Importance of Continuous and Simultaneous Monitoring of Both Electrode Voltages during Discharge/Charge Battery Tests: Application to Zn-Based Batteries
Abstract
:1. Introduction
- The normally obtained discharge/charge curve, i.e., Ew–Ec vs. capacity or time.
- The working electrode voltage, Ew, in this case, the positive electrode of the battery, is referenced to the reference electrode vs. capacity or time.
- The counter electrode voltage, Ec, here the negative electrode of the battery, is referenced to the reference electrode vs. capacity or time.

2. Experimental Section
3. Results
3.1. Zn/Air Battery Test
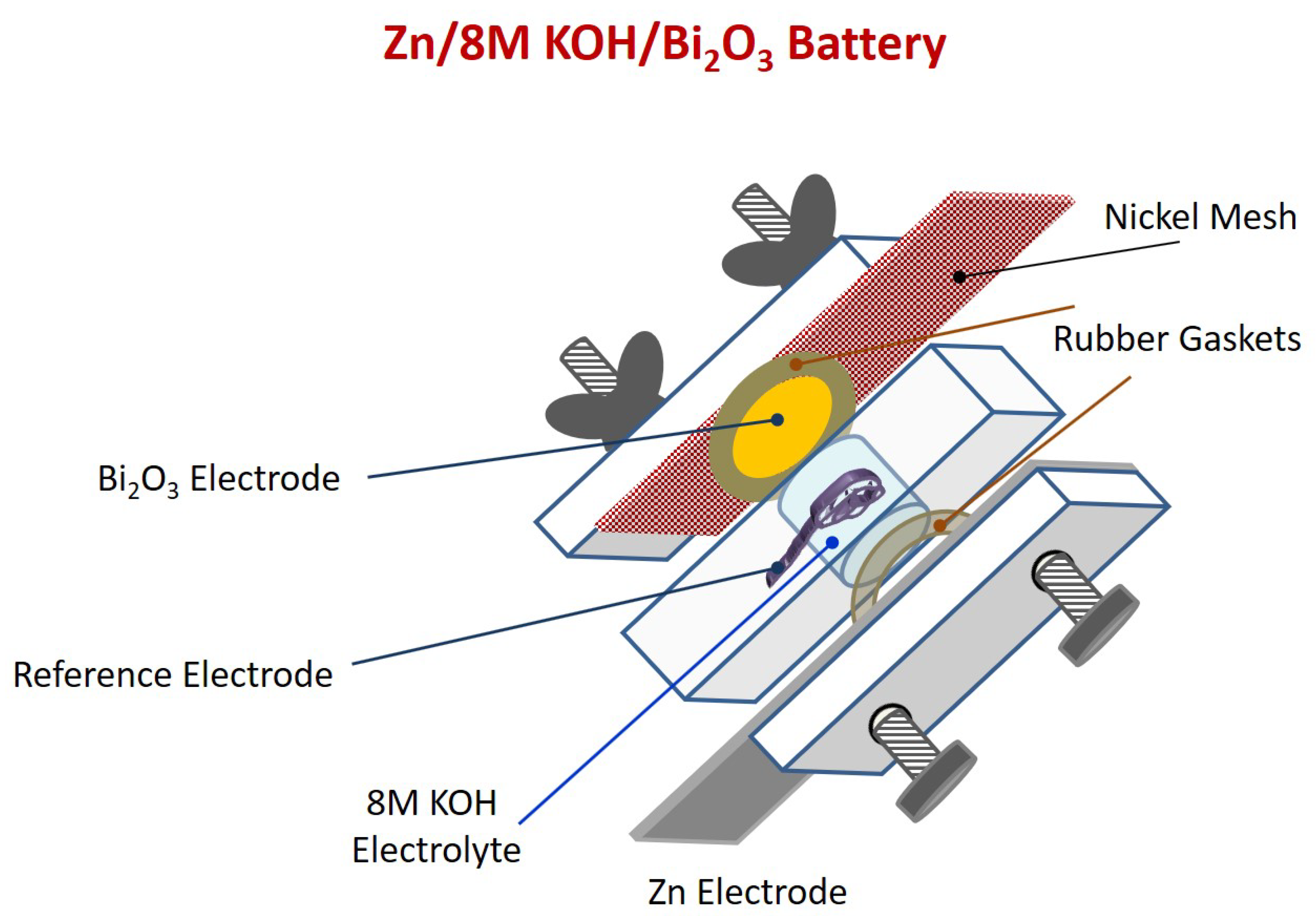
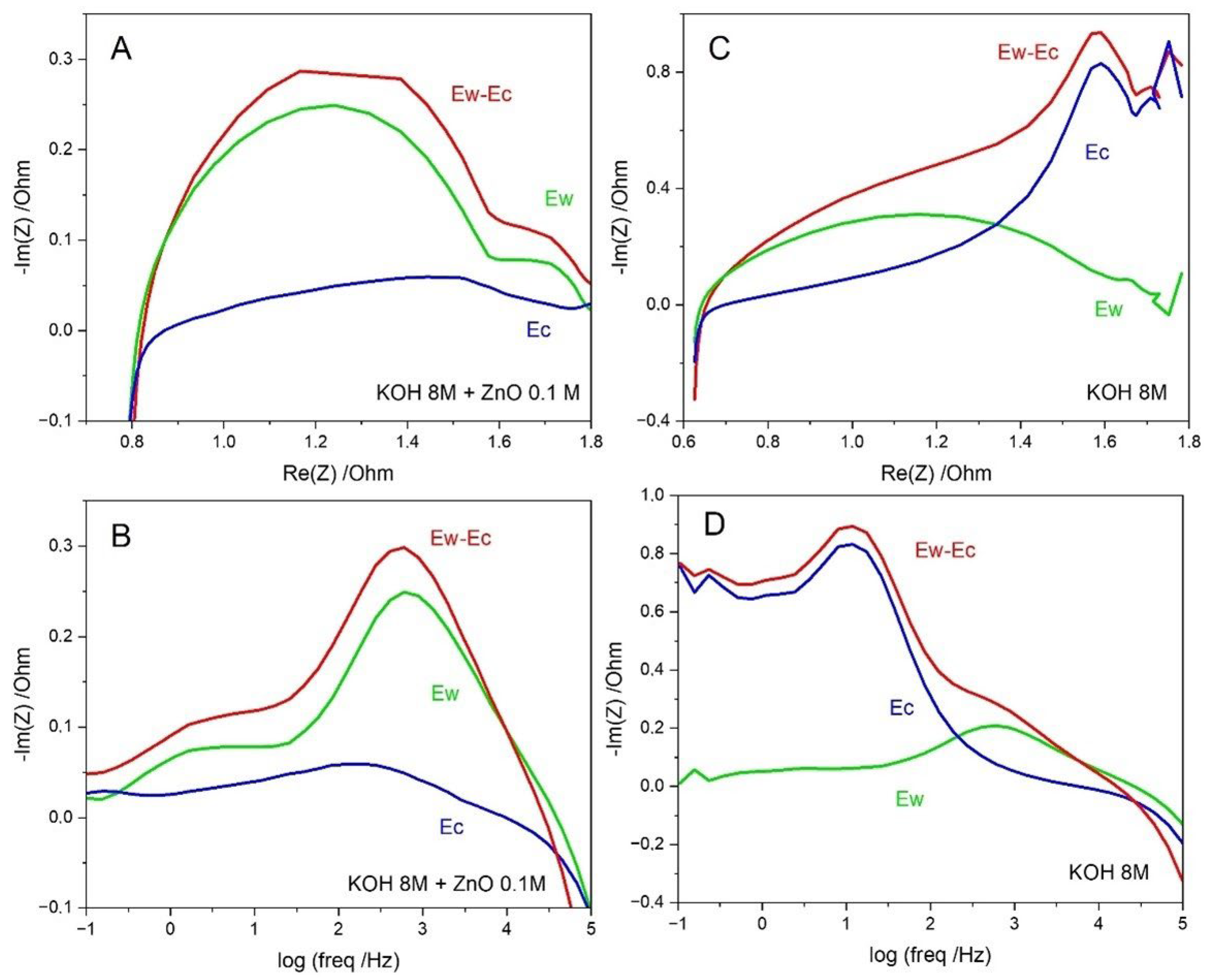
3.2. Zn/Bi2O3 Battery Test in a ~1 mL 8 M KOH Solution Cell
3.3. Zn/Bi2O3 Battery Test in a ~50 mL 8 M KOH Solution Cell
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Aremu, E.O.; Park, D.-J.; Ryu, K.-S. The effects of anode additives towards suppressing dendrite growth and hydrogen gas evolution reaction in Zn-air secondary batteries. Ionics 2019, 25, 4197–4207. [Google Scholar] [CrossRef]
- Lv, S.; Verhallen, T.; Vasileiadis, A.; Ooms, F.; Xu, Y.; Li, Z.; Li, Z.; Wagemaker, M. Operando monitoring the lithium spatial distribution of lithium metal anodes. Nat. Commun. 2018, 9, 2152. [Google Scholar] [CrossRef] [Green Version]
- Wood, K.N.; Steirer, K.X.; Hafner, S.E.; Ban, C.; Santhanagopalan, S.; Lee, S.-H.; Teeter, G. Operando X-ray photoelectron spectroscopy of solid electrolyte interphase formation and evolution in Li2S-P2S5 solid-state electrolytes. Nat. Commun. 2018, 9, 2490. [Google Scholar] [CrossRef] [Green Version]
- Santos, F.; Abad, J.; Vila, M.; Castro, G.R.; Urbina, A.; Romero, A.J.F. In situ synchrotron x-ray diffraction study of Zn/Bi2O3 electrodes prior to and during discharge of Zn-air batteries: Influence on ZnO deposition. Electrochim. Acta 2018, 281, 133–141. [Google Scholar] [CrossRef]
- Park, S.K.; Dose, W.M.; Boruah, B.D.; De Volder, M. In Situ and Operando Analyses of Reaction Mechanisms in Vanadium Oxides for Li-, Na-, Zn-, and Mg-Ions Batteries. Adv. Mater. Technol. 2021, 7, 2100799. [Google Scholar] [CrossRef]
- Inzelt, G. Handbook of Reference Electrodes; Inzelt, G., Lewenstam, A., Scholz, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020. [Google Scholar]
- Pino, M.; Cuadrado, C.; Chacón, J.; Rodríguez, P.; Fatás, E.; Ocón, P. The electrochemical characteristics of commercial aluminium alloy electrodes for Al/air batteries. J. Appl. Electrochem. 2014, 44, 1371–1380. [Google Scholar] [CrossRef]
- Lewis, T.; Spinks, G.M.; Wallace, G.; Mazzoldi, A.; De Rossi, D. Investigation of the applied potential limits for polypyrrole when employed as the active components of a two-electrode device. Synth. Met. 2001, 122, 379–385. [Google Scholar] [CrossRef]
- Padilla, J.; Seshadri, V.; Otero, T.; Sotzing, G. Electrochemical study of dual conjugated polymer electrochromic devices. J. Electroanal. Chem. 2007, 609, 75–84. [Google Scholar] [CrossRef]
- Hassab, S.; Shen, D.E.; Österholm, A.M.; Reynolds, J.R.; Padilla, J. Exploring unbalanced electrode configurations for electrochromic devices. J. Mater. Chem. C 2017, 6, 393–400. [Google Scholar] [CrossRef]
- Hassab, S.; Padilla, J. Using WO 3 as a transparent, optically-passive counter electrode in an unbalanced electrochromic configuration. Electrochem. Commun. 2016, 72, 87–90. [Google Scholar] [CrossRef]
- Soulsby, L.C.; Hayne, D.J.; Doeven, E.H.; Chen, L.; Hogan, C.F.; Kerr, E.; Adcock, J.L.; Francis, P.S. Electrochemically, Spectrally, and Spatially Resolved Annihilation-Electrogenerated Chemiluminescence of Mixed-Metal Complexes at Working and Counter Electrodes. ChemElectroChem 2018, 5, 1543–1547. [Google Scholar] [CrossRef]
- Costard, J.; Ender, M.; Weiss, M.; Ivers-Tiffée, E. Three-Electrode Setups for Lithium-Ion Batteries II. Experimental Study of Different Reference Electrode Designs and Their Implications for Half-Cell Impedance Spectra. J. Electrochem. Soc. 2017, 164, A80–A87. [Google Scholar] [CrossRef]
- Ender, M.; Illig, J.; Ivers-Tiffée, E. Three-Electrode Setups for Lithium-Ion Batteries I. Fem-Simulation of Different Reference Electrode Designs and Their Implications for Half-Cell Impedance Spectra. J. Electrochem. Soc. 2017, 164, A71–A79. [Google Scholar] [CrossRef]
- Raccichini, R.; Amores, M.; Hinds, G. Critical Review of the Use of Reference Electrodes in Li-Ion Batteries: A Diagnostic Perspective. Batteries 2019, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Middlemiss, L.A.; Rennie, A.J.; Sayers, R.; West, A.R. Characterisation of batteries by electrochemical impedance spectroscopy. Energy Rep. 2020, 6, 232–241. [Google Scholar] [CrossRef]
- Hwang, B.; Oh, E.-S.; Kim, K. Observation of electrochemical reactions at Zn electrodes in Zn-air secondary batteries. Electrochim. Acta 2016, 216, 484–489. [Google Scholar] [CrossRef]
- Liu, X.; Elia, G.A.; Passerini, S. Evaluation of counter and reference electrodes for the investigation of Ca battery materials. J. Power Sources Adv. 2020, 2, 100008. [Google Scholar] [CrossRef]
- Chu, Z.; Feng, X.; Liaw, B.; Li, Y.; Lu, L.; Li, J.; Han, X.; Ouyang, M. Testing Lithium-Ion Battery with the Internal Reference Electrode: An Insight into the Blocking Effect. J. Electrochem. Soc. 2018, 165, A3240–A3248. [Google Scholar] [CrossRef]
- Lim, M.B.; Lambert, T.N.; Ruiz, E.I. Effect of ZnO-Saturated Electrolyte on Rechargeable Alkaline Zinc Batteries at Increased Depth-of-Discharge. J. Electrochem. Soc. 2020, 167, 060508. [Google Scholar] [CrossRef]
- Bockelmann, M.; Becker, M.; Reining, L.; Kunz, U.; Turek, T. Passivation of Zinc Anodes in Alkaline Electrolyte: Part I. Determination of the Starting Point of Passive Film Formation. J. Electrochem. Soc. 2018, 165, A3048–A3055. [Google Scholar] [CrossRef]
- Wang, N.; Wan, H.; Duan, J.; Wang, X.; Tao, L.; Zhang, J. A review of zinc-based battery from alkaline to acid. Mater. Today Adv. 2021, 11, 100149. [Google Scholar] [CrossRef]
- Shin, J.; Lee, J.; Park, Y.; Choi, J.W. Aqueous zinc ion batteries: Focus on zinc metal anodes. Chem. Sci. 2020, 11, 2028–2044. [Google Scholar] [CrossRef] [Green Version]
- Mainar, A.R.; Leonet, O.; Bengoechea, M.; Boyano, I.; de Meatza, I.; Kvasha, A.; Guerfi, A.; Blázquez, J.A. Alkaline aqueous electrolytes for secondary zinc-air batteries: An overview. Int. J. Energy Res. 2016, 40, 1032–1049. [Google Scholar] [CrossRef]
- Liu, M.; Cook, G.M.; Yao, N.P. Passivation of Zinc Anodes in KOH Electrolytes. J. Electrochem. Soc. 1981, 128, 1663–1668. [Google Scholar] [CrossRef]
- Santos, F.; Romero, A.J.F. Hydration as a solution to zinc batteries. Nat. Sustain. 2022, 5, 179–180. [Google Scholar] [CrossRef]
- Yang, H. Improved discharge capacity and suppressed surface passivation of zinc anode in dilute alkaline solution using surfactant additives. J. Power Sources 2004, 128, 97–101. [Google Scholar] [CrossRef]
- Lorca, S.; Santos, F.; Abad, J.; Huttel, Y.; Urbina, A.; Romero, A.J.F. Characterization of a new rechargeable Zn/PVA-KOH/Bi2O3 battery: Structural changes of the Bi2O3 electrode. Sustain. Energy Fuels 2020, 4, 4497–4505. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorca, S.; Santos, F.; Padilla, J.; López Cascales, J.J.; Fernández Romero, A.J. Importance of Continuous and Simultaneous Monitoring of Both Electrode Voltages during Discharge/Charge Battery Tests: Application to Zn-Based Batteries. Batteries 2022, 8, 221. https://doi.org/10.3390/batteries8110221
Lorca S, Santos F, Padilla J, López Cascales JJ, Fernández Romero AJ. Importance of Continuous and Simultaneous Monitoring of Both Electrode Voltages during Discharge/Charge Battery Tests: Application to Zn-Based Batteries. Batteries. 2022; 8(11):221. https://doi.org/10.3390/batteries8110221
Chicago/Turabian StyleLorca, Sebastián, Florencio Santos, Javier Padilla, J. J. López Cascales, and Antonio J. Fernández Romero. 2022. "Importance of Continuous and Simultaneous Monitoring of Both Electrode Voltages during Discharge/Charge Battery Tests: Application to Zn-Based Batteries" Batteries 8, no. 11: 221. https://doi.org/10.3390/batteries8110221






