Preparing Co/N-Doped Carbon as Electrocatalyst toward Oxygen Reduction Reaction via the Ancient “Pharaoh’s Snakes” Reaction
Abstract
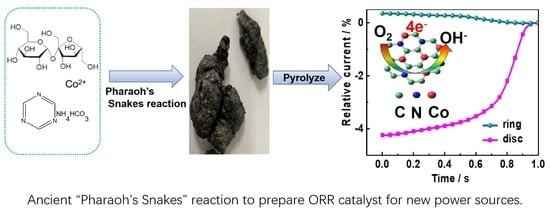
:1. Introduction
2. Experimental Section
2.1. Preparation of Electrocatalysts
2.2. Characterization
2.3. Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, D.; Pan, X.; Yang, P.; Li, R.; Xu, H.; Li, Y.; Meng, F.; Zhang, J.; An, M. Transition metal and nitrogen Co-doped carbon-based electrocatalysts for the oxygen reduction reaction: From active site insights to the rational design of precursors and structures. ChemSusChem 2021, 14, 33–55. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, X.L.; Fu, J.; Li, J.D.; Park, M.G.; Zhang, Y.N.; Lui, G.; Chen, Z.W. Pomegranate-inspired design of highly active and durable bifunctional electrocatalysts for rechargeable metal-air batteries. Angew. Chem. Int. Ed. 2016, 55, 4977–4982. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhao, Z.; Peng, B.; Duan, X.; Huang, Y. Beyond extended surfaces: Understanding the oxygen reduction reaction on nanocatalysts. J. Am. Chem. Soc. 2020, 142, 17812–17827. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hou, L.; Yu, M.; Li, Q.; Zhang, T.; Sun, H. Review and recent advances of oxygen transfer in Li-air batteries. ChemElectroChem 2021, 8, 3588–3603. [Google Scholar] [CrossRef]
- Liu, B.; Sun, Y.; Liu, L.; Xu, S.; Yan, X. Advances in manganese-mased oxides cathodic electrocatalysts for Li–Air batteries. Adv. Funct. Mater. 2018, 28, 1704973. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, J.; Wang, Y.; Zhang, W. A new promising catalytic activity on blue phosphorene nitrogen-doped nanosheets for the ORR as cathode in nonaqueous Li–air batteries. Appl. Surf. Sci. 2019, 488, 620–628. [Google Scholar] [CrossRef]
- Wroblowa, H.S.; Razumney, G. Electroreduction of oxygen: A new mechanistic criterion. J. Electroanal. Chem. Interfacial Electrochem. 1976, 69, 195–201. [Google Scholar] [CrossRef]
- Luo, E.; Chu, Y.; Liu, J.; Shi, Z.; Zhu, S.; Gong, L.; Ge, J.; Choi, C.H.; Liu, C.; Xing, W. Pyrolyzed M–N x catalysts for oxygen reduction reaction: Progress and prospects. Energy Environ. Sci. 2021, 14, 2158–2185. [Google Scholar] [CrossRef]
- Chen, Z.W.; Higgins, D.; Yu, A.P.; Zhang, L.; Zhang, J.J. A review on non-precious metal electrocatalysts for PEM fuel cells. Energy Environ. Sci. 2011, 4, 3167–3192. [Google Scholar] [CrossRef]
- Sun, Z.; Yuan, M.; Lin, L.; Yang, H.; Nan, C.; Sun, G.; Li, H.; Yang, X. Perovskite La0.5Sr0.5CoO3−δ grown on Ti3C2T x MXene nanosheets as bifunctional efficient hybrid catalysts for Li–Oxygen batteries. ACS Appl. Energy Mater. 2019, 2, 4144–4150. [Google Scholar] [CrossRef]
- Clark, S.; Latz, A.; Horstmann, B. A review of model-based design tools for metal-air batteries. Batteries 2018, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Ren, G.Y.; Gao, L.L.; Teng, C.; Li, Y.A.; Yang, H.Q.; Shui, J.L.; Lu, X.Y.; Zhu, Y.; Dai, L.M. Ancient chemistry "Pharaoh’s Snakes" for efficient Fe-/N-doped carbon electrocatalysts. ACS Appl. Mater. Interfaces 2018, 10, 10778–10785. [Google Scholar] [CrossRef] [PubMed]
- Zion, N.; Cullen, D.A.; Zelenay, P.; Elbaz, L. Heat-treated aerogel as a catalyst for the oxygen reduction reaction. Angew. Chem. Int. Ed. 2020, 132, 2504–2510. [Google Scholar] [CrossRef]
- Yang, L.; Zeng, X.; Wang, W.; Cao, D. Recent progress in MOF-derived, heteroatom-doped porous carbons as highly efficient electrocatalysts for oxygen reduction reaction in fuel cells. Adv. Funct. Mater. 2018, 28, 1704537. [Google Scholar] [CrossRef]
- Liang, H.-W.; Zhuang, X.; Brüller, S.; Feng, X.; Müllen, K. Hierarchically porous carbons with optimized nitrogen doping as highly active electrocatalysts for oxygen reduction. Nat. Commun. 2014, 5, 4973. [Google Scholar] [CrossRef] [Green Version]
- Ingo, G.M.; Guida, G.; Angelini, E.; Di Carlo, G.; Mezzi, A.; Padeletti, G. Ancient mercury-based plating methods: Combined use of surface analytical techniques for the study of manufacturing process and degradation phenomena. Acc. Chem. Res. 2013, 46, 2365–2375. [Google Scholar] [CrossRef]
- Buelens, L.C.; Galvita, V.V.; Poelman, H.; Detavernier, C.; Marin, G.B. Super-dry reforming of methane intensifies CO2 utilization via Le Chatelier’s principle. Science 2016, 354, 449–452. [Google Scholar] [CrossRef]
- Xie, X.H.; He, C.; Li, B.Y.; He, Y.H.; Cullen, D.A.; Wegener, E.C.; Kropf, A.J.; Martinez, U.; Cheng, Y.W.; Engelhard, M.H.; et al. Performance enhancement and degradation mechanism identification of a single-atom Co-N-C catalyst for proton exchange membrane fuel cells. Nat. Catal. 2020, 3, 1044–1054. [Google Scholar] [CrossRef]
- Wang, X.X.; Prabhakaran, V.; He, Y.; Shao, Y.; Wu, G. Iron-free cathode catalysts for proton-exchange-membrane fuel cells: Cobalt catalysts and the peroxide mitigation approach. Adv. Mater. 2019, 31, 1805126. [Google Scholar] [CrossRef]
- Wang, J.; Gao, R.; Zhou, D.; Chen, Z.; Wu, Z.; Schumacher, G.; Hu, Z.; Liu, X. Boosting the electrocatalytic activity of Co3O4 nanosheets for a Li-O2 battery through modulating inner oxygen vacancy and exterior Co3+/Co2+ ratio. ACS Catal. 2017, 7, 6533–6541. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Wang, Y.; Wu, H.; Liu, X.; Wang, L.; Yu, Q.; Li, A.; Wang, H.; Song, C.; Gao, Z.; et al. Construction of a sp3/sp2 carbon interface in 3D N-doped nanocarbons for the oxygen reduction reaction. Angew. Chem. Int. Ed. 2019, 58, 15089–15097. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Cui, X.; Yang, G.; Zheng, W. Amorphous carbon enriched with pyridinic nitrogen as an efficient metal-free electrocatalyst for oxygen reduction reaction. Chem. Commun. 2014, 50, 557–559. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Ma, N.; Zheng, Y.; Zhang, J.; Gui, J.; Guo, C.; An, H.; Tan, X.; Yin, Z.; Ma, D. Cobalt/nitrogen-doped porous carbon nanosheets derived from polymerizable ionic liquids as bifunctional electrocatalyst for oxygen evolution and oxygen reduction reaction. ChemCatChem 2017, 9, 1601–1609. [Google Scholar] [CrossRef]
- Wei, D.C.; Liu, Y.Q.; Wang, Y.; Zhang, H.L.; Huang, L.P.; Yu, G. Synthesis of N-doped graphene by chemical vapor deposition and its electrical properties. Nano Lett. 2009, 9, 1752–1758. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, D.; Wang, G.; Miao, S.; Wu, H.; Li, J.; Bao, X. Cobalt nanoparticles encapsulated in nitrogen-doped carbon as a bifunctional catalyst for water electrolysis. J. Mater. Chem. A 2014, 2, 20067–20074. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Z.; Xia, Z.; Dai, L. A metal-free bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Nat. Nanotechnol. 2015, 10, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Jia, Y.; Odedairo, T.; Zhao, X.; Jin, Z.; Zhu, Z.; Yao, X. Activated carbon becomes active for oxygen reduction and hydrogen evolution reactions. Chem. Commun. 2016, 52, 8156–8159. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hu, C.; Yu, C.; Wang, S.; Zhang, P.; Qiu, J. Organic amine-grafted carbon quantum dots with tailored surface and enhanced photoluminescence properties. Carbon 2015, 91, 291–297. [Google Scholar] [CrossRef]
- Hemamalini, S.; Manimekalai, R. Synthesis, structural, magnetic, textural, optical investigation and photocatalytic performance of undoped and doped cobaltite nanoparticles. J. Coord. Chem 2020, 73, 3431–3451. [Google Scholar] [CrossRef]
- Osbeck, S.; Bradley, R.; Liu, C.; Idriss, H.; Ward, S. Effect of an ultraviolet/ozone treatment on the surface texture and functional groups on polyacrylonitrile carbon fibres. Carbon 2011, 49, 4322–4330. [Google Scholar] [CrossRef]
- Chao, H.; Chang, Y.; Mingyu, L.; Xiuna, W.; Qiang, D.; Gang, W.; Jieshan, Q. Nitrogen-doped carbon dots decorated on graphene: A novel all-carbon hybrid electrocatalyst for enhanced oxygen reduction reaction. Chem. Commun. 2015, 51, 3419–3422. [Google Scholar]
- Tahir, M.; Mahmood, N.; Zhang, X.X.; Mahmood, T.; Butt, F.K.; Aslam, I.; Tanveer, M.; Idrees, F.; Khalid, S.; Shakir, I.; et al. Bifunctional catalysts of Co3O4@GCN tubular nanostructured (TNS) hybrids for oxygen and hydrogen evolution reactions. Nano Res. 2015, 8, 3725–3736. [Google Scholar] [CrossRef]
- Zhou, G.-W.; Wang, J.; Gao, P.; Yang, X.; He, Y.-S.; Liao, X.-Z.; Yang, J.; Ma, Z.-F. Facile spray drying route for the three-dimensional graphene-encapsulated Fe2O3 nanoparticles for lithium ion battery anodes. Ind. Eng. Chem. Res. 2013, 52, 1197–1204. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Xia, Z. Mechanisms of Oxygen Reduction Reaction on Nitrogen-Doped Graphene for Fuel Cells. J. Phys. Chem. C 2011, 115, 11170–11176. [Google Scholar] [CrossRef]
- Kurak, K.A.; Anderson, A.B. Nitrogen-treated graphite and oxygen electroreduction on pyridinic edge sites. J. Phys. Chem. C 2009, 113, 6730–6734. [Google Scholar] [CrossRef]
- Xiang, Z.; Xue, Y.; Cao, D.; Huang, L.; Chen, J.F.; Dai, L. Highly efficient electrocatalysts for oxygen reduction based on 2D covalent organic polymers complexed with non-precious metals. Angew. Chem. Int. Ed. 2014, 53, 2433–2437. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Yang, S.; Yan, X.; Leng, J.; Shuang, S.; Ajayan, P.M.; Zhang, Z. Pyridinic-nitrogen-Dominated graphene aerogels with Fe–N–C coordination for highly efficient oxygen reduction reaction. Adv. Funct. Mater. 2016, 26, 5708–5717. [Google Scholar] [CrossRef]
- Kang, G.-S.; Jang, J.-H.; Son, S.-Y.; Lee, Y.-K.; Lee, D.C.; Yoo, S.J.; Lee, S.; Joh, H.-I. Pyrrolic N wrapping strategy to maximize the number of single-atomic Fe-Nx sites for oxygen reduction reaction. J. Power Sources 2022, 520, 230904. [Google Scholar] [CrossRef]
- Zhu, J.; He, C.; Li, Y.; Kang, S.; Shen, P.K. One-step synthesis of boron and nitrogen-dual-self-doped graphene sheets as non-metal catalysts for oxygen reduction reaction. J. Mater. Chem. A 2013, 1, 14700–14705. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Z.; Jia, L.; Xiao, Z. Origin of the catalytic activity of graphite nitride for the electrochemical reduction of oxygen: Geometric factors vs. electronic factors. Phys. Chem. Chem. Phys. 2009, 11, 2730–2740. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Zhang, M.; Liu, H.; Wang, Y. Three-dimensional sulfur-doped graphene supported cobalt-molybdenum bimetallic sulfides nanocrystal with highly interfacial storage capability for supercapacitor electrodes. Electrochim. Acta 2019, 322, 134762. [Google Scholar] [CrossRef]
- Hong, Q.-L.; Zhai, Q.-G.; Liang, X.-L.; Yang, Y.; Li, F.-M.; Jiang, Y.-C.; Hu, M.-C.; Li, S.-N.; Chen, Y. Holey cobalt oxyhydroxide nanosheets for the oxygen evolution reaction. J. Mater. Chem. A 2021, 9, 3297–3302. [Google Scholar] [CrossRef]
- Ren, X.; Ge, R.; Zhang, Y.; Liu, D.; Wu, D.; Sun, X.; Du, B.; Wei, Q. Cobalt–borate nanowire array as a high-performance catalyst for oxygen evolution reaction in near-neutral media. J. Mater. Chem. A 2017, 5, 7291–7294. [Google Scholar] [CrossRef]
- Béjar, J.; Álvarez-Contreras, L.; Ledesma-García, J.; Arjona, N.; Arriaga, L.G. An advanced three-dimensionally ordered macroporous NiCo2O4 spinel as a bifunctional electrocatalyst for rechargeable Zn–air batteries. J. Mater. Chem. A 2020, 8, 8554–8565. [Google Scholar] [CrossRef]
- Liang, Z.; Dong, X. Co2P nanosheet cocatalyst-modified Cd0.5Zn0.5S nanoparticles as 2D-0D heterojunction photocatalysts toward high photocatalytic activity. J. Photochem. Photobiol. A Chem. 2021, 407, 113081. [Google Scholar] [CrossRef]
- Niu, K.; Yang, B.; Cui, J.; Jin, J.; Fu, X.; Zhao, Q.; Zhang, J. Graphene-based non-noble-metal Co/N/C catalyst for oxygen reduction reaction in alkaline solution. J. Power Sources 2013, 243, 65–71. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, G.; Jin, S.; Zhou, Y.; Ji, Q.; Lan, H.; Liu, H.; Qu, J. Graphitic N in nitrogen-doped carbon promotes hydrogen peroxide synthesis from electrocatalytic oxygen reduction. Carbon 2020, 163, 154–161. [Google Scholar] [CrossRef]
- Chen, P.; Wang, L.-K.; Wang, G.; Gao, M.-R.; Ge, J.; Yuan, W.-J.; Shen, Y.-H.; Xie, A.-J.; Yu, S.-H. Nitrogen-doped nanoporous carbon nanosheets derived from plant biomass: An efficient catalyst for oxygen reduction reaction. Energy Environ. Sci. 2014, 7, 4095–4103. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Y.; Zhou, W.; Li, L.; Huang, S.; Chen, S. Biomass-derived nitrogen self-doped porous carbon as effective metal-free catalysts for oxygen reduction reaction. Nanoscale 2015, 7, 6136–6142. [Google Scholar] [CrossRef]
- Chaudhari, K.N.; Song, M.Y.; Yu, J.S. Transforming hair into heteroatom-doped carbon with high surface area. Small 2014, 10, 2625–2636. [Google Scholar] [CrossRef] [PubMed]
- Borghei, M.; Laocharoen, N.; Kibena-Põldsepp, E.; Johansson, L.-S.; Campbell, J.; Kauppinen, E.; Tammeveski, K.; Rojas, O.J. Porous N, P-doped carbon from coconut shells with high electrocatalytic activity for oxygen reduction: Alternative to Pt-C for alkaline fuel cells. Appl. Catal. B Environ. 2017, 204, 394–402. [Google Scholar] [CrossRef]
- Wang, S.; Yu, D.; Dai, L. Polyelectrolyte functionalized carbon nanotubes as efficient metal-free electrocatalysts for oxygen reduction. J. Am. Chem. Soc. 2011, 133, 5182–5185. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, X. Facile preparation of porous carbon nanosheets without nemplate and their excellent electrocatalytic property. ACS Appl. Mater. Interfaces 2013, 5, 11597–11602. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Fellinger, T.-P.; Antonietti, M. Efficient metal-free oxygen reduction in alkaline medium on high-surface-area mesoporous nitrogen-doped carbons made from ionic liquids and nucleobases. J. Am. Chem. Soc. 2011, 133, 206–209. [Google Scholar] [CrossRef]
- Chen, P.; Zang, J.; Zhou, S.; Jia, S.; Tian, P.; Cai, H.; Gao, H.; Wang, Y. N-doped 3D porous carbon catalyst derived from biowaste Triarrhena sacchariflora panicle for oxygen reduction reaction. Carbon 2019, 146, 70–77. [Google Scholar] [CrossRef]
- Wu, C.-H.; Wang, K.-C.; Chang, S.-T.; Chang, Y.-C.; Chen, H.-Y.; Yamanaka, I.; Chiang, T.-C.; Huang, H.-C.; Wang, C.-H. High performance of metal-organic framework-derived catalyst supported by tellurium nanowire for oxygen reduction reaction. Renew. Energ. 2020, 158, 324–331. [Google Scholar] [CrossRef]
- Kim, S.; Myles, T.D.; Kunz, H.R.; Kwak, D.; Wang, Y.; Maric, R. The effect of binder content on the performance of a high temperature polymer electrolyte membrane fuel cell produced with reactive spray deposition technology. Electrochim. Acta 2015, 177, 190–200. [Google Scholar] [CrossRef]
- Lin, L.; Zhu, Q.; Xu, A.-W. Noble-metal-free Fe-N/C catalyst for highly efficient oxygen reduction reaction under both alkaline and acidic conditions. J. Am. Chem. Soc. 2014, 136, 11027–11033. [Google Scholar] [CrossRef]
- Li, S.; Zhang, L.; Liu, H.; Pan, M.; Zan, L.; Zhang, J. Heat-treated cobalt–tripyridyl triazine (Co–TPTZ) electrocatalysts for oxygen reduction reaction in acidic medium. Electrochim. Acta 2010, 55, 4403–4411. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Zhou, M.; Wang, X.; Wang, H.; Yin, Z.; Tan, X.; Li, Y. Preparing Co/N-Doped Carbon as Electrocatalyst toward Oxygen Reduction Reaction via the Ancient “Pharaoh’s Snakes” Reaction. Batteries 2022, 8, 150. https://doi.org/10.3390/batteries8100150
Gao J, Zhou M, Wang X, Wang H, Yin Z, Tan X, Li Y. Preparing Co/N-Doped Carbon as Electrocatalyst toward Oxygen Reduction Reaction via the Ancient “Pharaoh’s Snakes” Reaction. Batteries. 2022; 8(10):150. https://doi.org/10.3390/batteries8100150
Chicago/Turabian StyleGao, Jian, Mengxin Zhou, Xinyao Wang, Hong Wang, Zhen Yin, Xiaoyao Tan, and Yuan Li. 2022. "Preparing Co/N-Doped Carbon as Electrocatalyst toward Oxygen Reduction Reaction via the Ancient “Pharaoh’s Snakes” Reaction" Batteries 8, no. 10: 150. https://doi.org/10.3390/batteries8100150