Mechanical Coating of Zinc Particles with Bi2O3-Li2O-ZnO Glasses as Anode Material for Rechargeable Zinc-Based Batteries
Abstract
:1. Introduction
2. Experimental Setup
2.1. Synthesis of Bi2O3-Li2O-ZnO Glass
2.2. Synthesis of Zinc–Glass Composite Material
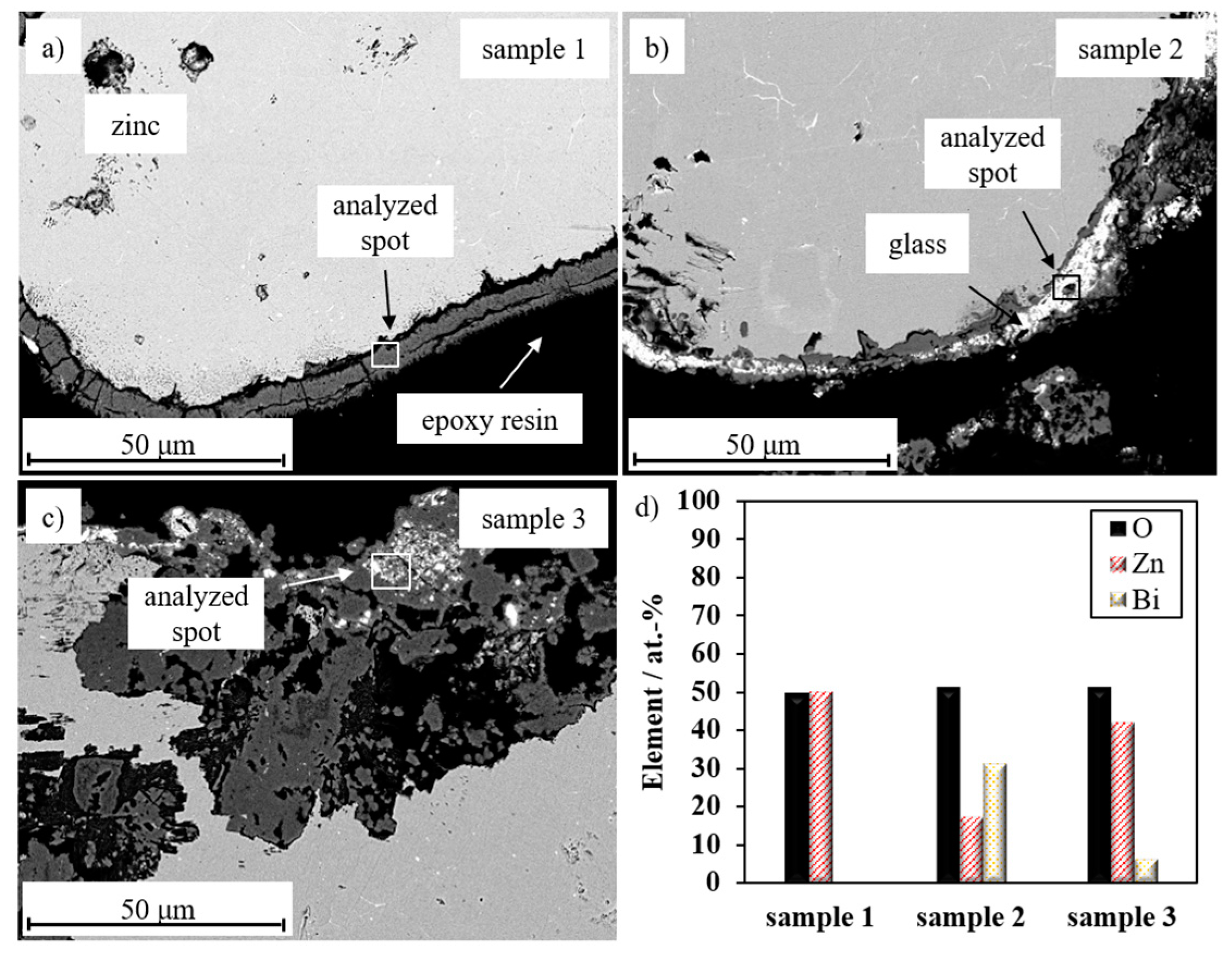
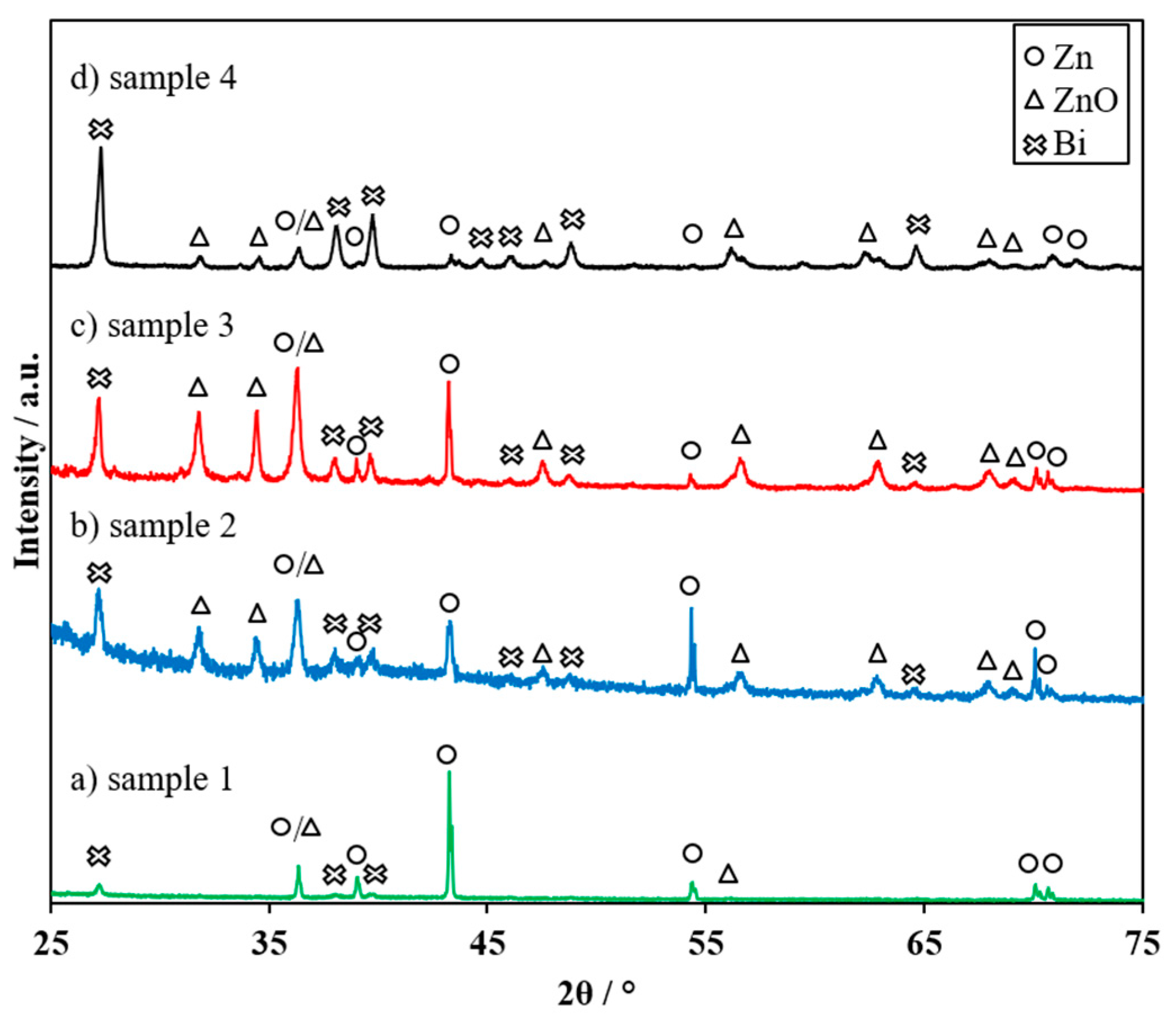
2.3. Analytical Methods
2.4. Electrochemical Characterization
3. Results and Discussion
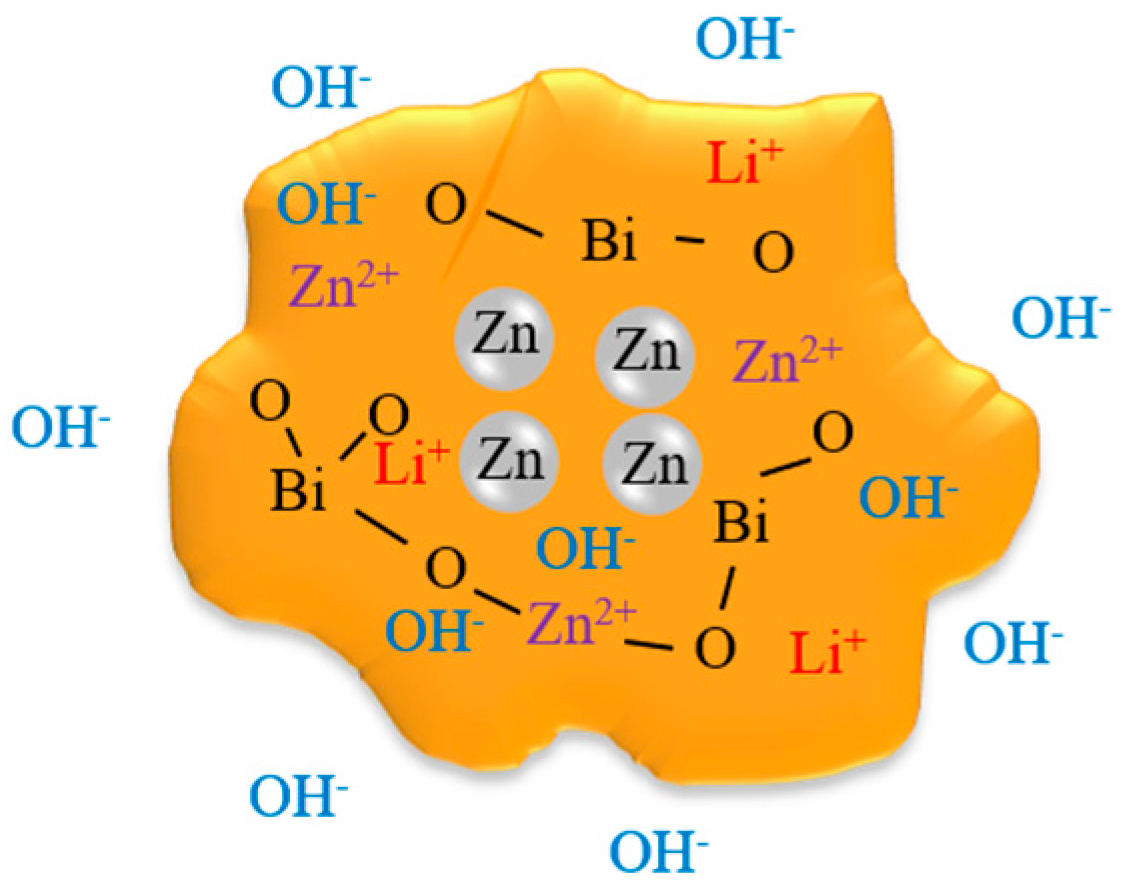
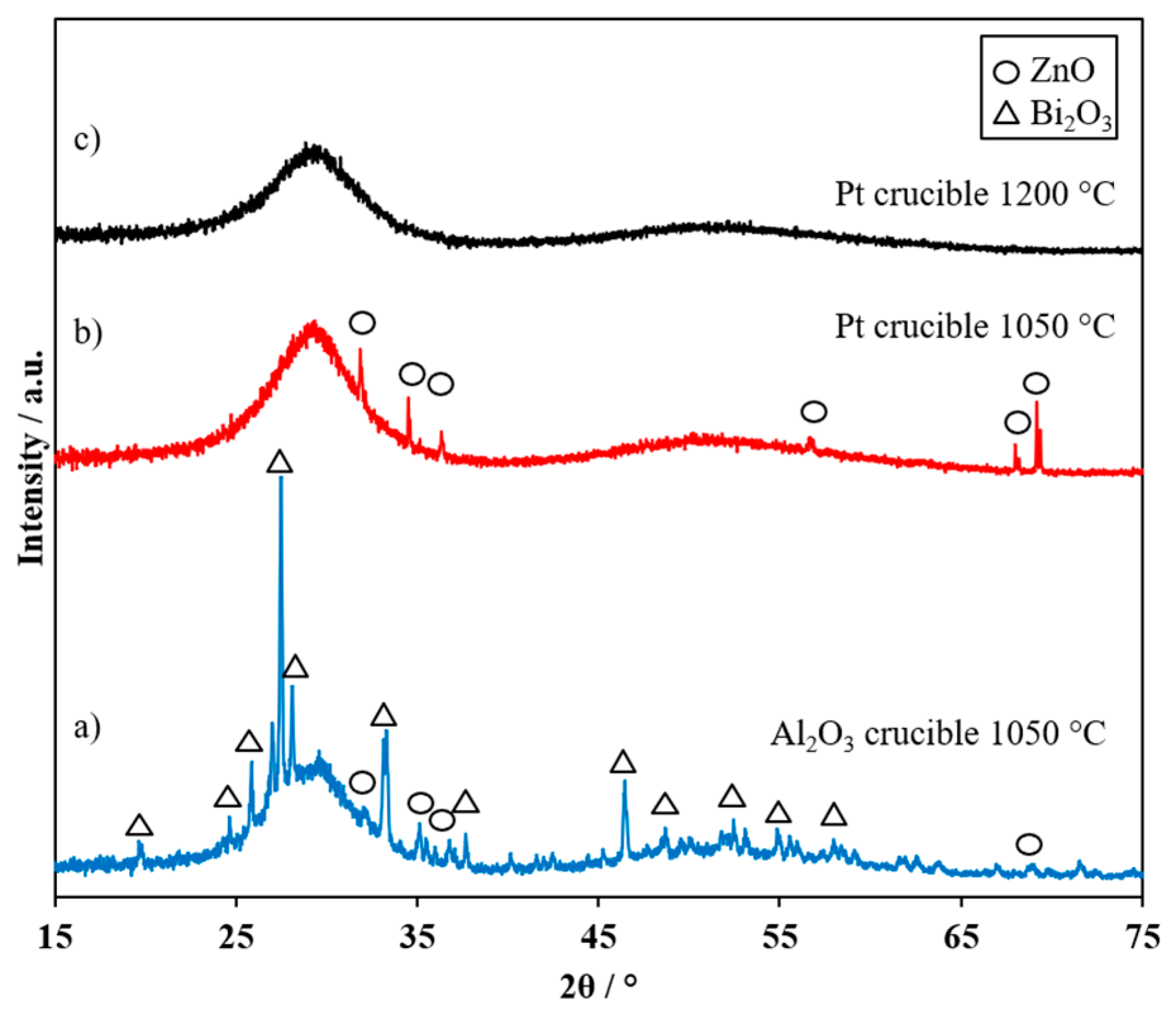
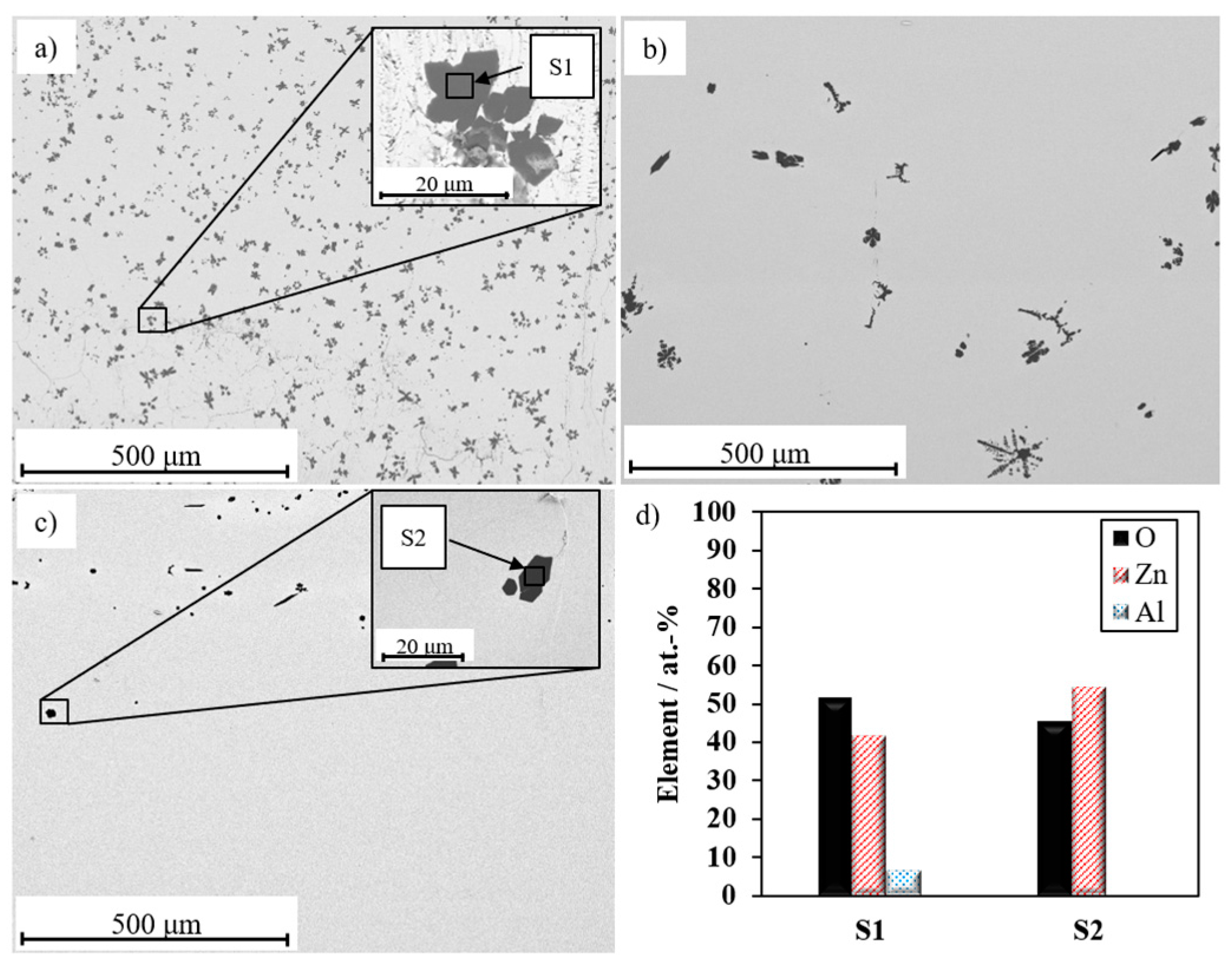
3.1. Optimization of Process Conditions of Bi2O3-Li2O-ZnO Glass
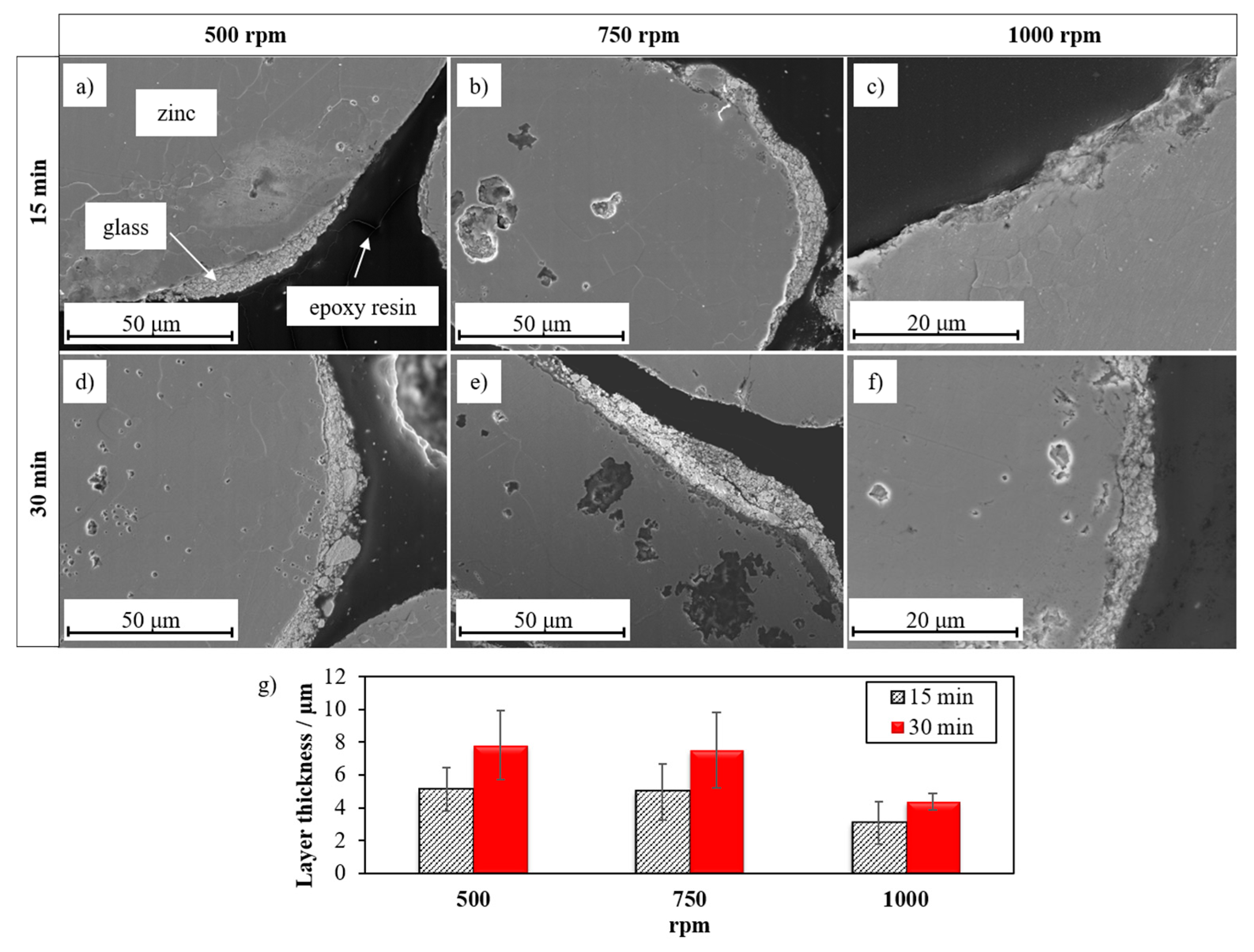
3.2. Optimization of the Coating Parameters for Zinc–Glass Composite Material
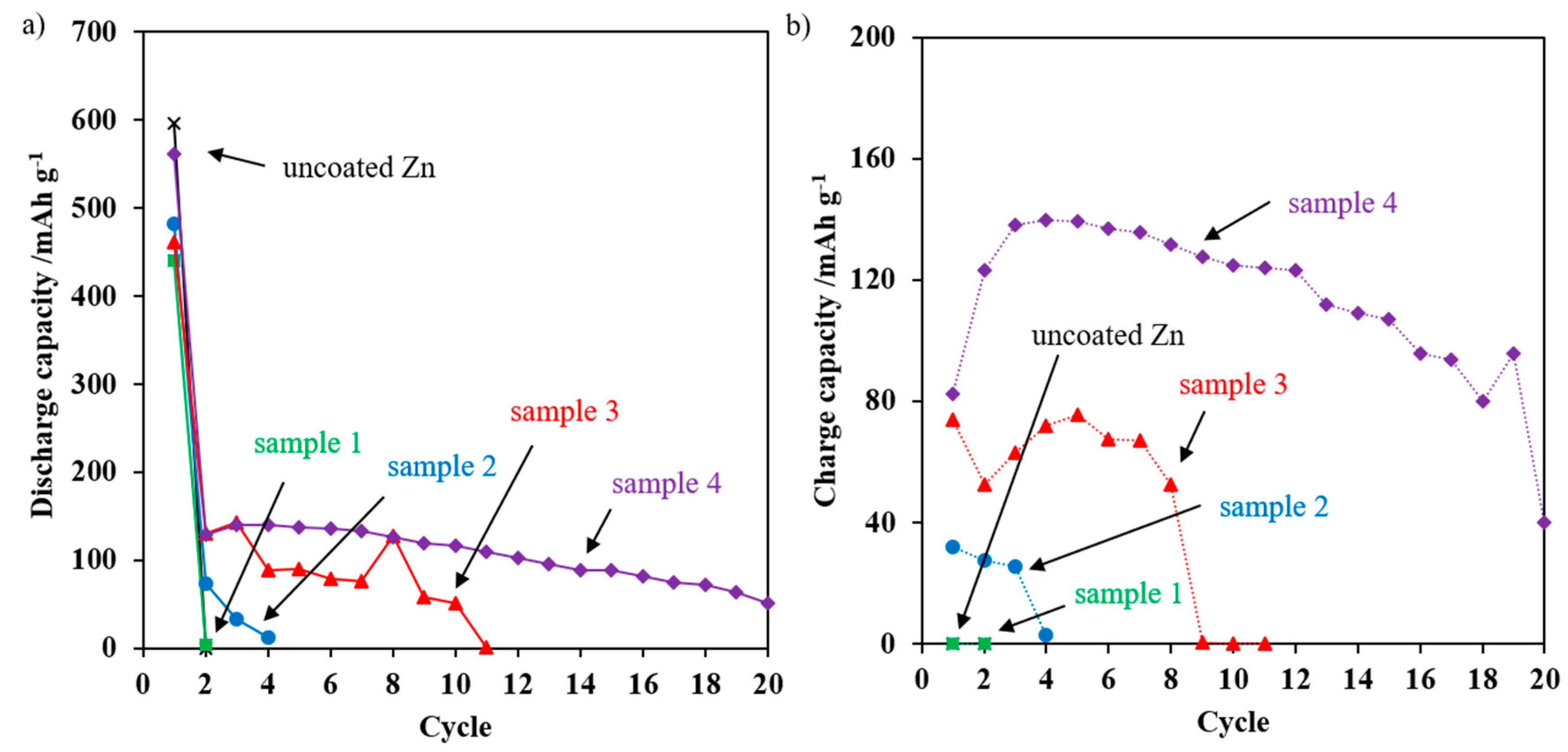
3.3. Electrochemical Performance
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ibrahim, H.; Ilinca, A.; Perron, J. Energy storage systems—Characteristics and comparisons. Renew. Sustain. Energy Rev. 2008, 12, 1221–1250. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Pei, P.; Wang, K.; Ma, Z. Technologies for extending zinc–air battery’s cyclelife: A review. Appl. Energy 2014, 128, 315–324. [Google Scholar] [CrossRef]
- Fu, J.; Cano, Z.P.; Park, M.G.; Yu, A.; Fowler, M.; Chen, Z. Electrically rechargeable zinc–air batteries: Progress, challenges, and perspectives. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.D.; Fey, G.T.K. Surface treatment of zinc anodes to improve discharge capacity and suppress hydrogen gas evolution. J. Power Sources 2008, 184, 610–616. [Google Scholar] [CrossRef]
- Xu, M.; Ivey, D.G.; Xie, Z.; Qu, W. Rechargeable Zn-air batteries: Progress in electrolyte development and cell configuration advancement. J. Power Sources 2015, 283, 358–371. [Google Scholar] [CrossRef]
- Larsson, F.; Rytinki, A.; Ahmed, I.; Albinsson, I.; Mellander, B.-E. Overcurrent Abuse of Primary Prismatic Zinc–Air Battery Cells Studying Air Supply Effects on Performance and Safety Shut-Down. Batteries 2017, 3, 1. [Google Scholar] [CrossRef]
- Garcia, G.; Schuhmann, W.; Ventosa, E. A Three-Electrode, Battery-Type Swagelok Cell for the Evaluation of Secondary Alkaline Batteries: The Case of the Ni–Zn Battery. ChemElectroChem 2016, 3, 592–597. [Google Scholar] [CrossRef]
- Ma, H.; Wang, B.; Fan, Y.; Hong, W. Development and Characterization of an Electrically Rechargeable Zinc-Air Battery Stack. Energies 2014, 7, 6549–6557. [Google Scholar] [CrossRef]
- Franke-Lang, R.; Arlt, T.; Manke, I.; Kowal, J. X-ray tomography as a powerful method for zinc-air battery research. J. Power Sources 2017, 370, 45–51. [Google Scholar] [CrossRef]
- Xu, N.; Qiao, J.; Nie, Q.; Wang, M.; Xu, H.; Wang, Y.; Zhou, X.D. CoFe2O4 nanoparticles decorated carbon nanotubes: Air-cathode bifunctional catalysts for rechargeable zinc-air batteries. Catal. Today 2017. [Google Scholar] [CrossRef]
- Stamm, J.; Varzi, A.; Latz, A.; Horstmann, B. Modeling nucleation and growth of zinc oxide during discharge of primary zinc-air batteries. J. Power Sources 2017, 360, 136–149. [Google Scholar] [CrossRef]
- Parker, J.F.; Chervin, C.N.; Pala, I.R.; Machler, M.; Burz, M.F.; Long, J.W.; Rolison, D.R. Rechargeable nickel–3D zinc batteries: An energy-dense, safer alternative to lithium-ion. Science 2017, 356, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Tai Kim, S.; Cao, R.; Choi, N.S.; Liu, M.; Lee, K.T.; Cho, J. Metal–air batteries with high energy density: Li–air versus Zn–air. Adv. Energy Mater. 2011, 1, 34–50. [Google Scholar] [CrossRef]
- Garcia, G.; Ventosa, E.; Schuhmann, W. Complete prevention of dendrite formation in Zn metal anodes by means of pulsed charging protocols. ACS Appl. Mater. Interfaces 2017, 9, 18691–18698. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Kim, E.J.; Kim, S.J.; Shin, H.C. Influence of ZnO precipitation on the cycling stability of rechargeable Zn–air batteries. J. Appl. Electrochem. 2015, 45, 335–342. [Google Scholar] [CrossRef]
- Yang, H.; Cao, Y.; Ai, X.; Xiao, L. Improved discharge capacity and suppressed surface passivation of zinc anode in dilute alkaline solution using surfactant additives. J. Power Sources 2004, 128, 97–101. [Google Scholar] [CrossRef]
- Shin, J.; You, J.M.; Lee, J.Z.; Kumar, R.; Yin, L.; Wang, J.; Meng, Y.S. Deposition of ZnO on bismuth species towards a rechargeable Zn-based aqueous battery. Phys. Chem. Chem. Phys. 2016, 18, 26376–26382. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cui, T.; Pulletikurthi, G.; Lahiri, A.; Carstens, T.; Olschewski, M.; Endres, F. Dendrite-Free Nanocrystalline Zinc Electrodeposition from an Ionic Liquid Containing Nickel Triflate for Rechargeable Zn-Based Batteries. Angew. Chem. Int. Ed. 2016, 55, 2889–2893. [Google Scholar] [CrossRef] [PubMed]
- McBreen, J.; Gannon, E. Bismuth oxide as an additive in pasted zinc electrodes. J. Power Sources 1985, 15, 169–177. [Google Scholar] [CrossRef]
- Huang, H.; Gu, S.J.; Gan, Y.P.; Tao, X.Y.; Zhang, W.K. ZnO/ZnO-Bi2O3 Nanocomposite as an Anode Material for Ni-Zn Rechargeable Battery. Adv. Mater. Res. 2012, 396, 1725–1729. [Google Scholar] [CrossRef]
- Yuan, Y.F.; Yu, L.Q.; Wu, H.M.; Yang, J.L.; Chen, Y.B.; Guo, S.Y.; Tu, J.P. Electrochemical performances of Bi based compound film-coated ZnO as anodic materials of Ni–Zn secondary batteries. Electrochim. Acta 2011, 56, 4378–4383. [Google Scholar] [CrossRef]
- Schmid, M.; Willert-Porada, M. Electrochemical behavior of zinc particles with silica based coatings as anode material for zinc air batteries with improved discharge capacity. J. Power Sources 2017, 351, 115–122. [Google Scholar] [CrossRef]
- Clark, S.; Latz, A.; Horstmann, B. Rational Development of Neutral Aqueous Electrolytes for Zinc-Air Batteries. ChemSusChem 2017, 10, 4735–4747. [Google Scholar] [CrossRef] [PubMed]
- Ingale, P.; Sakthivel, M.; Drillet, J.F. Test of Diethylmethylammonium Trifluoromethanesulfonate Ionic Liquid as Electrolyte in Electrically Rechargeable Zn/Air Battery. J. Electrochem. Soc. 2017, 164, 5224–5229. [Google Scholar] [CrossRef]
- Moser, F.; Fourgeot, F.; Rouget, R.; Crosnier, O.; Brousse, T. In situ X-ray diffraction investigation of zinc based electrode in Ni–Zn secondary batteries. Electrochim. Acta 2013, 109, 110–116. [Google Scholar] [CrossRef]
- Schmid, M.; Willert-Porade, M. Zinc particles coated with bismuth oxide based glasses as anode material for zinc air batteries with improved electrical rechargeability. Electrochim. Acta 2018, 260, 246–253. [Google Scholar] [CrossRef]
- Fu, J.; Yatsuda, H. New families of glasses based on Bi2O3. Phys. Chem. Glasses 1995, 36, 211–215. [Google Scholar]
| Component | Bi2O3 | Li2O | ZnO |
|---|---|---|---|
| Amount in mol % | 60 | 15 | 25 |
| Parameter | Value | |||
|---|---|---|---|---|
| Sample Name | Sample 1 (Figure 4c) | Sample 2 (Figure 4e) | Sample 3 (Figure 4f) | Sample 4 |
| Schematic illustration (see Figure 4) |  |  |  |  |
| Zinc powder/μm | 250 | 250 | 250 | 30 |
| Rotational speed/rpm | 1000 | 750 | 1000 | 1000 |
| Coating time/min | 15 | 30 | 30 | 30 |
| Layer thickness/μm (see Figure 4) | 3.0 1.3 | 7.5 2.1 | 4.4 0.5 | 2.0 ± 0.3 |
| Glass content/wt % | 1.61 | 4.39 | 4.83 | 10.72 |
| Zinc surface coverage with glass/g m−2 | 0.82 | 2.23 | 2.45 | 2.63 |
| Parameter | Value | ||||
|---|---|---|---|---|---|
| Sample Name | Pure Zinc | Sample 1 | Sample 2 | Sample 3 | Sample 4 |
| Ri before first discharge/Ω | 1.16 ± 0.02 | 1.03 ± 0.01 | 1.20 ± 0.01 | 1.14 ± 0.01 | 1.10 ± 0.01 |
| Ri after first discharge/Ω | 9.08 ± 0.39 | 11.29 ± 0.41 | 3.69 ± 0.77 | 1.68 ± 0.04 | 1.25 ± 0.02 |
| Ri after first recharge/Ω | 8.95 ± 0.38 * | 6.30 ± 0.15 * | 1.32 ± 0.01 | 1.35 ± 0.06 | 1.17 ± 0.02 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michlik, T.; Schmid, M.; Rosin, A.; Gerdes, T.; Moos, R. Mechanical Coating of Zinc Particles with Bi2O3-Li2O-ZnO Glasses as Anode Material for Rechargeable Zinc-Based Batteries. Batteries 2018, 4, 12. https://doi.org/10.3390/batteries4010012
Michlik T, Schmid M, Rosin A, Gerdes T, Moos R. Mechanical Coating of Zinc Particles with Bi2O3-Li2O-ZnO Glasses as Anode Material for Rechargeable Zinc-Based Batteries. Batteries. 2018; 4(1):12. https://doi.org/10.3390/batteries4010012
Chicago/Turabian StyleMichlik, Tobias, Manuela Schmid, Andreas Rosin, Thorsten Gerdes, and Ralf Moos. 2018. "Mechanical Coating of Zinc Particles with Bi2O3-Li2O-ZnO Glasses as Anode Material for Rechargeable Zinc-Based Batteries" Batteries 4, no. 1: 12. https://doi.org/10.3390/batteries4010012
APA StyleMichlik, T., Schmid, M., Rosin, A., Gerdes, T., & Moos, R. (2018). Mechanical Coating of Zinc Particles with Bi2O3-Li2O-ZnO Glasses as Anode Material for Rechargeable Zinc-Based Batteries. Batteries, 4(1), 12. https://doi.org/10.3390/batteries4010012





