Highly Flexible Stencil Printed Alkaline Ag2O-Zn Battery for Wearable Electronics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Used
2.2. Battery Fabrication Procedure
2.3. Characterization Methods
3. Results
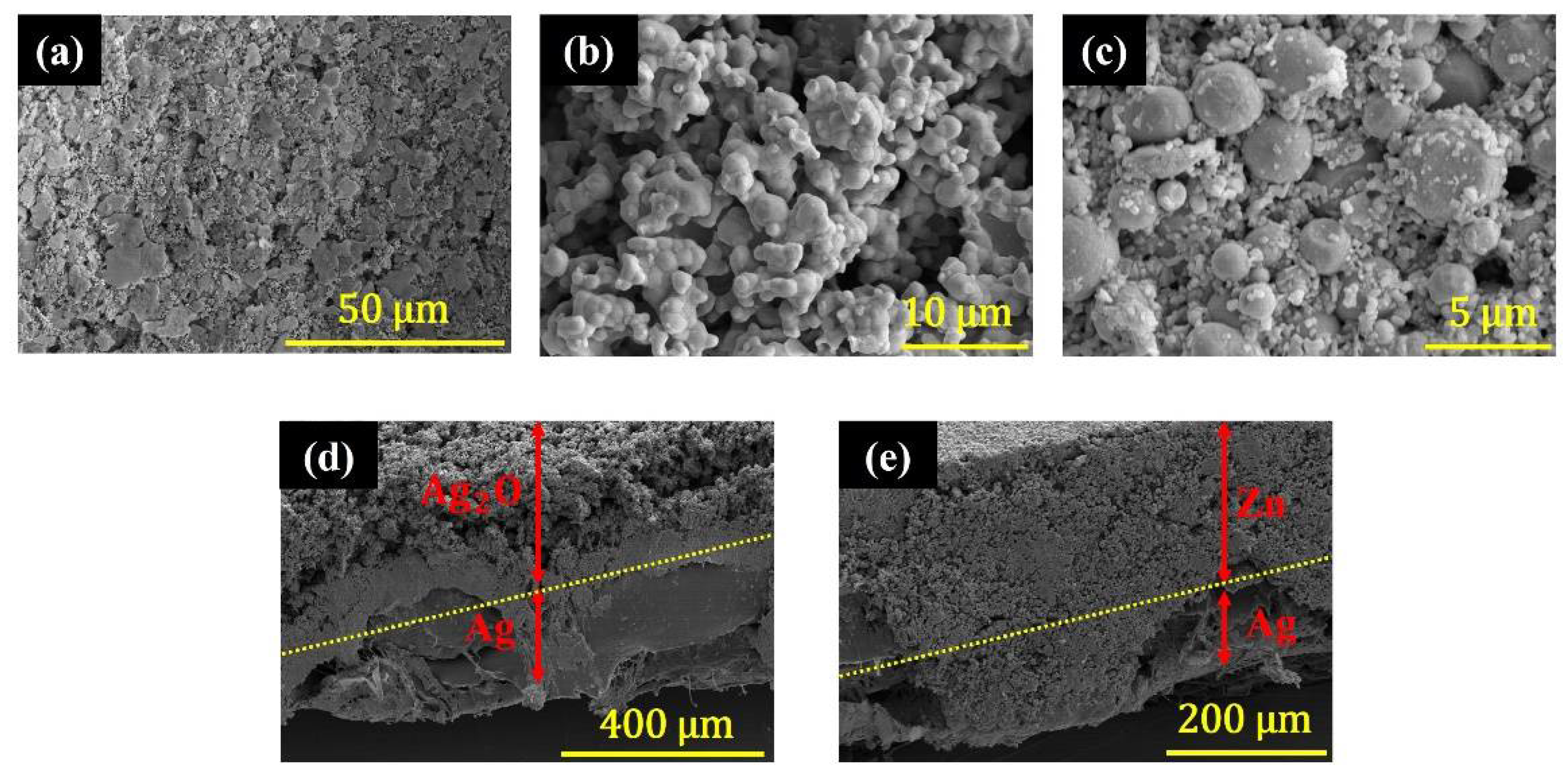
3.1. HRSEM Characterization
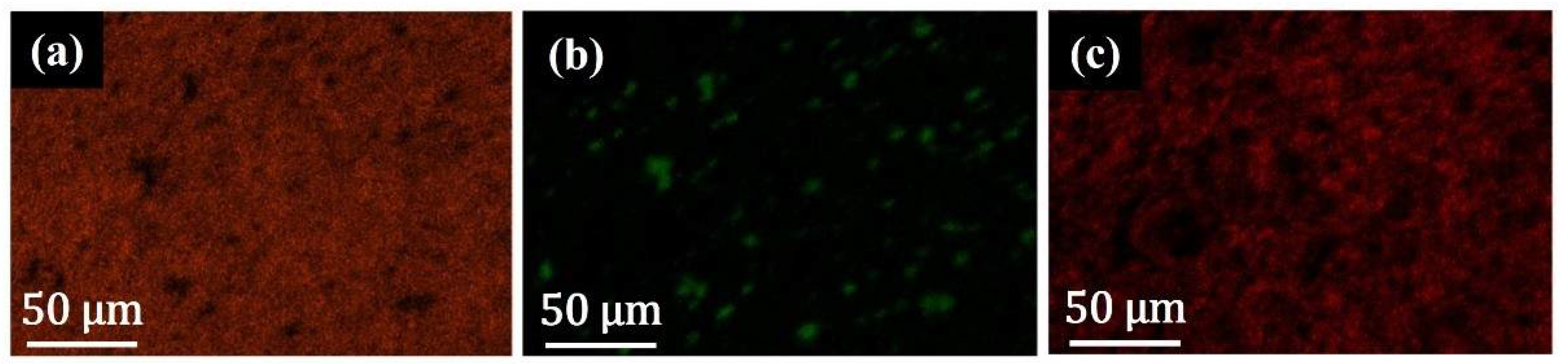
3.2. EDX Characterization
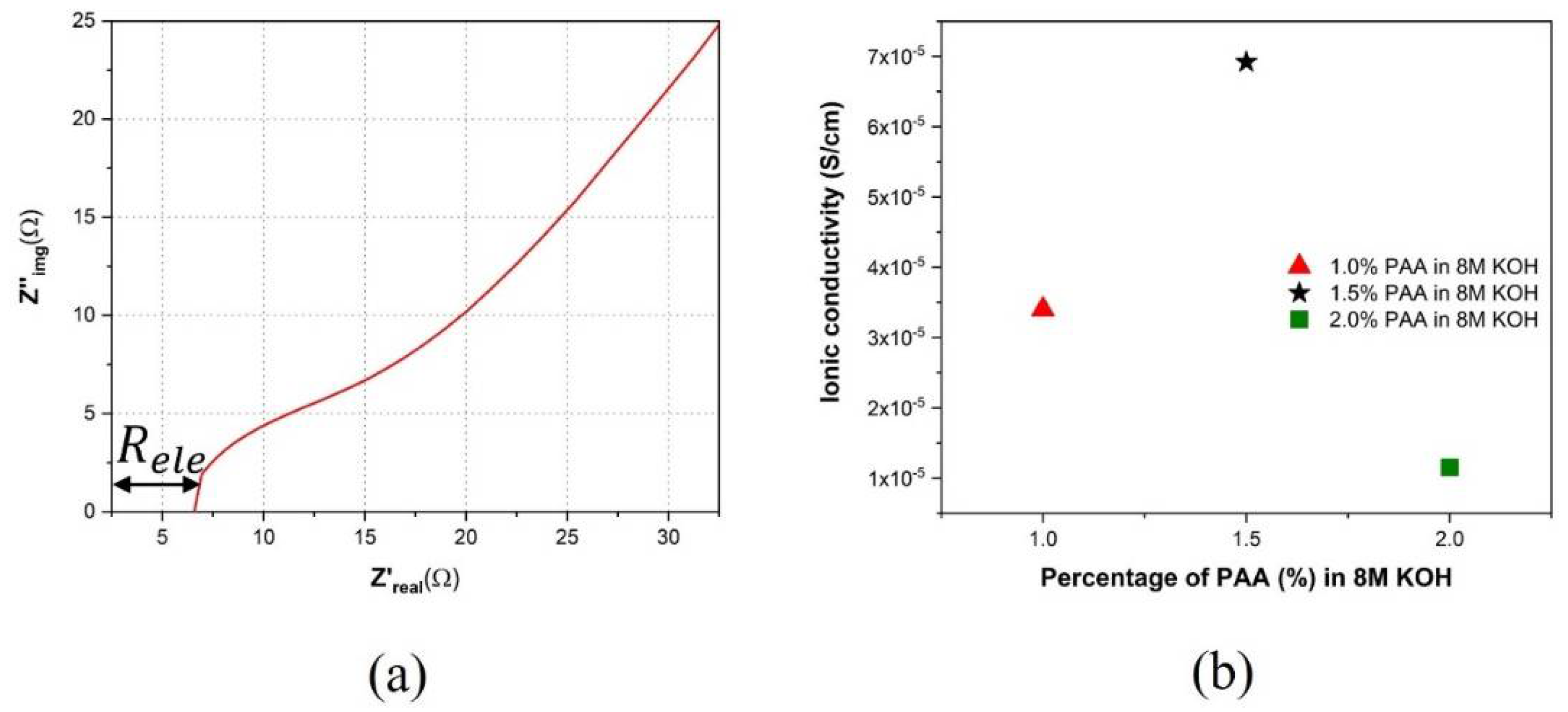
3.3. EIS Characterization
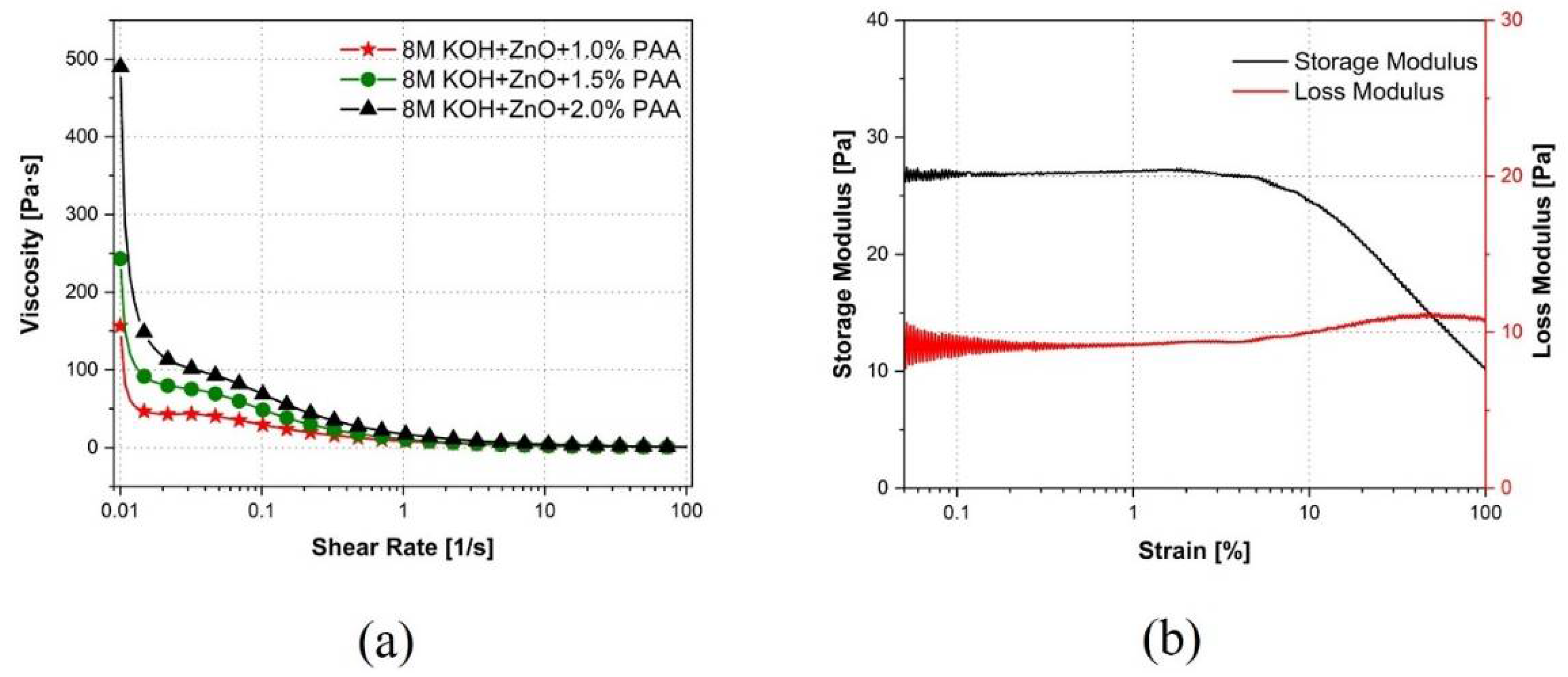
3.4. Rheology Characterization
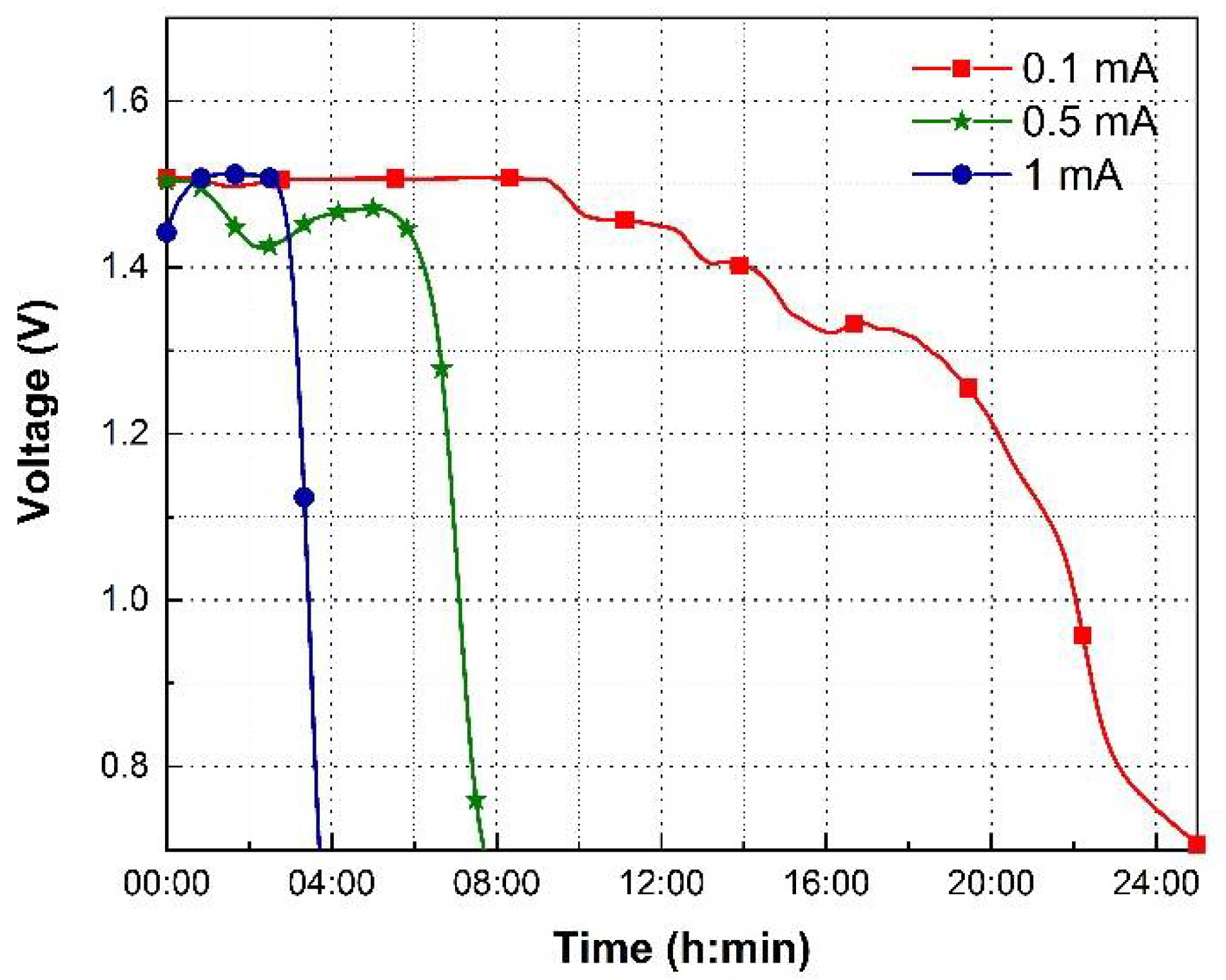
3.5. Discharge Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ershad, F.; Thukral, A.; Yue, J.; Comeaux, P.; Lu, Y.; Shim, H.; Sim, K.; Kim, N.; Rao, Z.; Guevara, R. Ultra-Conformal Drawn-on-Skin Electronics for Multifunctional Motion Artifact-Free Sensing and Point-of-Care Treatment. Nat. Commun. 2020, 11, 3823. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.; Wang, J. Wearable Biosensors for Healthcare Monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, Y.; Mahmood, M.; Kwon, S.; Zavanelli, N.; Kim, H.S.; Rim, Y.S.; Epps, F.; Yeo, W. Fully Integrated, Stretchable, Wireless Skin-Conformal Bioelectronics for Continuous Stress Monitoring in Daily Life. Adv. Sci. 2020, 7, 2000810. [Google Scholar] [CrossRef] [PubMed]
- Whiting, G.L.; Arias, A.C. Chemically Modified Ink-Jet Printed Silver Electrodes for Organic Field-Effect Transistors. Appl. Phys. Lett. 2009, 95, 328. [Google Scholar] [CrossRef]
- Kim, D.; Kim, Y.; Amsden, J.; Panilaitis, B.; Kaplan, D.L.; Omenetto, F.G.; Zakin, M.R.; Rogers, J.A. Silicon Electronics on Silk as a Path to Bioresorbable, Implantable Devices. Appl. Phys. Lett. 2009, 95, 133701. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.; Duan, L.; Zhang, L.M.; Wang, L.D.; Dong, G.F.; Wang, Z.L. Flexible Organic Tribotronic Transistor Memory for a Visible and Wearable Touch Monitoring System. Adv. Mater. 2016, 28, 106–110. [Google Scholar] [CrossRef]
- Eshkeiti, A.; Reddy, A.S.; Emamian, S.; Narakathu, B.B.; Joyce, M.; Joyce, M.; Fleming, P.D.; Bazuin, B.J.; Atashbar, M.Z. Screen Printing of Multilayered Hybrid Printed Circuit Boards on Different Substrates. IEEE Trans. Compon. Packag. Manuf. Technol. 2015, 5, 415–421. [Google Scholar] [CrossRef]
- Lee, J.M.; Choi, C.; Kim, J.H.; de Andrade, M.J.; Baughman, R.H.; Kim, S.J. Biscrolled Carbon Nanotube Yarn Structured Silver-Zinc Battery. Sci. Rep. 2018, 8, 11150. [Google Scholar] [CrossRef]
- Kwon, Y.H.; Woo, S.; Jung, H.; Yu, H.K.; Kim, K.; Oh, B.H.; Ahn, S.; Lee, S.; Song, S.; Cho, J. Cable-type Flexible Lithium Ion Battery Based on Hollow Multi-helix Electrodes. Adv. Mater. 2012, 24, 5192–5197. [Google Scholar] [CrossRef]
- Rao, J.; Liu, N.; Li, L.; Su, J.; Long, F.; Zou, Z.; Gao, Y. A High Performance Wire-Shaped Flexible Lithium-Ion Battery Based on Silicon Nanoparticles within Polypyrrole/Twisted Carbon Fibers. RSC Adv. 2017, 7, 26601–26607. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, Y.; Bai, W.; Chen, X.; Zhang, Z.; Fang, X.; Weng, W.; Wang, Y.; Peng, H. Rücktitelbild: Elastic and Wearable Wire-Shaped Lithium-Ion Battery with High Electrochemical Performance. Angew. Chem. 2014, 126, 8092. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, X.; Han, J.; Zhang, X.; Sun, X.; Li, C.; Liu, W.; Li, Q.; Ma, Y. High-Performance Cable-Type Flexible Rechargeable Zn Battery Based on MnO2@ CNT Fiber Microelectrode. ACS Appl. Mater. Interfaces 2018, 10, 24573–24582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bai, W.; Ren, J.; Weng, W.; Lin, H.; Zhang, Z.; Peng, H. Super-Stretchy Lithium-Ion Battery Based on Carbon Nanotube Fiber. J. Mater. Chem. A 2014, 2, 11054–11059. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, J.; Lin, H.; Huang, W. Flexible Fiber and Fabric Batteries. Adv. Mater. Technol. 2018, 3, 1700302. [Google Scholar] [CrossRef]
- Liu, J.; Guan, C.; Zhou, C.; Fan, Z.; Ke, Q.; Zhang, G.; Liu, C.; Wang, J. A Flexible Quasi-solid-state Nickel–zinc Battery with High Energy and Power Densities Based on 3D Electrode Design. Adv. Mater. 2016, 28, 8732–8739. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Zhao, W.; Hu, Y.; Ke, Q.; Li, X.; Zhang, H.; Wang, J. High-performance Flexible Solid-state Ni/Fe Battery Consisting of Metal Oxides Coated Carbon Cloth/Carbon Nanofiber Electrodes. Adv. Energy Mater. 2016, 6, 1601034. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Z.; Bramnik, N.; Mitra, S. Fabrication of High-performance Flexible Alkaline Batteries by Implementing Multiwalled Carbon Nanotubes and Copolymer Separator. Adv. Mater. 2014, 26, 970–976. [Google Scholar] [CrossRef]
- Wang, Z.; Mitra, S. Development of Flexible Secondary Alkaline Battery with Carbon Nanotube Enhanced Electrodes. J. Power Sources 2014, 266, 296–303. [Google Scholar] [CrossRef]
- Gaikwad, A.M.; Whiting, G.L.; Steingart, D.A.; Arias, A.C. Highly Flexible, Printed Alkaline Batteries Based on Mesh-embedded Electrodes. Adv. Mater. 2011, 23, 3251–3255. [Google Scholar] [CrossRef]
- Gaikwad, A.M.; Steingart, D.A.; Nga Ng, T.; Schwartz, D.E.; Whiting, G.L. A Flexible High Potential Printed Battery for Powering Printed Electronics. Appl. Phys. Lett. 2013, 102, 233302. [Google Scholar] [CrossRef]
- Kumar, R.; Johnson, K.M.; Williams, N.X.; Subramanian, V. Scaling Printable Zn–Ag2O Batteries for Integrated Electronics. Adv. Energy Mater. 2019, 9, 1803645. [Google Scholar] [CrossRef]
- Kumar, R.; Shin, J.; Yin, L.; You, J.; Meng, Y.S.; Wang, J. All-printed, Stretchable Zn-Ag2O Rechargeable Battery Via Hyperelastic Binder for Self-powering Wearable Electronics. Adv. Energy Mater. 2017, 7, 1602096. [Google Scholar] [CrossRef]
- Kum, L.W.; Gogia, A.; Vallo, N.; Singh, D.K.; Kumar, J. Enhancing Electrochemical Performances of Rechargeable Lithium-Ion Batteries Via Cathode Interfacial Engineering. ACS Appl. Mater. Interfaces 2022, 14, 4100–4110. [Google Scholar] [CrossRef]
- Kim, D.; Xiao, J.; Song, J.; Huang, Y.; Rogers, J.A. Stretchable, Curvilinear Electronics Based on Inorganic Materials. Adv. Mater. 2010, 22, 2108–2124. [Google Scholar] [CrossRef] [PubMed]
- Hiralal, P.; Imaizumi, S.; Unalan, H.E.; Matsumoto, H.; Minagawa, M.; Rouvala, M.; Tanioka, A.; Amaratunga, G.A. Nanomaterial-Enhanced all-Solid Flexible Zinc–Carbon Batteries. ACS Nano 2010, 4, 2730–2734. [Google Scholar] [CrossRef] [PubMed]
- Long, J.W.; Dunn, B.; Rolison, D.R.; White, H.S. Three-Dimensional Battery Architectures. Chem. Rev. 2004, 104, 4463–4492. [Google Scholar] [CrossRef]
- Kota, A.; Gogia, A.; Neidhard-Doll, A.T.; Chodavarapu, V.P. Printed Textile-Based Ag2O–Zn Battery for Body Conformal Wearable Sensors. Sensors 2021, 21, 2178. [Google Scholar] [CrossRef]
- Khan, Z.; Ail, U.; Nadia, A.F.; Phopase, J.; Ullah, K.Z.; Kim, N.; Nilsson, J.; Inganäs, O.; Berggren, M.; Crispin, X. Water-in-Polymer Salt Electrolyte for Slow Self-Discharge in Organic Batteries. Adv. Energy Sustain. Res. 2022, 3, 2100165. [Google Scholar]
- Khan, Z.; Ail, U.; Nadia, A.F.; Phopase, J.; Ullah, K.Z.; Kim, N.; Kumar, D.; Nilsson, J.; Inganäs, O.; Berggren, M.; et al. Towards Printable Water-in-Polymer Salt Electrolytes for High Power Organic Batteries. J. Power Sources 2022, 524, 231103. [Google Scholar]
- Faes, M.; Vleugels, J.; Vogeler, F.; Ferraris, E. Extrusion-Based Additive Manufacturing of ZrO2 using Photoinitiated Polymerization. CIRP J. Manuf. Sci. Technol. 2016, 14, 28–34. [Google Scholar]
- Wang, L.; Luo, Z.; Yang, L.; Wang, H.; Zhong, J. Effect of Styrene Content on Mechanical and Rheological Behavior of Styrene Butadiene Rubber. Mater. Res. Express 2020, 8, 015302. [Google Scholar]
- Enfucell. Available online: https://www.enfucell.com/softbattery (accessed on 17 February 2022).






| Case | Discharge Current | Discharge Capacity | Specific Capacity | Areal Capacity |
|---|---|---|---|---|
| Without bending (Flat) | 0.1 | 2.5 | 86.21 | 0.78 |
| Bend radius of 1.25 cm | 0.1 | 1.75 | 60.34 | 0.55 |
| Bend radius of 0.8 cm | 0.1 | 1.6 | 55.17 | 0.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kota, A.; Kum, L.W.; Vallurupalli, K.; Gogia, A.; Neidhard-Doll, A.T.; Chodavarapu, V.P. Highly Flexible Stencil Printed Alkaline Ag2O-Zn Battery for Wearable Electronics. Batteries 2022, 8, 74. https://doi.org/10.3390/batteries8070074
Kota A, Kum LW, Vallurupalli K, Gogia A, Neidhard-Doll AT, Chodavarapu VP. Highly Flexible Stencil Printed Alkaline Ag2O-Zn Battery for Wearable Electronics. Batteries. 2022; 8(7):74. https://doi.org/10.3390/batteries8070074
Chicago/Turabian StyleKota, Akash, Lenin W. Kum, Kavya Vallurupalli, Ashish Gogia, Amy T. Neidhard-Doll, and Vamsy P. Chodavarapu. 2022. "Highly Flexible Stencil Printed Alkaline Ag2O-Zn Battery for Wearable Electronics" Batteries 8, no. 7: 74. https://doi.org/10.3390/batteries8070074







