed]
- Kurian, M. Recent Progress in the Chemical Reduction of Graphene Oxide by Green Reductants–A Mini Review. Carbon Trends 2021, 5, 100120. [Google Scholar] [CrossRef]
- Yi, M.; Shen, Z. A Review on Mechanical Exfoliation for the Scalable Production of Graphene. J. Mater. Chem. A 2015, 3, 11700–11715. [Google Scholar] [CrossRef]
- Liu, F.; Wang, C.; Sui, X.; Riaz, M.A.; Xu, M.; Wei, L.; Chen, Y. Synthesis of Graphene Materials by Electrochemical Exfoliation: Recent Progress and Future Potential. Carbon Energy 2019, 1, 173–199. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Z.; Xia, J.; Li, F.; He, W.; Li, G.; Huang, J. Preparing Graphene from Anode Graphite of Spent Lithium-Ion Batteries. Front. Environ. Sci. Eng. 2017, 11, 6. [Google Scholar] [CrossRef]
- Yu, J.; Lin, M.; Tan, Q.; Li, J. High-Value Utilization of Graphite Electrodes in Spent Lithium-Ion Batteries: From 3D Waste Graphite to 2D Graphene Oxide. J. Hazard. Mater. 2021, 401, 123715. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, X.; Wan, C.; Ye, X.; Wu, F. Soluble Graphene Nanosheets from Recycled Graphite of Spent Lithium Ion Batteries. J. Mater. Eng. Perform. 2018, 27, 875–880. [Google Scholar] [CrossRef]
- Natarajan, S.; Rao Ede, S.; Bajaj, H.C.; Kundu, S. Environmental Benign Synthesis of Reduced Graphene Oxide (RGO) from Spent Lithium-Ion Batteries (LIBs) Graphite and Its Application in Supercapacitor. Colloids Surf. A Physicochem. Eng. Asp. 2018, 543, 98–108. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, Y.; Peng, W.; Li, Y.; Zhang, G.; Zhang, F.; Fan, X. Direct Exfoliation of the Anode Graphite of Used Li-Ion Batteries into Few-Layer Graphene Sheets: A Green and High Yield Route to High-Quality Graphene Preparation. J. Mater. Chem. A 2017, 5, 5880–5885. [Google Scholar] [CrossRef]
- He, K.; Zhang, Z.-Y.; Zhang, F.-S. Synthesis of Graphene and Recovery of Lithium from Lithiated Graphite of Spent Li-Ion Battery. Waste Manag. 2021, 124, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.; Bhattarai, R.M.; Sudhakaran, M.S.P.; Mok, Y.S.; Kim, S.J. Recycling of Spent Graphite and Copper Current Collector for Lithium-Ion and Sodium-Ion Batteries. J. Power Sources 2023, 577, 233170. [Google Scholar] [CrossRef]
- Liang, H.-J.; Hou, B.-H.; Li, W.-H.; Ning, Q.-L.; Yang, X.; Gu, Z.-Y.; Nie, X.-J.; Wang, G.; Wu, X.-L. Staging Na/K-Ion de-/Intercalation of Graphite Retrieved from Spent Li-Ion Batteries: In Operando X-Ray Diffraction Studies and an Advanced Anode Material for Na/K-Ion Batteries. Energy Environ. Sci. 2019, 12, 3575–3584. [Google Scholar] [CrossRef]
- Meng, Y.-F.; Liang, H.-J.; Zhao, C.-D.; Li, W.-H.; Gu, Z.-Y.; Yu, M.-X.; Zhao, B.; Hou, X.-K.; Wu, X.-L. Concurrent Recycling Chemistry for Cathode/Anode in Spent Graphite/LiFePO4 Batteries: Designing a Unique Cation/Anion-Co-Workable Dual-Ion Battery. J. Energy Chem. 2022, 64, 166–171. [Google Scholar] [CrossRef]
- Yang, J.-L.; Zhao, X.-X.; Li, W.-H.; Liang, H.-J.; Gu, Z.-Y.; Liu, Y.; Du, M.; Wu, X.-L. Advanced Cathode for Dual-Ion Batteries: Waste-to-Wealth Reuse of Spent Graphite from Lithium-Ion Batteries. eScience 2022, 2, 95–101. [Google Scholar] [CrossRef]

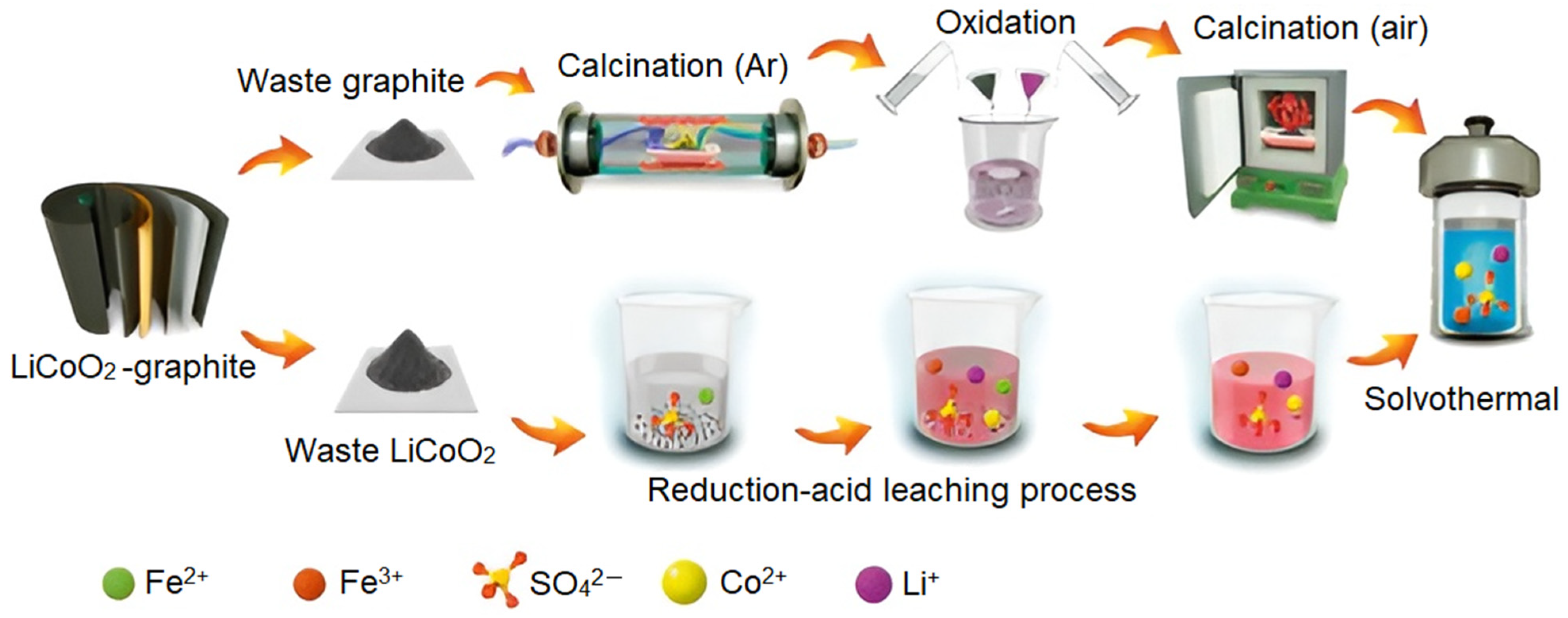
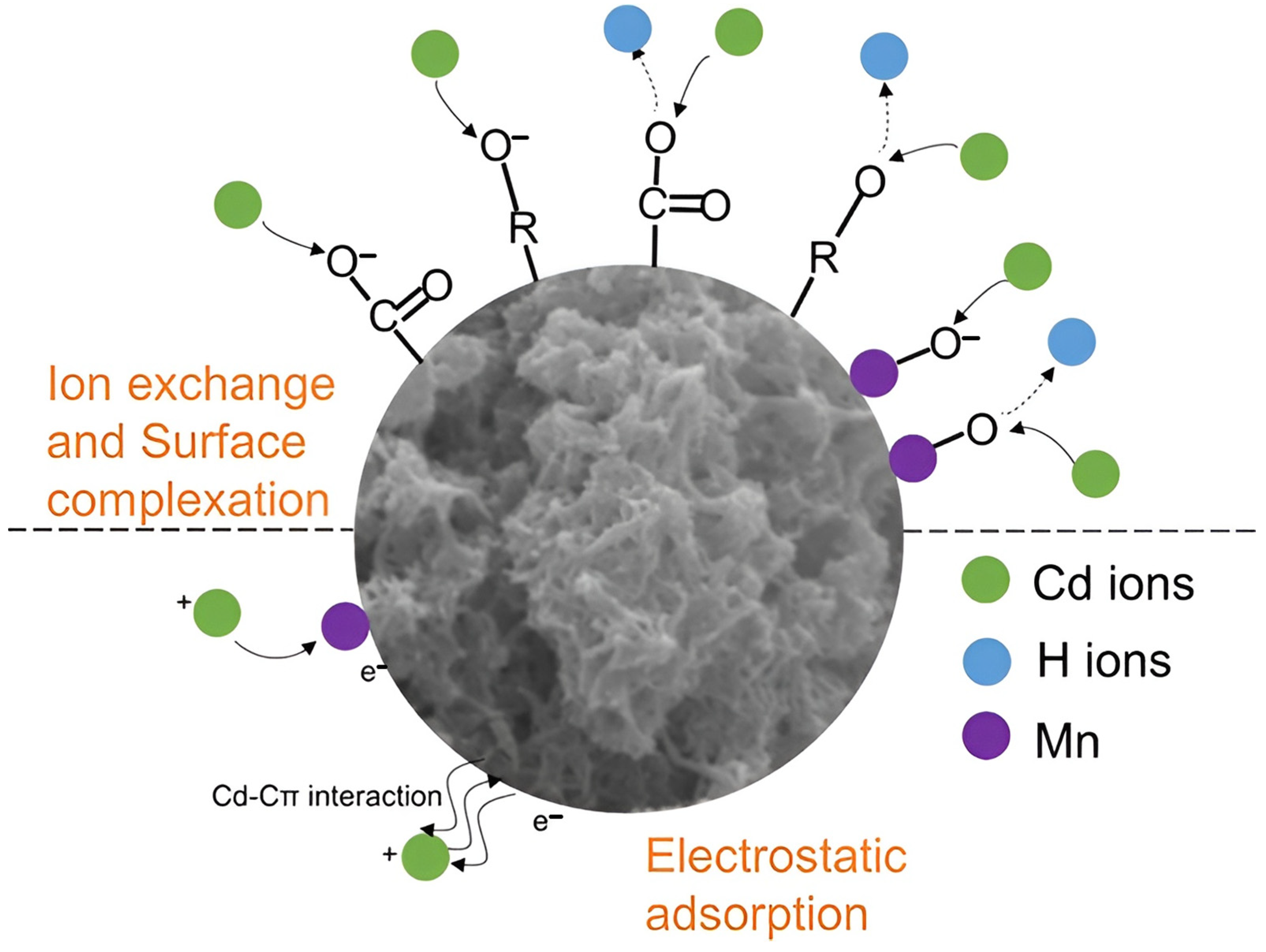


| Recovery Strategy | Advantages | Disadvantages |
|---|---|---|
| Hydrometallurgical strategy with deionized water leaching solution | Effective at removing intercalated lithium and solid electrolyte interface (SEI) layers from graphite waste. Simple and environmentally friendly exothermic reaction with lithium releases gaseous H2, facilitating SEI layer separation from waste graphite anodes. | Limited effectiveness in removing all lithium salts, particularly LiF and ROCO2Li. May not address all impurities present in the spent graphite. |
| Hydrometallurgical strategy with mineral acid leaching solution | Highly effective in removing almost all lithium salts and metallic residue from spent graphite, resulting in high purity of recovered graphite. Improves electrochemical and cycling performance of recovered graphite. Well-established and widely used process. | The use of strong mineral acids can pose environmental and safety concerns. Requires careful handling of chemicals and waste disposal. Energy-intensive due to heating requirements for the process. |
| Hydrometallurgical strategy with sulfuric acid and hydrogen peroxide leaching followed by sodium hydroxide sintering | Effective in extracting metal impurities from both spent cathode and anode materials. Enables the removal of PVDF binders from waste graphite anodes. Induces oxidation and expansion of spent graphite layers, leading to improved electrochemical performance of recovered graphite. | Complex multi-step process with multiple reagents and heating requirements. Potential environmental concerns related to chemical usage and waste disposal. Energy-intensive due to the sintering process. |
| Hydrometallurgical strategy with organic acid leaching solution | Environmentally friendly with the use of organic acids. High lithium extraction efficiency from waste graphite anodes. Lower environmental and safety risks compared to mineral acid leaching solutions. | Requires longer leaching times compared to some other methods. May not remove all impurities from spent graphite anodes. |
| High-temperature pyrometallurgy | Efficient in removing impurities from spent graphite anodes and restoring the crystal structure of graphite. Can improve the electrochemical behavior of recovered graphite. | Requires very high temperatures, which may be energy-intensive. Complex process with inert atmosphere and time considerations. |
| Pyro-hydrometallurgy | Combines acid leaching with high-temperature sintering for high-purity and structural repair of graphite. High-temperature sintering may not be required at extremely high temperatures. Catalytic additives can be used to enhance the recovery process. | Complex multi-step process with multiple reagents and heating requirements. Energy-intensive in some cases. |
| Electrolysis | Efficiently recovers graphite anodes and liberates lithium. Reduces the need for high-temperature processes. Effective in separating graphite from copper foil. Utilizes relatively safe and readily available electrolytes. | May require additional post-processing to obtain high-purity recovered graphite. May not address other impurities present in the spent graphite. |
| Mechanical-hydrometallurgical process with subcritical CO2-assisted electrolyte extraction prior to thermal treatment | Efficient in removing electrolyte salts before heat treatment. Prevents the formation of phosphorous compounds on recovered graphite. | Lowers the crystallinity of the recovered graphite. May require further processing to improve the electrochemical performance of the recovered graphite. |
| Microwave technology | Less energy consumption and shorter reaction times. Efficiently removes electrolyte residue and the binder, recovering nearly 100% of spent graphite. Reconstructs graphite structure, creating open areas for ion diffusion. | May require specialized equipment for microwave irradiation. Possible variations in recovered graphite properties due to the rapid heating process. |
| Process Conditions | Electrochemical Performance | Ref. |
|---|---|---|
| Recovery approach | ||
| 1.5 M hydrochloric acid 60 min ratio of solid/liquid phase—100 g/L | 540–591 mAh·g−1 at 37.2 mA·g−1 485–510 mAh·g−1 at 74.4 mA·g−1 305–335 mAh·g−1 at 186 mA·g−1 165–190 mAh·g−1 at 372 mA·g−1 | [37] |
| 1. 5 mol/L H2SO4, 35 w/w %H2O2 2. Heat treatment: 500 °C, 40 min with addition of NaOH | 377.3 mAh·g−1 at a current rate of 0.1C | [42] |
| 0.2 mol/L citric acid at 90 °C, 50 min | 330 mAh·g−1 at a current rate of 0.5C after 80 cycles | [43] |
| 2600 °C, Ar atmosphere | 263 mAh·g−1 at a current rate of 1C after 300 cycles | [47] |
| 3000 °C, 6 h | 360.8 mAh·g−1 at a current rate of 0.15C after 100 cycles | [48] |
| H2SO4 + 1500 °C for 2 h | 349 mAh·g−1 at 0.1C | [49] |
| H2SO4 + 900 °C | 358.1 mAh·g−1 at a current rate of 0.1C | [50] |
| 1. H2SO4 2. Sintering with Co(NO3)2 at 900 °C for 4 h under N2 | 358 mAh·g−1 at a current rate of 0.1C at 1st cycle 245.4 mAh·g−1 at a current rate of 0.1C after 500 cycles | [51] |
| Electrolysis: 30 V, 1.5 g/L Na2SO4, 25 min | 427.81 mAh·g−1 at a current rate of 0.1C | [52] |
| 1. Leaching sulfuric acid H2SO4, 200 g/L, 90 °C, 4 h 2. Microwave irradiation 800 W, 15 s | ≥400 mAh·g−1 | [54] |
| 1. Water leaching 2. Microwave irradiation 800 W 20–30 s | 438.6 mAh·g−1 at a current rate of 0.1C 320 mAh·g−1 after 100 cycles at a current rate of 0.5C | [55] |
| Recycle approach | ||
| 1. Air calcination 2. Graphite—300 mg Cellulose gum—100 mg C6H12O6—150 mg Conditions: 800 °C, 5 h under nitrogen atmosphere | 424.7 mAh·g−1 at a current rate of 0.1C after 270 cycles | [51] |
| 1. Heat treatment (HT) in air, acid leaching, HT under Ar 2. Graphite and pitch mixture, the weight ratio 9: 1, at 1000 °C, 2 h under nitrogen atmosphere | 325 mAh·g−1 at a current rate of 0.5C after 250 cycles | [57] |
| 1. Acid leaching and HT with alkalis 2. Graphite and pitch mixture Conditions: 1100 °C under nitrogen atmosphere, duration 2 h | 105.3 mAh·g−1 at a current rate of 1C after 500 cycles | [58] |
| 1. Acid leaching, HT under nitrogen atmosphere 2. Polyethylene glycol 400 monooleate acid—0.02 L, Graphite—1 g Zinc chloride—2 g Conditions: 1000 °C, 2 h under nitrogen atmosphere | 730.8 mAh·g−1 at a current rate of 0.1C at 1st cycle 420 mAh·g−1 at a current rate of 0.1C after 100 cycles | [59] |
| 1. Acid leaching and HT in air atmosphere 2. Graphite—10 g, Phenolic resin–ethanol solution—0.02 L, Conditions: solidification at 120 °C,1 h, Then 950 °C, 1 h under nitrogen atmosphere | 342.9 mAh·g−1 after 50 cycles | [60] |
| 1. Acid leaching 2. Graphite—1 g Urea—5 g Conditions: 550 °C, 3 h Then 800 °C, 1 h under argon atmosphere | 465.8 mAh·g−1 at 0.1A·g−1 after 200 cycles | [61] |
| 1. Acid leaching + air HT 2. Sintering at 1000 °C, 2 h, under N2 with pitch, graphite and Si | 774.5 mAh·g−1 at 0.05A·g−1 | [62] |
| 1. NMP + scrapping 2. Ball-milling with nano Si, sintering at 800 °C, 3 h under argon atmosphere | 1321.8 mAh·g−1 at 0.05 A·g−1 | [63] |
| 1. Acid leaching for cathode, oxidation-intercalation for anode 2. Solvothermal synthesis at 160 °C for 12 h | 890 after 700 cycles at 1 A·g−1 | [64] |
| 1. Scrapping and intercalation 2. Gas sulfidation at 600 °C, 2 h, under Ar/N2 | NCMS/C—900.4 mAh·g−1 after 200 cycles at 0.2 A·g−1 NCAS/C—830.5 mAh·g−1 after 200 cycles at 0.2 A·g−1 | [65] |
| Recovery Approach | Recycle Approach | ||
|---|---|---|---|
| Advantages | Disadvantages | Advantages | Disadvantages |
| Higher purity can be achieved, which is crucial for certain applications, such as lithium-ion battery anodes. | Recovery processes can be resource-intensive, especially when using strong acids or high-temperature treatments. | More eco-friendly and potentially energy-efficient, particularly if the emphasis is on reusing the recovered materials rather than achieving high purity. | May not yield the same level of purity as the recovery approach, limiting its suitability for certain applications. |
| Well-established recovery methods using hydrometallurgical processes can be more readily adopted in industrial settings. | Chemical handling and waste disposal can pose environmental and safety challenges. | Reduces the need for extensive chemical treatment, making it a greener option. | The electrochemical performance of recycled graphite may not match that of newly manufactured graphite, especially for high-performance batteries. |
| Offers the potential to produce high-quality graphite with enhanced electrochemical properties. | May require multiple steps, leading to complexity in the overall process. | Suitable for applications where high purity is not a strict requirement, such as in some energy storage systems or composite materials. | |
| Application of the Final Material | Performance/Application | Ref. | |
|---|---|---|---|
| Adsorbents | Spent graphite | Pb—43.5 mg/g Cd—11.0 mg/g Ba—24.7 mg/g DCP—6.5 mg/g TNT—2.6 mg/g DNT—2.3 mg/g | [66] |
| MnO2-loaded graphite | Ag—67.8 mg/g Cd—29.5 mg/g Pb—99.9 mg/g | [67] | |
| MnO2-coated amorphous carbon | Cd—135.81 mg/g | [68] | |
| Capacitors | Li-ion capacitor with LiC6 | 185.84 Wh/kg | [69] |
| Glyme-based Li-ion capacitor with LiC6 | 46.40 Wh/kg | [70] | |
| Glyme-based Na-ion capacitor with NaC6 | 59.93 Wh/kg | [71] | |
| Supercapacitor with cobalt-copper nanowire as positive electrode and graphite as negative electrode | 42 F/g | [72] | |
| Catalysts | Spent graphite | Catalytic redox degradation of organic compounds | [66] |
| Spent graphite | Polysulfide trapping and catalytic for LiS battery | [73] | |
| rGO | Catalytic ozonation of organic pollutants | [74] | |
| ZVI-EG | Heterogeneous Fenton rection for 4-chlorphenol removal | [75] | |
| ZVFe-Cu supported on graphite | Reduction and heterogeneous Fenton rection for 4-chlorphenol removal | [76] | |
| GO/CuO | Photodegradation for methylene blue | [77] | |
| Graphene | Graphene | Decrease in H2SO4 and KMnO4 consumption in graphene synthesis | [83] |
| 2D graphene oxide | Synthesis without pre-calcination and acid leaching | [84] | |
| Soluble graphene nanosheets | Excellent solubility in water, ethanol and other polar solvents | [85] | |
| rGO | Supercapacitor | [86] | |
| High-quality graphene | Conductive ink application | [87] | |
| 1–4-layered graphene 2–4-layered graphene Battery-grade Li2CO3 | Reduction in the graphene production cost (USD 540 per ton graphene) Li recovery from spent graphite anode | [88] | |
| Rechargeable batteries | Cu-BTC MOF | Application in LIB/SIB 208.9 mAh/g at 100 mA/g | [89] |
| Heat-treated spent graphite | Application in SIB/KIB For SIB 162 mAh/g at 0.2 A/g; KIB 320 mAh/g at 0.05 A/g | [90] | |
| Joint cation-anion electrode RLFPG | Application in DIB 117.4 mAh/g at 24 mA/g; 78% after 1000 cycles at 100 mA/g | [91] | |
| Transformed spent graphite | Application in DIB 87 mAh/g at 200 mA/g | [92] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosenko, A.; Pushnitsa, K.; Pavlovskii, A.A.; Novikov, P.; Popovich, A.A. The Review of Existing Strategies of End-of-Life Graphite Anode Processing Using 3Rs Approach: Recovery, Recycle, Reuse. Batteries 2023, 9, 579. https://doi.org/10.3390/batteries9120579
Kosenko A, Pushnitsa K, Pavlovskii AA, Novikov P, Popovich AA. The Review of Existing Strategies of End-of-Life Graphite Anode Processing Using 3Rs Approach: Recovery, Recycle, Reuse. Batteries. 2023; 9(12):579. https://doi.org/10.3390/batteries9120579
Chicago/Turabian StyleKosenko, Alexandra, Konstantin Pushnitsa, Alexander A. Pavlovskii, Pavel Novikov, and Anatoliy A. Popovich. 2023. "The Review of Existing Strategies of End-of-Life Graphite Anode Processing Using 3Rs Approach: Recovery, Recycle, Reuse" Batteries 9, no. 12: 579. https://doi.org/10.3390/batteries9120579






