Recent Development of Carbonaceous Materials for Lithium–Sulphur Batteries
Abstract
:1. Introduction
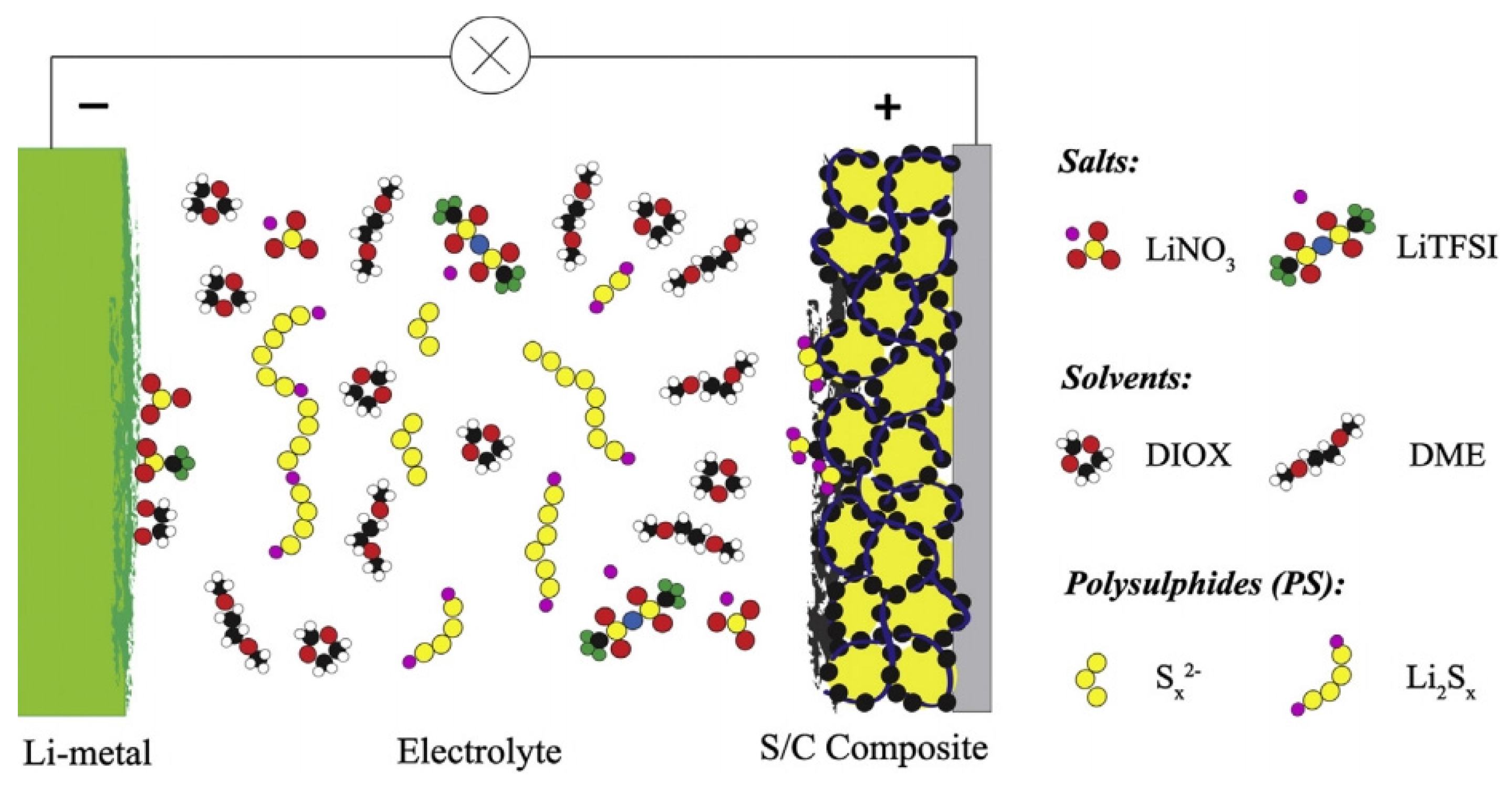
1.1. Working Mechanism of Lithium–Sulphur Batteries
1.2. The Challenges of Lithium–Sulphur Batteries for Commercialization
1.2.1. Poor Electrical Conductivity of Sulphur and Li2S/Li2S2
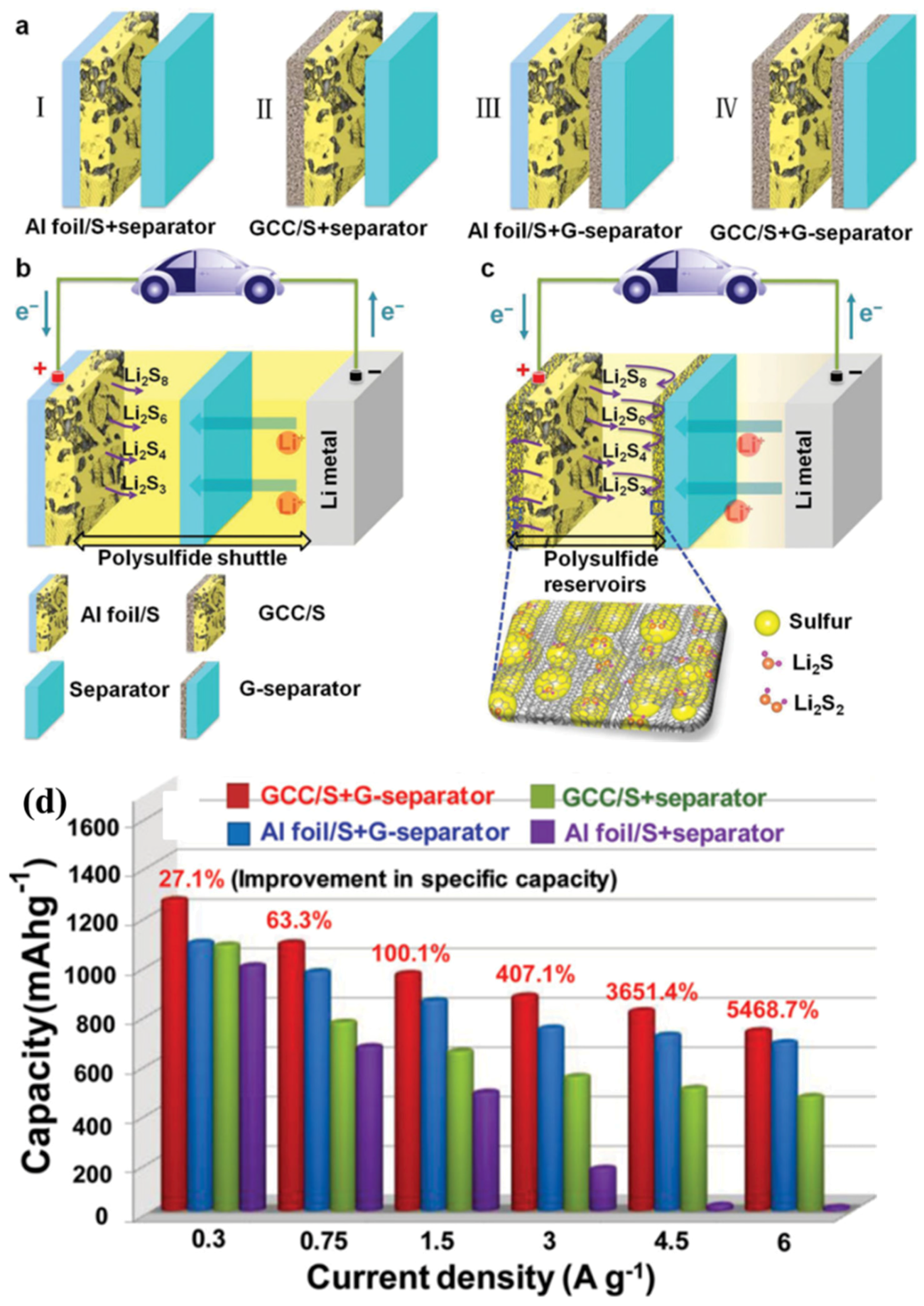
1.2.2. Dissolution of Polysulphides and the Related Shuttle Effect
1.2.3. Self-Discharge
1.2.4. Volume Expansion
1.3. Strategies for Improving the Performances of Lithium–Sulphur Batteries
2. Carbonaceous Materials for the Cathode of Lithium–Sulphur Batteries
2.1. Porous Carbon for the Lithium–Sulphur Electrode
2.1.1. Microporous Carbon–Sulphur Composites
2.1.2. Mesoporous Carbon–Sulphur Composites
2.1.3. Hierarchical Porous Carbon–Sulphur Composites
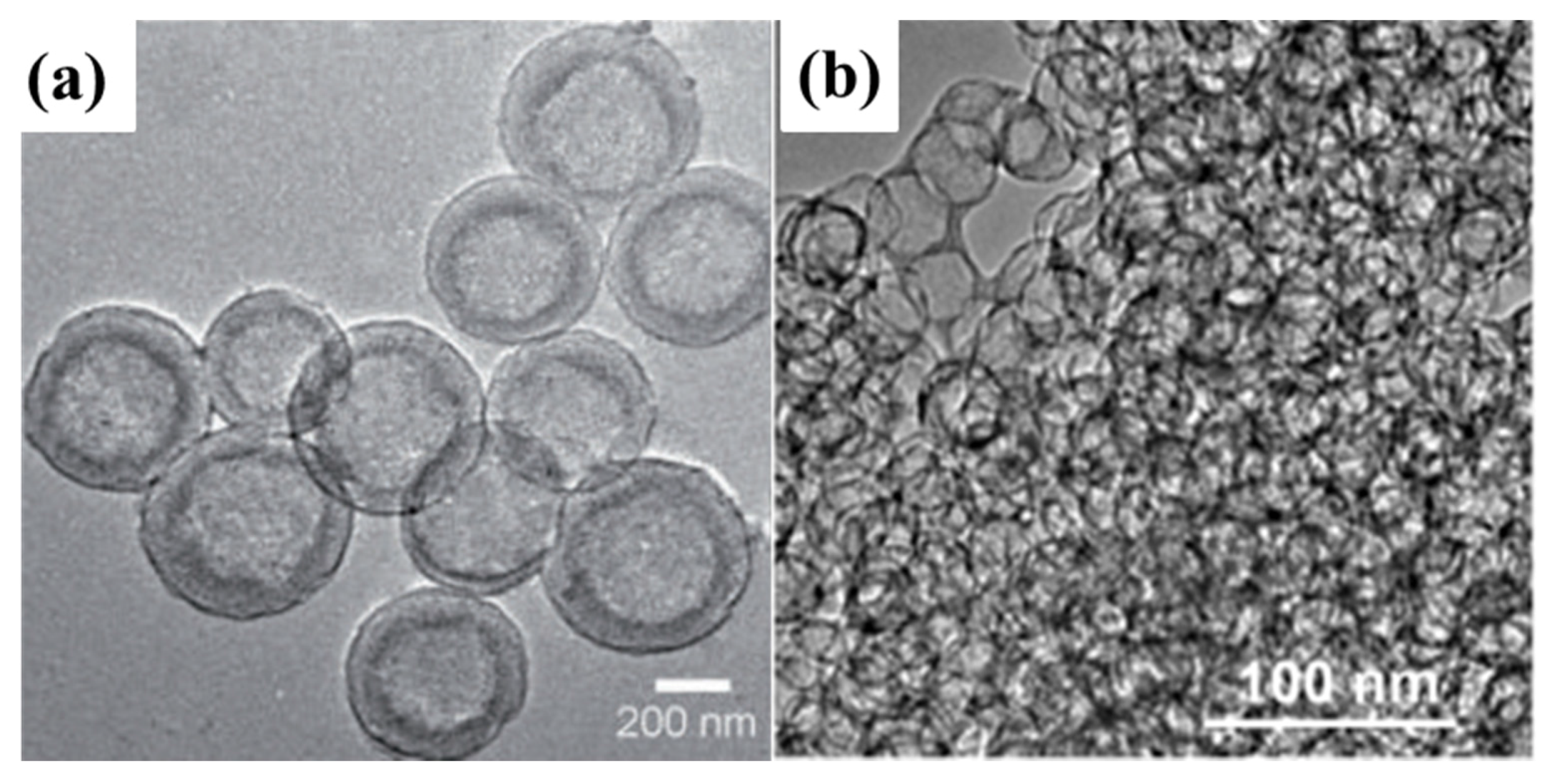
2.1.4. Hollow Carbon–Sulphur Composites
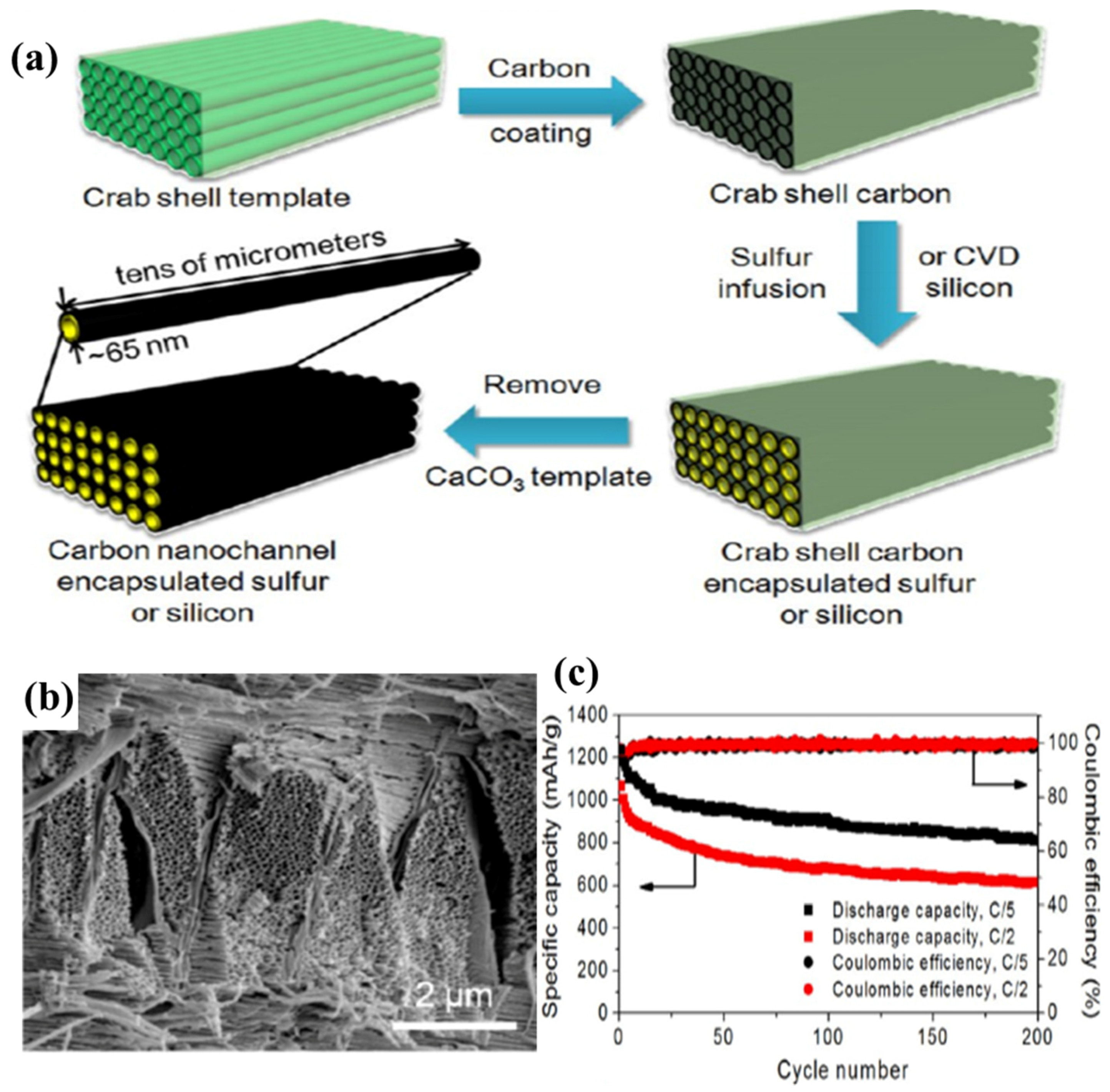
2.1.5. Biochar–Sulphur Composites
2.2. One-Dimensional Carbon Nanostructure/Sulphur Electrodes
2.2.1. Carbon Nanofiber–Sulphur Composites
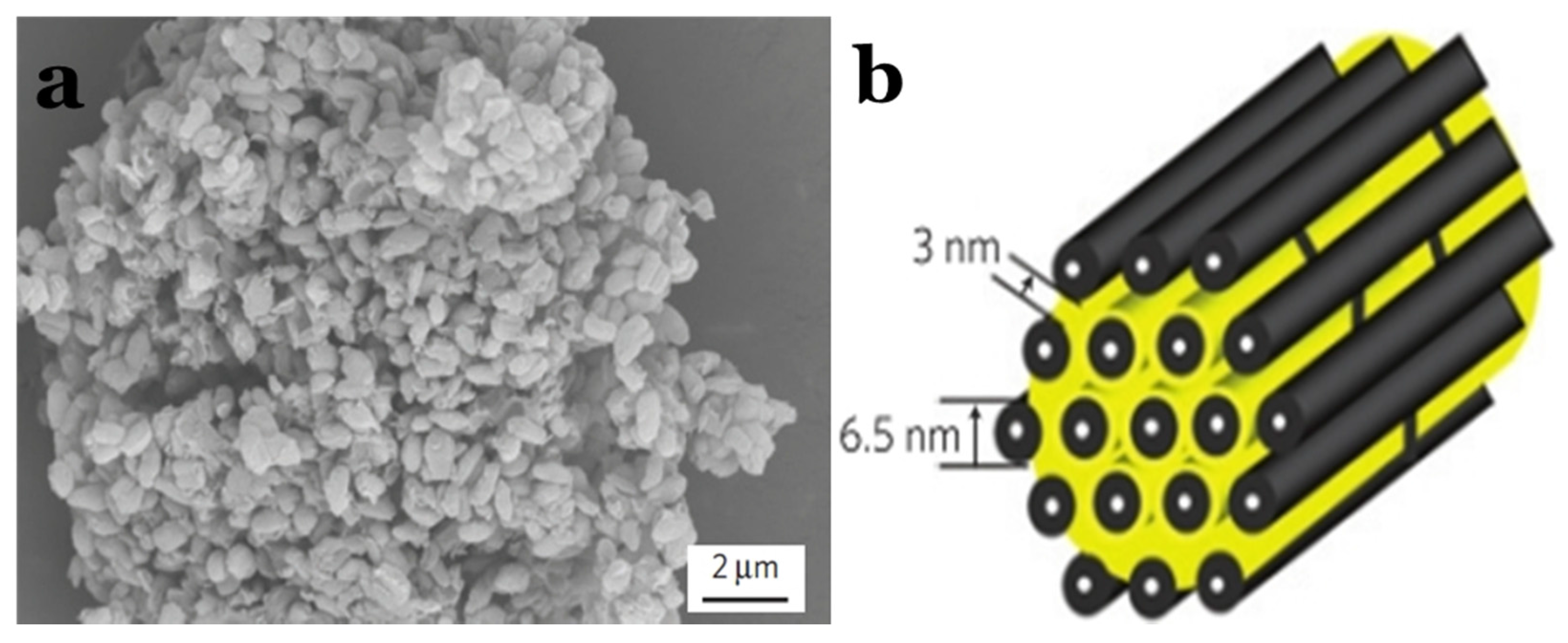
2.2.2. Carbon Nanotube–Sulphur Composites
2.3. Two-Dimensional Carbon Nanostructure/Sulphur Electrodes
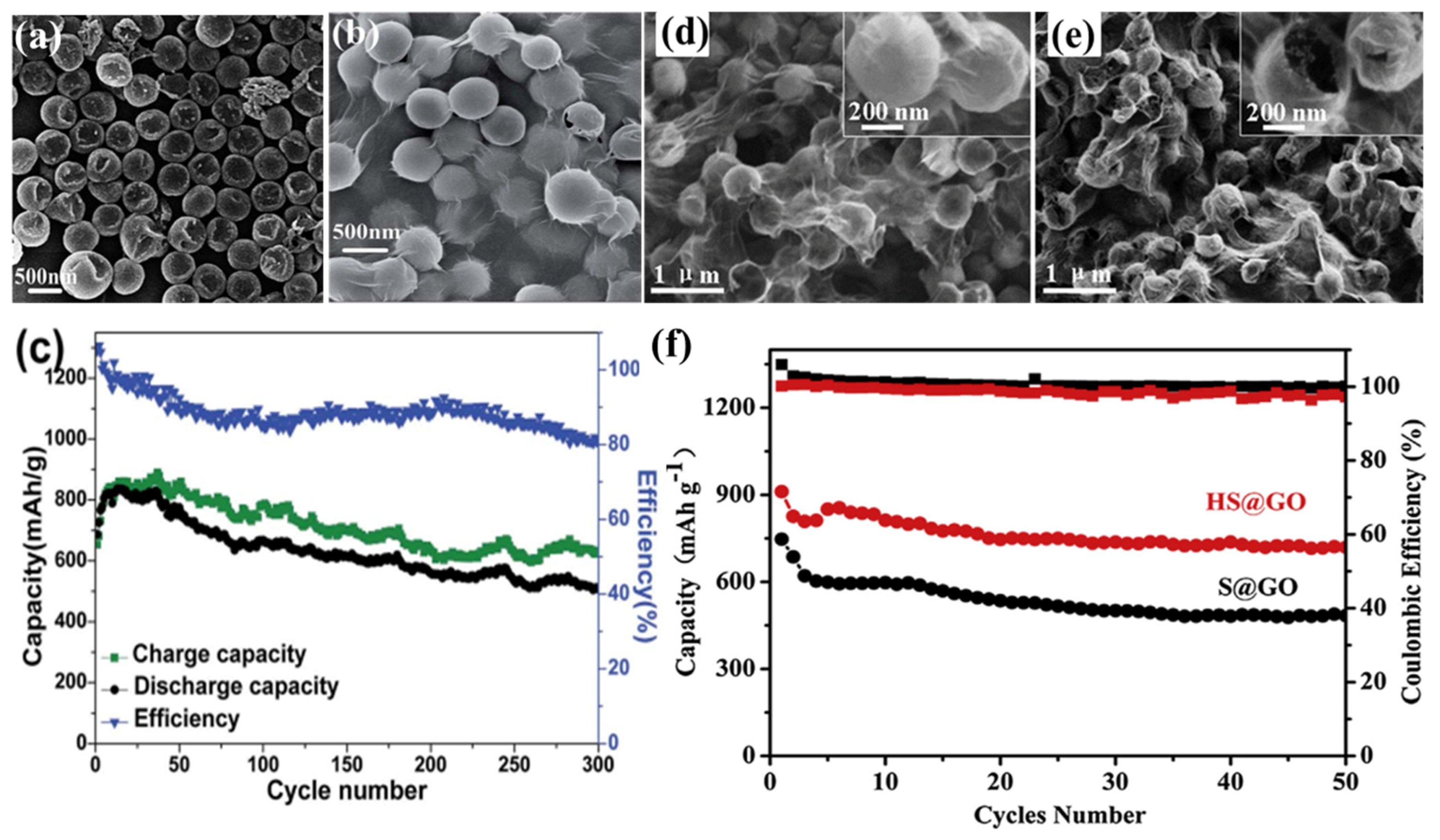
2.3.1. Graphene Oxide–Sulphur Composite Cathodes
2.3.2. Graphene/Reduced Graphene Oxide–Sulphur Composite Cathodes
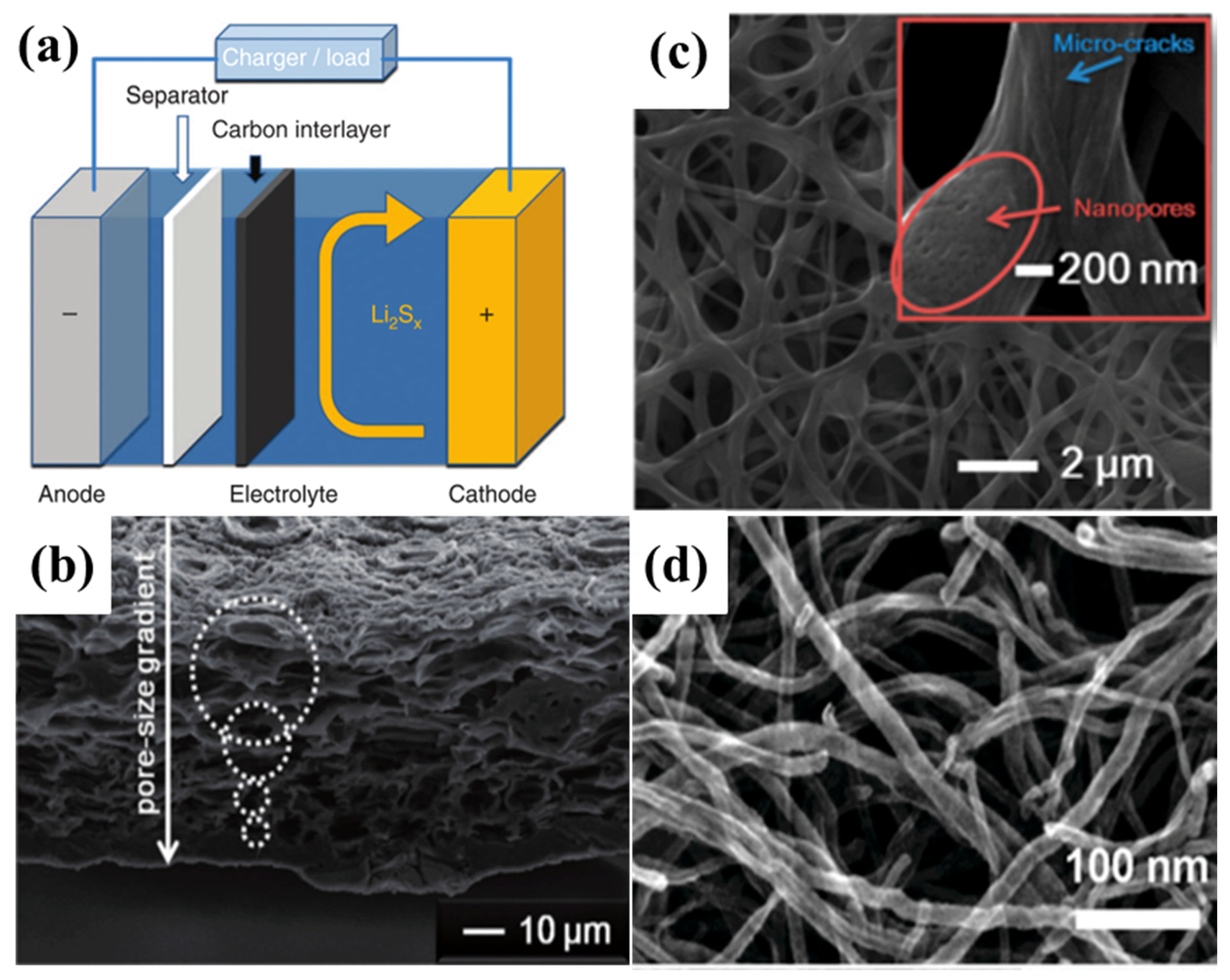
2.4. Carbon Interlayers for Lithium–Sulphur Batteries
2.5. Carbon-Modified Separators
3. Summary and Perspective
3.1. Materials Manipulation (Without Sacrificing Sulphur Content)
3.2. Novel Cell Configurations
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, J.-G.; Xie, K.; Wei, B. Advanced engineering of nanostructured carbons for lithium-sulphur batteries. Nano Energy 2015, 15, 413–444. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.W.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical energy storage for green grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef] [PubMed]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.M. Li-O2 and Li–S batteries with high energy storage. Nat. Mater. 2012, 11, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.X.; Zhang, S.Q.; Hou, Y.L. Graphene-based sulphur composites for energy storage and conversion in Li–S batteries. Chin. J. Chem. 2016, 34, 11–31. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, G.; Cui, Y. Nanostructured sulphur cathodes. Chem. Soc. Rev. 2013, 42, 3018–3032. [Google Scholar] [CrossRef] [PubMed]
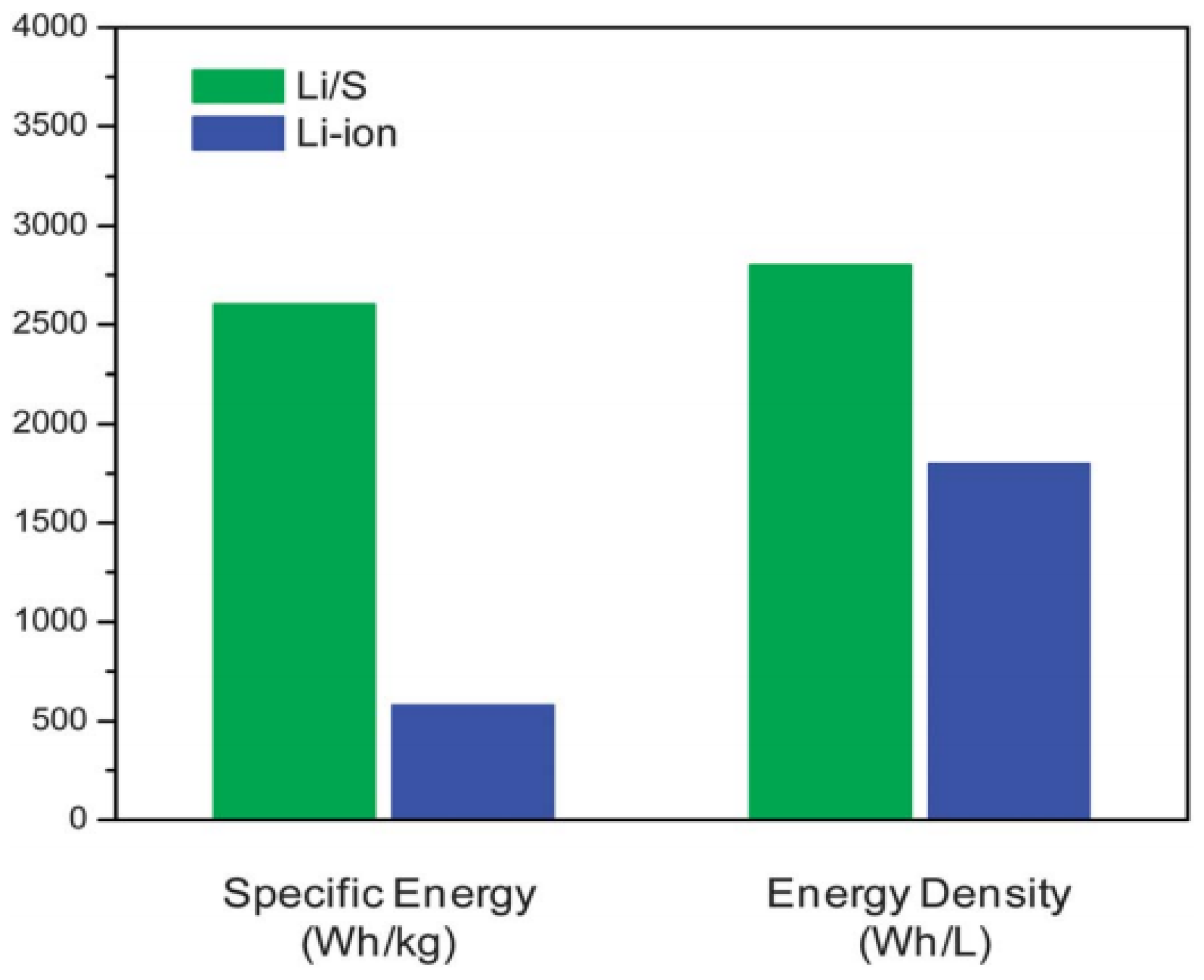
- Song, M.K.; Cairns, E.J.; Zhang, Y. Lithium/sulphur batteries with high specific energy: Old challenges and new opportunities. Nanoscale 2013, 5, 2186–2204. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Tong, C.-J.; Lai, C.; Qiu, J.; Huang, X.; Yang, W.; Wen, B.; Liu, L.-M.; Hou, Y.; Zhang, S. Porous nitrogen and phosphorous dual doped graphene blocking layer for high performance Li–S batteries. J. Mater. Chem. A 2015, 3, 16670–16678. [Google Scholar] [CrossRef]
- Scheers, J.; Fantini, S.; Johansson, P. A review of electrolytes for lithium–sulphur batteries. J. Power Sources 2014, 255, 204–218. [Google Scholar] [CrossRef]
- Jayaprakash, N.; Shen, J.; Moganty, S.S.; Corona, A.; Archer, L.A. Porous hollow carbon@sulphur composites for high-power lithium-sulphur batteries. Angew. Chem. Int. Ed. 2011, 50, 5904–5908. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Cao, Y.; Xiao, J.; Schwenzer, B.; Engelhard, M.H.; Saraf, L.V.; Nie, Z.; Exarhos, G.J.; Liu, J. A soft approach to encapsulate sulphur: polyaniline nanotubes for lithium-sulphur batteries with long cycle life. Adv. Mater. 2012, 24, 1176–1181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiang, J.; Dong, Z.; Liu, Y.; Wu, Y.; Xu, C.; Du, G. Biomass derived activated carbon with 3D connected architecture for rechargeable lithium-sulphur batteries. Electrochim. Acta 2014, 116, 146–151. [Google Scholar] [CrossRef]
- Lin, Z.; Liang, C. Lithium-sulphur batteries: from liquid to solid cells. J. Mater. Chem. A 2015, 3, 936–958. [Google Scholar] [CrossRef]
- Mikhaylik, Y.V.; Akridge, J.R. Polysulphide shuttle study in the Li/S battery system. J. Electrochem. Soc. 2004, 151, A1969–A1976. [Google Scholar] [CrossRef]
- Gu, X.; Wang, Y.; Lai, C.; Qiu, J.; Li, S.; Hou, Y.; Martens, W.; Mahmood, N.; Zhang, S. Microporous bamboo biochar for lithium-sulphur batteries. Nano Res. 2015, 8, 129–139. [Google Scholar] [CrossRef]
- Rehman, S.; Gu, X.; Khan, K.; Mahmood, N.; Yang, W.; Huang, X.; Guo, S.; Hou, Y. 3D vertically aligned and interconnected porous carbon nanosheets as sulphur immobilizers for high performance lithium-sulphur batteries. Adv. Energy Mater. 2016, 6, 1502518–1502525. [Google Scholar] [CrossRef]
- Xin, S.; Yin, Y.-X.; Wan, L.-J.; Guo, Y.-G. Encapsulation of sulphurin a hollow porous carbon substrate for superior Li–S batteries with long lifespan. Part. Part. Syst. Charact. 2013, 30, 321–325. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, Y.; Yuan, L.; Yi, Z.; Wu, C.; Liu, Y.; Strasser, P.; Huang, Y. A highly ordered meso@microporous carbon-supported sulphur@smaller sulphur core-shell structured cathode for Li–S batteries. ACS Nano 2014, 8, 9295–9303. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Lai, C.; Liu, F.; Yang, W.; Hou, Y.; Zhang, S. A conductive interwoven bamboo carbon fiber membrane for Li–S batteries. J. Mater. Chem. A 2015, 3, 9502–9509. [Google Scholar] [CrossRef]
- Gu, X.; Tong, C.J.; Rehman, S.; Liu, L.M.; Hou, Y.; Zhang, S. Multifunctional nitrogen-doped loofah sponge carbon blocking layer for high-performance rechargeable lithium batteries. ACS Appl. Mater. Interface 2016, 8, 15991–16001. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Manthiram, A. Enhanced cyclability of lithium-sulphur batteries by a polymer acid-doped polypyrrole mixed ionic-electronic conductor. Chem. Mater. 2012, 24, 3081–3087. [Google Scholar] [CrossRef]
- Li, G.C.; Hu, J.J.; Li, G.R.; Ye, S.H.; Gao, X.P. Sulphur/activated-conductive carbon black composites as cathode materials for lithium/sulphur battery. J. Power Sources 2013, 240, 598–605. [Google Scholar] [CrossRef]
- Su, Y.-S.; Manthiram, A. A facile in situ sulphur deposition route to obtain carbon-wrapped sulphur composite cathodes for lithium–sulphur batteries. Electrochim. Acta 2012, 77, 272–278. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Xie, J.; Xu, N. A novel conductive polymer-sulphur composite cathode material for rechargeable lithium-sulphur batteries. Adv. Mater. 2002, 14, 963–965. [Google Scholar] [CrossRef]
- Zhou, W.; Yu, Y.; Chen, H.; DiSalvo, F.J.; Abruna, H.D. Yolk-shell structure of polyaniline-coated sulphurfor lithium-sulphur batteries. J. Am. Chem. Soc. 2013, 135, 16736–16743. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Tong, C.-J.; Wen, B.; Liu, L.-M.; Lai, C.; Zhang, S. Ball-milling synthesis of ZnO@sulphur/carbon nanotubes and Ni(OH)2@sulphur/carbon nanotubes composites for high-performance lithium-sulphur batteries. Electrochim. Acta 2016, 196, 369–376. [Google Scholar] [CrossRef]
- Liang, X.; Nazar, L.F. In situ reactive assembly of scalable core–shell sulphur–MnO2 composite cathodes. ACS Nano 2016, 10, 4192–4198. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Ou, X.; Yue, X.; Yang, Y.; Wang, Z.; Rooney, D.; Sun, K. A simply effective double-coating cathode with MnO2 nanosheets/graphene as functionalized interlayer for high performance lithium-sulphur batteries. Electrochim. Acta 2016, 207, 198–206. [Google Scholar] [CrossRef]
- Yu, M.; Ma, J.; Song, H.; Wang, A.; Tian, F.; Wang, Y.; Qiu, H.; Wang, R. Atomic layer deposited TiO2 on a nitrogen-doped graphene/sulphur electrode for high performance lithium-sulphur batteries. Energy Environ. Sci. 2016, 9, 1495–1503. [Google Scholar] [CrossRef]
- Demir-Cakan, R.; Morcrette, M.; Nouar, F.; Davoisne, C.; Devic, T.; Gonbeau, D.; Dominko, R.; Serre, C.; Ferey, G.; Tarascon, J.M. Cathode composites for Li–S batteries via the use of oxygenated porous architectures. J. Am. Chem. Soc. 2011, 133, 16154–16160. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Chen, F.; Xia, Y.; Huang, H.; Gan, Y.; Chen, X.; Zhang, W. Decoration of sulphur with porous metal nanostructures: An alternative strategy for improving the cyclability of sulphur cathode materials for advanced lithium-sulphur batteries. Chem. Commun. 2013, 49, 4513–4515. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Tian, J.; Wu, D.; Gu, M.; Xu, W.; Wang, C.; Gao, F.; Engelhard, M.H.; Zhang, J.G.; Liu, J.; et al. Lewis acid-base interactions between polysulphides and metal organic framework in lithium sulphur batteries. Nano Lett. 2014, 14, 2345–2352. [Google Scholar] [CrossRef] [PubMed]
- Demir-Cakan, R.; Morcrette, M.; Gueguen, A.; Dedryvere, R.; Tarascon, J.M. Li–S batteries: Simple approaches for superior performance. Energy Environ. Sci. 2013, 6, 176–182. [Google Scholar] [CrossRef]
- Li, G.; Ling, M.; Ye, Y.; Li, Z.; Guo, J.; Yao, Y.; Zhu, J.; Lin, Z.; Zhang, S. Acacia senegal-inspired bifunctional binder for longevity of lithium–sulfur batteries. Adv. Energy Mater. 2015, 5. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, Z.; Fu, W.; Dudney, N.J.; Liang, C. Phosphorous pentasulfide as a novel additive for high-performance lithium-sulphur batteries. Adv. Funct. Mater. 2013, 23, 1064–1069. [Google Scholar] [CrossRef]
- Suo, L.; Hu, Y.S.; Li, H.; Armand, M.; Chen, L. A new class of solvent-in-salt electrolyte for high-energy rechargeable metallic lithium batteries. Nat. Commun. 2013, 4, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Bao, W.; Lu, H.; Jia, M.; Xie, K.; Lai, Y.; Li, J. Water-soluble polyacrylic acid as a binder for sulphur cathode in lithium-sulphur battery. ECS Electrochem. Lett. 2012, 1, A34–A37. [Google Scholar] [CrossRef]
- He, B.; Li, W.C.; Yang, C.; Wang, S.Q.; Lu, A.H. Incorporating sulphur inside the pores of carbons for advanced lithium-sulphur batteries: An electrolysis approach. ACS Nano 2016, 10, 1633–1639. [Google Scholar] [CrossRef] [PubMed]
- Xi, K.; Cao, S.; Peng, X.; Ducati, C.; Kumar, R.V.; Cheetham, A.K. Carbon with hierarchical pores from carbonized metal-organic frameworks for lithium sulphur batteries. Chem. Commun. 2013, 49, 2192–2194. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wei, Y.; Wang, J.; Qiao, W.; Ling, L.; Long, D. Controllable nitrogen doping of high-surface-area microporous carbons synthesized from an organic-inorganic sol-gel approach for Li–S cathodes. ACS Appl. Mater. Interface 2015, 7, 21188–21197. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yuan, L.; Yi, Z.; Sun, Y.; Liu, Y.; Jiang, Y.; Shen, Y.; Xin, Y.; Zhang, Z.; Huang, Y. Insight into the electrode mechanism in lithium-sulphur batteries with ordered microporous carbon confined sulphur as the cathode. Adv. Energy Mater. 2014, 4, 1301473–1301480. [Google Scholar] [CrossRef]
- Rao, M.; Li, W.; Cairns, E.J. Porous carbon-sulfur composite cathode for lithium/sulfur cells. Electrochem. Commun. 2012, 17, 1–5. [Google Scholar] [CrossRef]
- Ryu, H.S.; Park, J.W.; Park, J.; Ahn, J.-P.; Kim, K.-W.; Ahn, J.-H.; Nam, T.-H.; Wang, G.; Ahn, H.-J. High capacity cathode materials for Li–S batteries. J. Mater. Chem. A 2013, 1, 1573–1578. [Google Scholar] [CrossRef]
- Xu, G.L.; Xu, Y.F.; Fang, J.C.; Peng, X.X.; Fu, F.; Huang, L.; Li, J.T.; Sun, S.G. Porous graphitic carbon loading ultra high sulphuras high-performance ccathode of rechargeable lithium-sulphur batteries. ACS Appl. Mater. Interface 2013, 5, 10782–10793. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wen, Y.; Zhu, Y.; Gaskell, K.; Cychosz, K.A.; Eichhorn, B.; Xu, K.; Wang, C. Confined sulphur in microporous carbon renders superior cycling stability in Li/S batteries. Adv. Funct. Mater. 2015, 25, 4312–4320. [Google Scholar] [CrossRef]
- Zhang, B.; Qin, X.; Li, G.R.; Gao, X.P. Enhancement of long stability of sulphur cathode by encapsulating sulphur into micropores of carbon spheres. Energy Environ. Sci. 2010, 3, 1531–1537. [Google Scholar] [CrossRef]
- Zhang, W.; Qiao, D.; Pan, J.; Cao, Y.; Yang, H.; Ai, X. A Li+-conductive microporous carbon–sulphur composite for Li–S batteries. Electrochim. Acta 2013, 87, 497–502. [Google Scholar] [CrossRef]
- Zhou, L.; Huang, T.; Yu, A. Three-dimensional flower-shaped activated porous carbon/sulphur composites as cathode materials for lithium-sulphur batteries. ACS Sustain. Chem. Eng. 2014, 2, 2442–2447. [Google Scholar] [CrossRef]
- Wang, J.L.; Yang, J.; Xie, J.Y.; Xu, N.X.; Li, Y. Sulphur-carbon nano-composite as cathode for rechargeable lithium battery based on gel electrolyte. Electrochem. Commun. 2002, 4, 499–502. [Google Scholar] [CrossRef]
- Wang, J.; Chew, S.Y.; Zhao, Z.W.; Ashraf, S.; Wexler, D.; Chen, J.; Ng, S.H.; Chou, S.L.; Liu, H.K. Sulphur-mesoporous carbon composites in conjunction with a novel ionic liquid electrolyte for lithium rechargeable batteries. Carbon 2008, 46, 229–235. [Google Scholar] [CrossRef]
- Ji, X.; Lee, K.T.; Nazar, L.F. A highly ordered nanostructured carbon–sulphur cathode for lithium–sulphur batteries. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Weng, W.; Ren, J.; Peng, H. A cable-shaped lithium sulphur battery. Adv. Mater. 2016, 28, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Evers, S.; Black, R.; Nazar, L.F. Stabilizing lithium-sulphur cathodes using polysulphide reservoirs. Nat. Commun. 2011, 2, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, T.; Jeong, Y.C.; Lee, K.; Park, K.T.; Yang, S.J.; Park, C.R. Stabilization of insoluble discharge products by facile aniline modification for high performance Li–S batteries. Adv. Energy Mater. 2015, 5, 1500268–1500277. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, C.; Ji, Z.; Han, B.; Feng, L.; Wu, J. High-performance lithium/sulphur batteries by decorating CMK-3/S cathodes with DNA. J. Mater. Chem. A 2015, 3, 7241–7247. [Google Scholar] [CrossRef]
- Nagao, M.; Imade, Y.; Narisawa, H.; Kobayashi, T.; Watanabe, R.; Yokoi, T.; Tatsumi, T.; Kanno, R. All-solid-state Li-sulphur batteries with mesoporous electrode and thio-LISICON solid electrolyte. J. Power Sources 2013, 222, 237–242. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Tu, J.P.; Lu, Y.; Cai, J.B.; Zhang, Y.J.; Wang, X.L.; Gu, C.D. Graphene-coated mesoporous carbon/sulphur cathode with enhanced cycling stability. Electrochim. Acta 2013, 113, 256–262. [Google Scholar] [CrossRef]
- Zhou, X.; Xie, J.; Yang, J.; Zou, Y.; Tang, J.; Wang, S.; Ma, L.; Liao, Q. Improving the performance of lithium-sulphur batteries by graphene coating. J. Power Sources 2013, 243, 993–1000. [Google Scholar] [CrossRef]
- Chen, S.-R.; Zhai, Y.-P.; Xu, G.-L.; Jiang, Y.-X.; Zhao, D.-Y.; Li, J.-T.; Huang, L.; Sun, S.-G. Ordered mesoporous carbon/sulphur nanocomposite of high performances as cathode for lithium-sulphur battery. Electrochim. Acta 2011, 56, 9549–9555. [Google Scholar] [CrossRef]
- He, G.; Ji, X.; Nazar, L. High “C” rate Li–S cathodes: Sulphur imbibed bimodal porous carbons. Energy Environ. Sci. 2011, 4, 2878–2883. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, H.; Wang, M.; Zhang, H.; Qu, C. Tri-modal mesoporous carbon/sulphur nanocomposite for high performance Li–S battery. Electrochim. Acta 2016, 190, 322–328. [Google Scholar] [CrossRef]
- Li, J.; Qin, F.; Zhang, L.; Zhang, K.; Li, Q.; Lai, Y.; Zhang, Z.; Fang, J. Mesoporous carbon from biomass: One-pot synthesis and application for Li–S batteries. J. Mater. Chem. A 2014, 2, 13916–13922. [Google Scholar] [CrossRef]
- Li, L.-Y.; Chen, Y.-X.; Zhong, B.-H. Synthesis and electrochemical performance of a simple and low-cost sulphur/porous carbon composite cathode for rechargeable lithium sulphur battery. Compos. Part A Appl. Sci. Manuf. 2014, 62, 26–31. [Google Scholar] [CrossRef]
- Li, X.; Cao, Y.; Qi, W.; Saraf, L.V.; Xiao, J.; Nie, Z.; Mietek, J.; Zhang, J.-G.; Schwenzer, B.; Liu, J. Optimization of mesoporous carbon structures for lithium–sulphur battery applications. J. Mater. Chem. 2011, 21, 16603–16610. [Google Scholar] [CrossRef]
- Liang, X.; Wen, Z.; Liu, Y.; Zhang, H.; Huang, L.; Jin, J. Highly dispersed sulphurin ordered mesoporous carbon sphere as a composite cathode for rechargeable polymer Li/S battery. J. Power Sources 2011, 196, 3655–3658. [Google Scholar] [CrossRef]
- Liu, J.; Yang, T.; Wang, D.W.; Lu, G.Q.; Zhao, D.; Qiao, S.Z. A facile soft-template synthesis of mesoporous polymeric and carbonaceous nanospheres. Nat. Commun. 2013, 4, 2798–2804. [Google Scholar] [CrossRef]
- Park, M.S.; Jeong, B.O.; Kim, T.J.; Kim, S.; Kim, K.J.; Yu, J.S.; Jung, Y.; Kim, Y.J. Disordered mesoporous carbon as polysulphide reservoir for improved cyclic performance of lithium–sulphur batteries. Carbon 2014, 68, 265–272. [Google Scholar] [CrossRef]
- Schuster, J.; He, G.; Mandlmeier, B.; Yim, T.; Lee, K.T.; Bein, T.; Nazar, L.F. Spherical ordered mesoporous carbon nanoparticles with high porosity for lithium-sulphur batteries. Angew. Chem. Int. Ed. 2012, 51, 3591–3595. [Google Scholar] [CrossRef] [PubMed]
- See, K.A.; Jun, Y.S.; Gerbec, J.A.; Sprafke, J.K.; Wudl, F.; Stucky, G.D.; Seshadri, R. Sulphur-functionalized mesoporous carbons as sulphur hosts in Li–S batteries: Increasing the affinity of polysulphide intermediates to enhance performance. ACS Appl. Mater. Interface 2014, 6, 10908–10916. [Google Scholar] [CrossRef] [PubMed]
- Sohn, H.; Gordin, M.L.; Xu, T.; Chen, S.; Lv, D.; Song, J.; Manivannan, A.; Wang, D. Porous spherical carbon/sulphur nanocomposites by aerosol-assisted synthesis: The effect of pore structure and morphology on their electrochemical performance as lithium/sulphur battery cathodes. ACS Appl. Mater. Interface 2014, 6, 7596–7606. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Xu, T.; Gordin, M.L.; Zhu, P.; Lv, D.; Jiang, Y.-B.; Chen, Y.; Duan, Y.; Wang, D. Nitrogen-doped mesoporous carbon promoted chemical adsorption of sulphur and fabrication of high-areal-capacity sulphur cathode with exceptional cycling stability for lithium-sulphur batteries. Adv. Funct. Mater. 2014, 24, 1243–1250. [Google Scholar] [CrossRef]
- Sun, X.G.; Wang, X.; Mayes, R.T.; Dai, S. Lithium–sulphur batteries based on nitrogen-doped carbon and an ionic-liquid electrolyte. ChemSusChem 2012, 5, 2079–2085. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Chen, X.; Xia, Y.; Huang, H.; Gan, Y.; Wu, R.; Chen, F.; Zhang, W. Highly mesoporous carbon foams synthesized by a facile, cost-effective and template-free Pechini method for advanced lithium–sulphur batteries. J. Mater. Chem. A 2013, 1, 3295–3301. [Google Scholar] [CrossRef]
- Wei, Y.; Tao, Y.; Zhang, C.; Wang, J.; Qiao, W.; Ling, L.; Long, D. Layered carbide-derived carbon with hierarchically porous structure for high rate lithium-sulphur batteries. Electrochim. Acta 2016, 188, 385–392. [Google Scholar] [CrossRef]
- Weng, W.; Pol, V.G.; Amine, K. Ultrasound assisted design of sulphur/carbon cathodes with partially fluorinated ether electrolytes for highly efficient Li/S batteries. Adv. Mater. 2013, 25, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Jia, M.; Mao, C.; Liu, S.; Bao, S.; Jiang, J.; Liu, Y.; Lu, Z. Aspergillus flavus conidia-derived carbon/sulfur composite as a cathode material for high performance lithium–sulfur battery. Sci. Rep. 2016, 6, 18739–18747. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.; Agrawal, M.; Formanek, P.; Jehnichen, D.; Fischer, D.; Krause, B.; Albrecht, V.; Stamm, M.; Ionov, L. Nanoporous cathodes for high-energy Li–S batteries from gyroid block copolymer templates. ACS Nano 2015, 9, 6147–6157. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Yuan, C.; Shen, L.; Xu, G.; Nie, P.; Zhang, X. Encapsulating sulphur into hierarchically ordered porous carbon as a high-performance cathode for lithium-sulphur batteries. Chem. Eur. J. 2013, 19, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-W.; Zeng, Q.; Zhou, G.; Yin, L.; Li, F.; Cheng, H.-M.; Gentle, I.R.; Lu, G.Q.M. Carbon–sulphur composites for Li–S batteries: Status and prospects. J. Mater. Chem. A 2013, 1, 9382–9394. [Google Scholar] [CrossRef]
- Lai, C.; Gao, X.P.; Zhang, B.; Yan, T.Y.; Zhou, Z. Synthesis and electrochemical performance of sulphur/highly porous carbon composites. J. Phys. Chem. C 2009, 113, 4712–4716. [Google Scholar] [CrossRef]
- Liang, C.; Dudney, N.J.; Howe, J.Y. Hierarchically structured sulphur/carbon nanocomposite material for high-energy lithium battery. Chem. Mater. 2009, 21, 4724–4730. [Google Scholar] [CrossRef]
- Qu, Y.; Zhang, Z.; Zhang, X.; Ren, G.; Wang, X.; Lai, Y.; Liu, Y.; Li, J. Synthesis of hierarchical porous honeycomb carbon for lithium-sulphur battery cathode with high rate capability and long cycling stability. Electrochim. Acta 2014, 137, 439–446. [Google Scholar] [CrossRef]
- Yu, L.; Brun, N.; Sakaushi, K.; Eckert, J.; Titirici, M.M. Hydrothermal nanocasting: Synthesis of hierarchically porous carbon monoliths and their application in lithium–sulphur batteries. Carbon 2013, 61, 245–253. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, L.; Zhao, H.; Krall, A.; Wen, Z.; Chen, J.; Hurley, P.; Jiang, J.; Li, Y. Sulphur-infiltrated porous carbon microspheres with controllable multi-modal pore size distribution for high energy lithium–sulphur batteries. Nanoscale 2014, 6, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Ding, B.; Nie, P.; Shen, L.; Dou, H.; Zhang, X. Hierarchically porous carbon encapsulating sulphur as a superior cathode material for high performance lithium–sulphur batteries. ACS Appl. Mater. Interface 2014, 6, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fang, X.; Guo, X.; Wang, Z.; Chen, L. Sulphur in hierarchically pore-structured carbon pillars as cathode material for lithium–sulphur batteries. Electrochim. Acta 2013, 97, 238–243. [Google Scholar] [CrossRef]
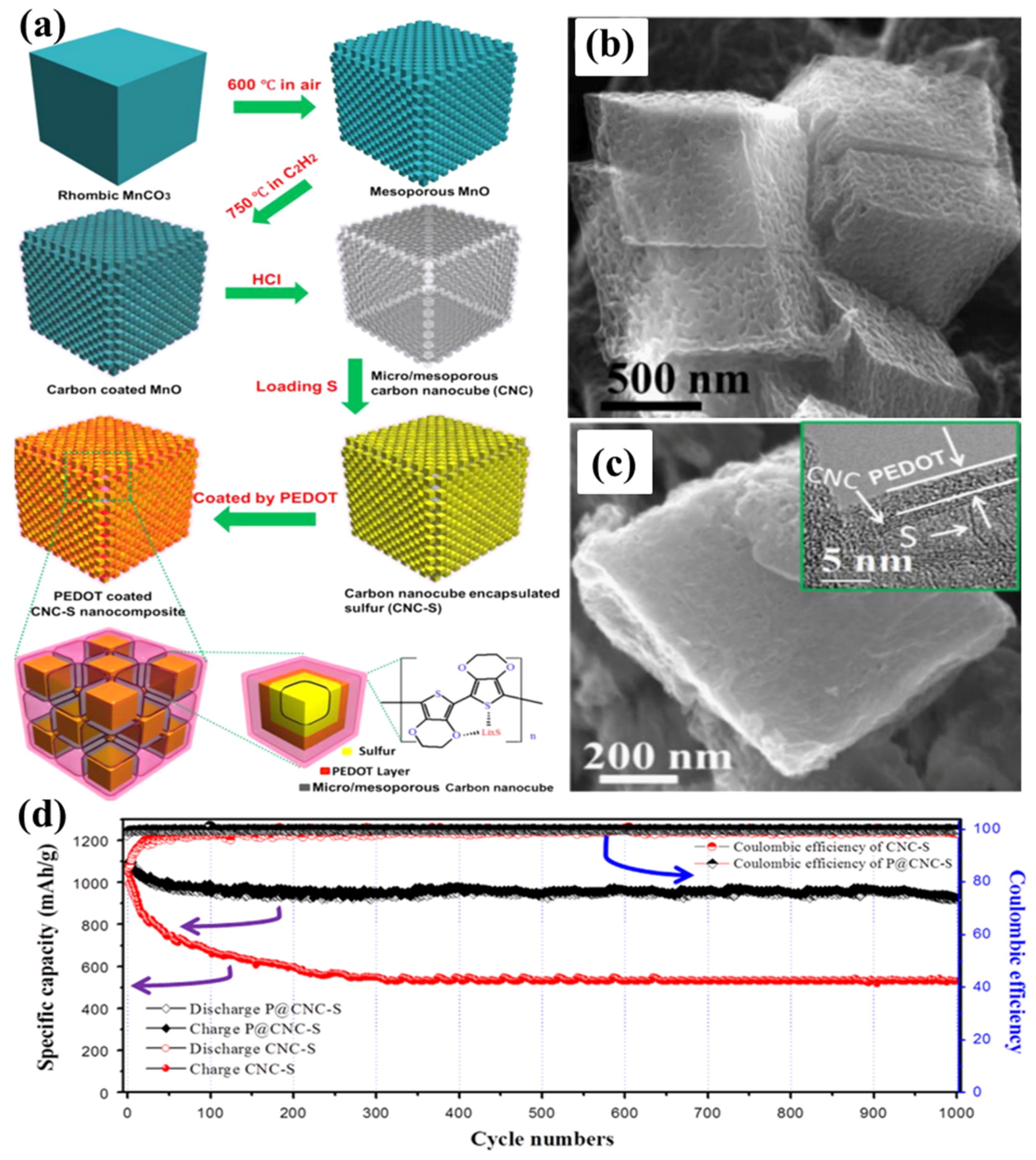
- Chen, S.; Sun, B.; Xie, X.; Mondal, A.K.; Huang, X.; Wang, G. Multi-chambered micro/mesoporous carbon nanocubes as new polysulphides reserviors for lithium–sulphur batteries with long cycle life. Nano Energy 2015, 16, 268–280. [Google Scholar] [CrossRef]
- Brückner, J.; Thieme, S.; Böttger-Hiller, F.; Bauer, I.; Grossmann, H.T.; Strubel, P.; Althues, H.; Spange, S.; Kaskel, S. Carbon-based anodes for lithium sulphur full cells with high cycle stability. Adv. Funct. Mater. 2013, 24, 1284–1289. [Google Scholar] [CrossRef]
- Brun, N.; Sakaushi, K.; Eckert, J.; Titirici, M.M. Carbohydrate-derived nanoarchitectures: On a synergistic effect toward an improved performance in lithium–sulphur batteries. ACS Sustain. Chem. Eng. 2014, 2, 126–129. [Google Scholar] [CrossRef]
- Brun, N.; Sakaushi, K.; Yu, L.; Giebeler, L.; Eckert, J.; Titirici, M.M. Hydrothermal carbon-based nanostructured hollow spheres as electrode materials for high-power lithium–sulphur batteries. Phys. Chem. Chem. Phys. 2013, 15, 6080–6087. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hao, B.; Yuan, C.; Guo, Z.; Lou, X.D. Confining sulphur in double-shelled hollow carbon spheres for lithium–sulphur batteries. Angew. Chem. Int. Ed. 2012, 124, 9730–9733. [Google Scholar] [CrossRef]
- He, G.; Evers, S.; Liang, X.; Cuisinier, M.; Garsuch, A.; Nazar, L.F. Tailoring porosity in carbon nanospheres for lithium–sulphur battery cathodes. ACS Nano 2013, 7, 10920–10930. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhang, H.; Zhang, Y.; Wang, M.; Qu, C.; Yi, J. Biomineralization-induced self-assembly of porous hollow carbon nanocapsule monoliths and their application in Li–S batteries. Chem. Commun. 2015, 51, 1085–1088. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Han, F.; Wang, S.; Cheng, F.; Sun, Q.; Li, W.C. High sulphur loading cathodes fabricated using peapodlike, large pore volume mesoporous carbon for lithium–sulphur battery. ACS Appl. Mater. Interface 2013, 5, 2208–2213. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.S.; Kim, M.S.; Cho, W.I.; Oh, S.H. Sulphur/graphitic hollow carbon sphere nano-composite as a cathode material for high-power lithium-sulphur battery. Nanoscale Res. Lett. 2013, 8, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhao, Q.; Tao, Z.; Chen, J. Composite of sulphur impregnated in porous hollow carbon spheres as the Cathode of Li–S batteries with high performance. Nano Res. 2012, 6, 38–46. [Google Scholar] [CrossRef]
- Yao, H.; Zheng, G.; Li, W.; McDowell, M.T.; Seh, Z.; Liu, N.; Lu, Z.; Cui, Y. Crab shells as sustainable templates from nature for nanostructured battery electrodes. Nano Lett. 2013, 13, 3385–3390. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Zhang, H.; Huang, Y.; Wang, W.; Xia, Y.; Yu, Z. Pig bone derived hierarchical porous carbon and its enhanced cycling performance of lithium–sulphur batteries. Energy Environ. Sci. 2011, 4, 736–740. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, Y.; Zhong, Q.; Lai, D.; Yao, J. Porous nitrogen-doped carbon derived from silk fibroin protein encapsulating sulphur as a superior cathode material for high-performance lithium–sulphur batteries. Nanoscale 2015, 7, 17791–17797. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, C.; Wang, W.; Zhang, H.; Gao, M.; Xiong, X.; Wang, A.; Yuan, K.; Huang, Y.; Wang, F. A novel porous nanocomposite of sulphur/carbon obtained from fish scales for lithium–sulphur batteries. J. Mater. Chem. A 2013, 1, 3334–3339. [Google Scholar] [CrossRef]
- Chung, S.-H.; Manthiram, A. Eggshell membrane-derived polysulphide absorbents for highly stable and reversible lithium–sulphur cells. ACS Sustain. Chem. Eng. 2014, 2, 2248–2252. [Google Scholar] [CrossRef]
- Tao, X.Y.; Zhang, J.T.; Xia, Y.; Huang, H.; Du, J.; Xiao, H.; Zhang, W.K.; Gan, Y.P. Bio-inspired fabrication of carbon nanotiles for high performance cathode of Li–S batteries. J. Mater. Chem. A 2014, 2, 2290–2296. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Konarov, A.; Li, Z.; Chen, P. Effect of mesoporous carbon microtube prepared by carbonizing the poplar catkin on sulphur cathode performance in Li/S batteries. J. Alloy. Compd. 2015, 619, 298–302. [Google Scholar] [CrossRef]
- Yang, S.T.; Yan, C.; Cao, Z.-X.; Shi, M.-J.; Li, Y.-L.; Yin, Y.-H. Preparation of hierarchical porous carbon/sulphur composite based on lotus-leaves and its property for Li–S batteries. J. Inorg. Mater. 2016, 31, 135–140. [Google Scholar]
- Pang, Q.; Tang, J.; Huang, H.; Liang, X.; Hart, C.; Tam, K.C.; Nazar, L.F. A nitrogen and sulphur dual-doped carbon derived from polyrhodanine@cellulose for advanced lithium–sulphur batteries. Adv. Mater. 2015, 27, 6021–6028. [Google Scholar] [CrossRef] [PubMed]
- Schipper, F.; Vizintin, A.; Ren, J.; Dominko, R.; Fellinger, T.P. Biomass-derived heteroatom-doped carbon aerogels from a salt melt sol-gel synthesis and their performance in Li–S batteries. ChemSusChem 2015, 8, 3077–3083. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.; Song, X.; Cairns, E.J. Nano-carbon/sulphur composite cathode materials with carbon nanofiber as electrical conductor for advanced secondary lithium/sulphur cells. J. Power Sources 2012, 205, 474–478. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Kim, K.-W.; Ahn, H.-J.; Ahn, J.-H. Improvement of cycle property of sulphur electrode for lithium/sulphur battery. J. Alloy. Compd. 2008, 449, 313–316. [Google Scholar] [CrossRef]
- Elazari, R.; Salitra, G.; Garsuch, A.; Panchenko, A.; Aurbach, D. Sulphur-impregnated activated carbon fiber cloth as a binder-free cathode for rechargeable Li–S batteries. Adv. Mater. 2011, 23, 5641–5644. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Rao, M.; Aloni, S.; Wang, L.; Cairns, E.J.; Zhang, Y. Porous carbon nanofiber–sulfur composite electrodes for lithium/sulfur cells. Energy Environ. Sci. 2011, 4, 5053–5059. [Google Scholar] [CrossRef]
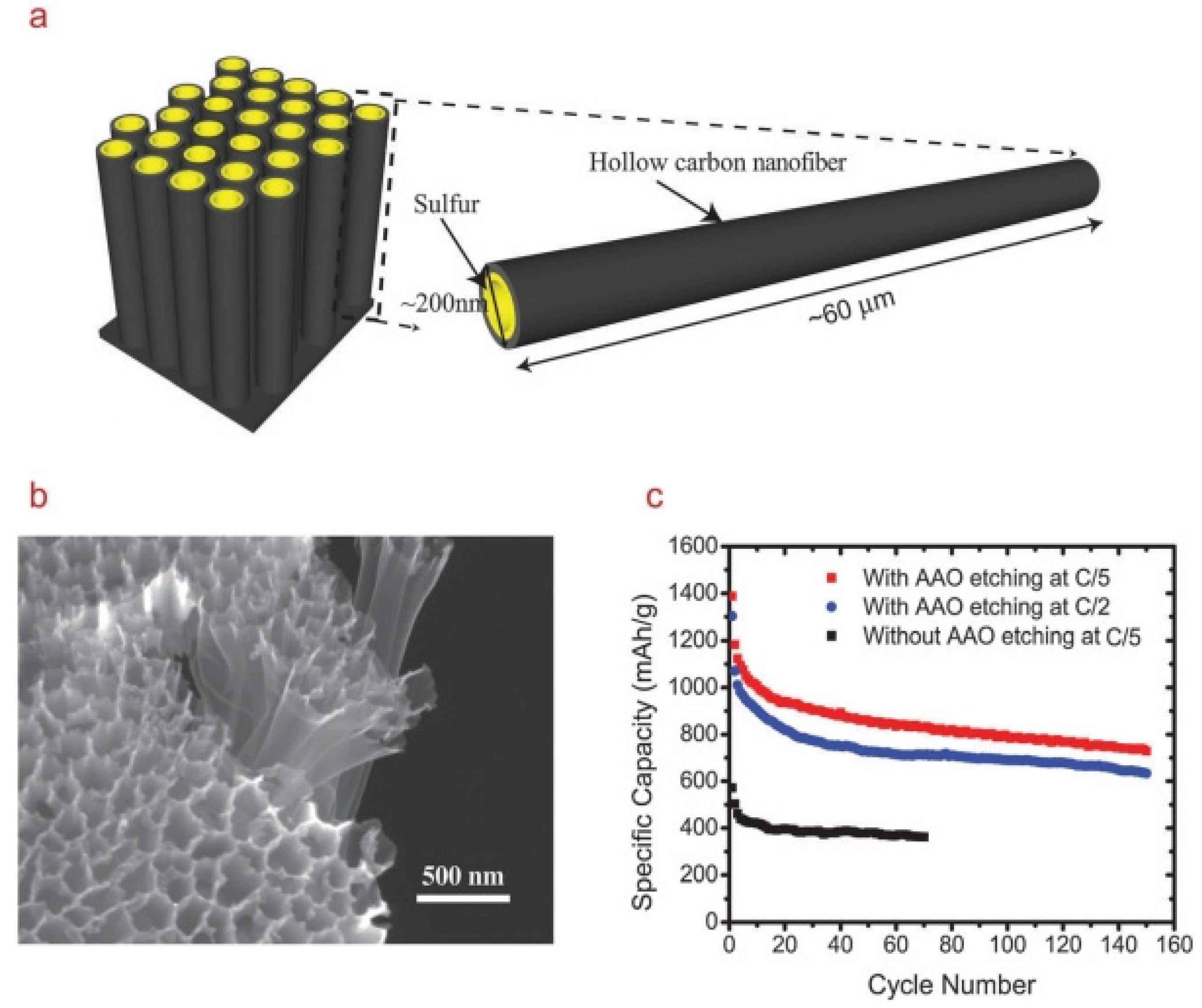
- Zheng, G.; Yang, Y.; Cha, J.J.; Hong, S.S.; Cui, Y. Hollow carbon nanofiber-encapsulated sulphur cathodes for high specific capacity rechargeable lithium batteries. Nano Lett. 2011, 11, 4462–4467. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Zhang, Q.; Cha, J.J.; Yang, Y.; Li, W.; Seh, Z.W.; Cui, Y. Amphiphilic surface modification of hollow carbon nanofibers for improved cycle life of lithium sulphur batteries. Nano Lett. 2013, 13, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Zhang, Z.; Lai, Y.; Liu, J.; Liu, Y.; Li, J. A sulphur–carbon composite for lithium/sulphur battery based on activated vapor-grown carbon fiber. Solid State Ion. 2013, 238, 44–49. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, M.; Li, X.; Liu, Y.; Pan, H. Preparation of mesohollow and microporous carbon nanofiber and its application in cathode material for lithium–sulphur batteries. J. Alloy. Compd. 2014, 608, 220–228. [Google Scholar] [CrossRef]
- Yang, J.; Xie, J.; Zhou, X.; Zou, Y.; Tang, J.; Wang, S.; Chen, F.; Wang, L. Functionalized N-doped porous carbon nanofiber webs for a lithium–sulphur battery with high capacity and rate performance. J. Phys. Chem. C 2014, 118, 1800–1807. [Google Scholar] [CrossRef]
- Han, S.-C.; Song, M.-S.; Lee, H.; Kim, H.-S.; Ahn, H.-J.; Lee, J.-Y. Effect of multiwalled carbon nanotubes on electrochemical properties of lithium/sulphur rechargeable batteries. J. Electrochem. Soc. 2003, 150, A889–A893. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, Y.W.; Hu, X.G.; Zhang, C.F. Novel nanosized adsorbing sulphur composite cathode materials for the advanced secondary lithium batteries. Electrochim. Acta 2006, 51, 1330–1335. [Google Scholar] [CrossRef]
- Yuan, L.; Yuan, H.; Qiu, X.; Chen, L.; Zhu, W. Improvement of cycle property of sulphur-coated multi-walled carbon nanotubes composite cathode for lithium/sulphur batteries. J. Power Sources 2009, 189, 1141–1146. [Google Scholar] [CrossRef]
- Chen, J.-J.; Jia, X.; She, Q.-J.; Wang, C.; Zhang, Q.; Zheng, M.-S.; Dong, Q.-F. The preparation of nano-sulphur/MWCNTs and its electrochemical performance. Electrochim. Acta 2010, 55, 8062–8066. [Google Scholar]
- Ahn, W.; Kim, K.-B.; Jung, K.-N.; Shin, K.-H.; Jin, C.-S. Synthesis and electrochemical properties of a sulphur-multi walled carbon nanotubes composite as a cathode material for lithium sulphur batteries. J. Power Sources 2012, 202, 394–399. [Google Scholar] [CrossRef]
- Geng, X.; Rao, M.; Li, X.; Li, W. Highly dispersed sulphur in multi-walled carbon nanotubes for lithium/sulphur battery. J. Solid State Electrochem. 2012, 17, 987–992. [Google Scholar] [CrossRef]
- Guo, J.; Xu, Y.; Wang, C. Sulfur-impregnated disordered carbon nanotubes cathode for lithium–sulfur batteries. Nano Lett. 2011, 11, 4288–4294. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.Z.; Jin, B.; Xin, P.M.; Wang, H.H. Multiwalled carbon nanotubes-sulphur composites with enhanced electrochemical performance for lithium/sulphur batteries. Appl. Surf. Sci. 2014, 307, 346–350. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Thomas, M.L.; Byon, H.R. In situ synthesis of bipyramidal sulphur with 3D carbon nanotube framework for lithium–sulphur batteries. Adv. Funct. Mater. 2014, 24, 2248–2252. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Huang, J.-Q.; Zhang, Q.; Peng, H.-J.; Zhao, M.-Q.; Wei, F. Aligned carbon nanotube/sulphur composite cathodes with high sulphur content for lithium–sulphur batteries. Nano Energy 2014, 4, 65–72. [Google Scholar] [CrossRef]
- Jin, K.; Zhou, X.; Zhang, L.; Xin, X.; Wang, G.; Liu, Z. Sulfur/carbon nanotube composite film as a flexible cathode for lithium–sulfur batteries. J. Phys. Chem. C 2013, 117, 21112–21119. [Google Scholar] [CrossRef]
- Moon, S.; Jung, Y.H.; Jung, W.K.; Jung, D.S.; Choi, J.W.; Kim, D.K. Encapsulated monoclinic sulphur for stable cycling of Li–S rechargeable batteries. Adv. Mater. 2013, 25, 6547–6553. [Google Scholar] [CrossRef] [PubMed]
- Hagen, M.; Dörfler, S.; Althues, H.; Tübke, J.; Hoffmann, M.J.; Kaskel, S.; Pinkwart, K. Lithium–sulphur batteries–binder free carbon nanotubes electrode examined with various electrolytes. J. Power Sources 2012, 213, 239–248. [Google Scholar] [CrossRef]
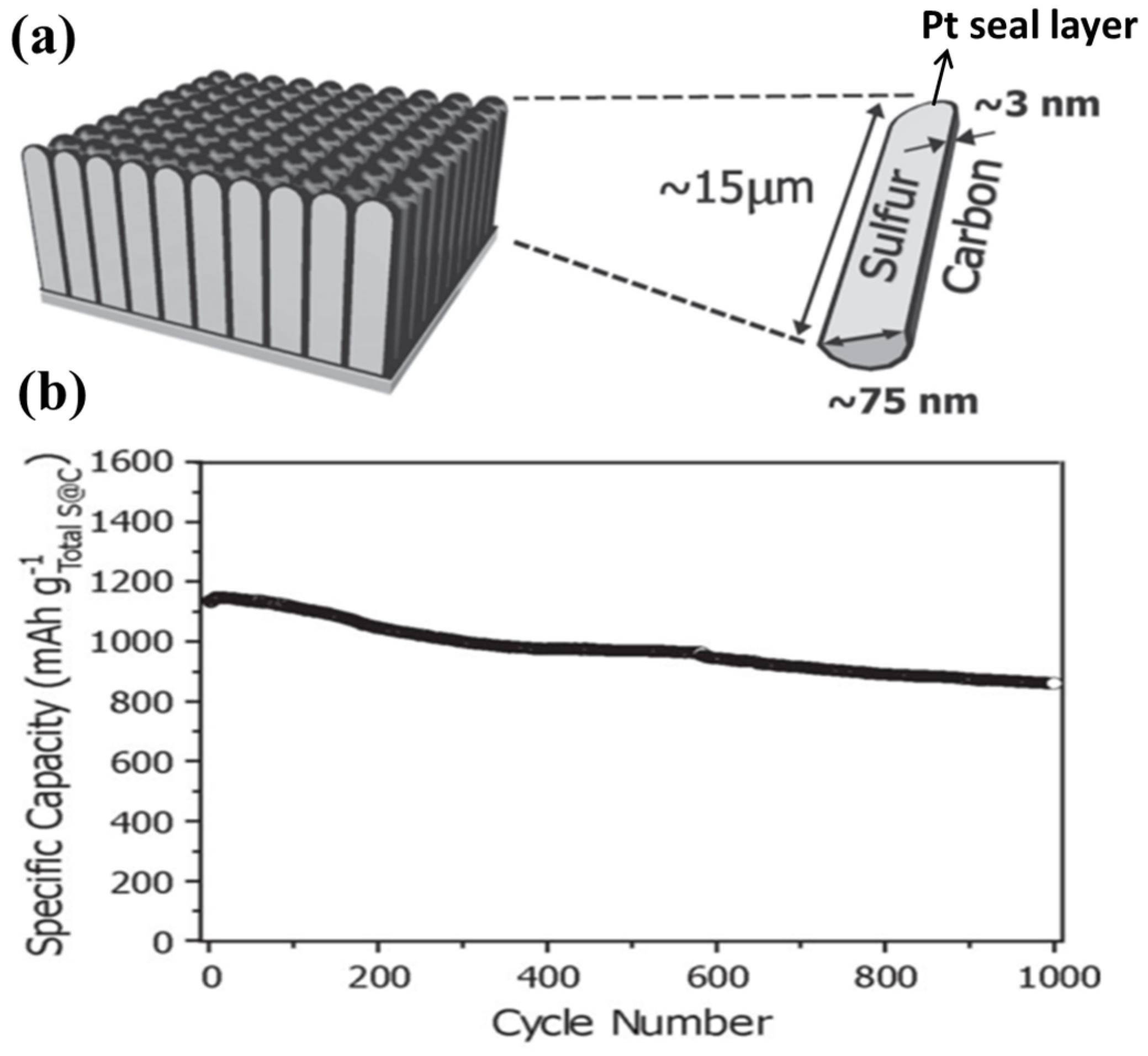
- Dorfler, S.; Hagen, M.; Althues, H.; Tubke, J.; Kaskel, S.; Hoffmann, M.J. High capacity vertical aligned carbon nanotube/sulfur composite cathodes for lithium–sulfur batteries. Chem. Commun. 2012, 48, 4097–4099. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-Q.; Zhang, Q.; Zhang, S.-M.; Liu, X.-F.; Zhu, W.; Qian, W.-Z.; Wei, F. Aligned sulfur-coated carbon nanotubes with a polyethylene glycol barrier at one end for use as a high efficiency sulfur cathode. Carbon 2013, 58, 99–106. [Google Scholar] [CrossRef]
- Wang, L.; Dong, Z.; Wang, D.; Zhang, F.; Jin, J. Covalent bond glued sulphur nanosheet-based cathode integration for long-cycle-life Li–S batteries. Nano Lett. 2013, 13, 6244–6250. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.C.; Wang, J.L.; Yang, J.; Nuli, Y.N. A novel pyrolyzed polyacrylonitrile-sulphur@MWCNT composite cathode material for high-rate rechargeable lithium/sulfur batteries. J. Mater. Chem. 2011, 21, 6807–6810. [Google Scholar] [CrossRef]
- Bao, W.; Zhang, Z.; Zhou, C.; Lai, Y.; Li, J. Multi-walled carbon nanotubes @ mesoporous carbon hybrid nanocomposites from carbonized multi-walled carbon nanotubes @ metal–organic framework for lithium sulfur battery. J. Power Sources 2014, 248, 570–576. [Google Scholar] [CrossRef]
- Chen, R.; Zhao, T.; Lu, J.; Wu, F.; Li, L.; Chen, J.; Tan, G.; Ye, Y.; Amine, K. Graphene-based three-dimensional hierarchical sandwich-type architecture for high-performance Li/S batteries. Nano Lett. 2013, 13, 4642–4649. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.-F.; Zhang, Z.-A.; Lu, H.; Lai, Y.-Q.; Liu, J.; Li, J.; Liu, Y.-X. Vapor-grown carbon fibers enhanced sulphur-multi walled carbon nanotubes composite cathode for lithium/sulphur batteries. Trans. Nonferrous Met. Soc. 2014, 24, 158–163. [Google Scholar] [CrossRef]
- Wei, W.; Wang, J.; Zhou, L.; Yang, J.; Schumann, B.; NuLi, Y. CNT enhanced sulfur composite cathode material for high rate lithium battery. Electrochem. Commun. 2011, 13, 399–402. [Google Scholar] [CrossRef]
- Xie, J.; Yang, J.; Zhou, X.; Zou, Y.; Tang, J.; Wang, S.; Chen, F. Preparation of three-dimensional hybrid nanostructure-encapsulated sulphur cathode for high-rate lithium sulphur batteries. J. Power Sources 2014, 253, 55–63. [Google Scholar] [CrossRef]
- Xu, T.; Song, J.; Gordin, M.L.; Sohn, H.; Yu, Z.; Chen, S.; Wang, D. Mesoporous carbon–carbon nanotube–sulfur composite microspheres for high-areal-capacity lithium–sulfur battery cathodes. ACS Appl. Mater. Interface 2013, 5, 11355–11362. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Zhang, Q.; Shi, Y.N.; Qin, L.L.; Cao, Y.; Zheng, M.S.; Dong, Q.F. A Hierarchical architecture S/MWCNT nanomicrosphere with large pores for lithium sulphur batteries. Phys. Chem. Chem. Phys. 2012, 14, 5376–5382. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Q.; Huang, J.; Zhang, S.; Peng, H.; Wei, F. Hierarchical nanostructured composite cathode with carbon nanotubes as conductive scaffold for lithium-sulfur batteries. J. Energy Chem. 2013, 22, 341–346. [Google Scholar] [CrossRef]
- Xu, G.; Ding, B.; Nie, P.; Shen, L.; Wang, J.; Zhang, X. Porous nitrogen-doped carbon nanotubes derived from tubular polypyrrole for energy-storage applications. Chem. Eur. J. 2013, 19, 12306–12312. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, Y.; Yuan, L.; Hao, Z.; Huang, Y. Status and prospects in sulfur–carbon composites as cathode materials for rechargeable lithium–sulfur batteries. Carbon 2015, 92, 41–63. [Google Scholar] [CrossRef]
- Ji, L.; Rao, M.; Zheng, H.; Zhang, L.; Li, Y.; Duan, W.; Guo, J.; Cairns, E.J.; Zhang, Y. Graphene oxide as a sulphur immobilizer in high performance lithium/sulphur cells. J. Am. Chem. Soc. 2011, 133, 18522–18525. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Huang, Y.; Zong, M.; Ding, X.; Ding, J.; Sun, X. Electrostatic self-assembly of graphene oxide wrapped sulfur particles for lithium–sulfur batteries. Mater. Res. Bull. 2015, 64, 12–16. [Google Scholar] [CrossRef]
- Xiao, M.; Huang, M.; Zeng, S.; Han, D.; Wang, S.; Sun, L.; Meng, Y. Sulfur@graphene oxide core–shell particles as a rechargeable lithium–sulfur battery cathode material with high cycling stability and capacity. RSC Adv. 2013, 3, 4914–4916. [Google Scholar] [CrossRef]
- Rong, J.; Ge, M.; Fang, X.; Zhou, C. Solution ionic strength engineering as a generic strategy to coat graphene oxide (GO) on various functional particles and its application in high-performance lithium–sulphur (Li–S) batteries. Nano Lett. 2014, 14, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Shi, L.; Fan, C.; Fu, X.; Ren, Z.; Qian, G.; Wang, Z. Bowl-like sulfur particles wrapped by graphene oxide as cathode material of lithium–sulfur batteries. RSC Adv. 2015, 5, 28832–28835. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, N.; Yang, X.; Li, S.; Yao, J.; Cai, Y. Hollow sulphur@graphene oxide core-shell composite for high-performance Li–S batteries. J. Alloy. Compd. 2015, 650, 604–609. [Google Scholar] [CrossRef]
- Wang, J.-Z.; Lu, L.; Choucair, M.; Stride, J.A.; Xu, X.; Liu, H.-K. Sulphur-graphene composite for rechargeable lithium batteries. J. Power Sources 2011, 196, 7030–7034. [Google Scholar] [CrossRef]
- Wei, Z.K.; Chen, J.J.; Qin, L.L.; Nemage, A.W.; Zheng, M.S.; Dong, Q.F. Two-step hydrothermal method for synthesis of sulphur-graphene hybrid and its application in lithium sulphur batteries. J. Electrochem. Soc. 2012, 159, A1236–A1239. [Google Scholar] [CrossRef]
- Li, H.; Yang, X.; Wang, X.; Liu, M.; Ye, F.; Wang, J.; Qiu, Y.; Li, W.; Zhang, Y. Dense integration of graphene and sulphur through the soft approach for compact lithium/sulphur battery cathode. Nano Energy 2015, 12, 468–475. [Google Scholar] [CrossRef]
- Lu, S.; Chen, Y.; Wu, X.; Wang, Z.; Li, Y. Three-dimensional sulfur/graphene multifunctional hybrid sponges for lithium-sulfur batteries with large areal mass loading. Sci. Rep. 2014, 4, 4629–4632. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wu, Y.; Zhao, X.; Wang, X.; Du, G.; Zhang, J.; Tu, J. Sulfur/three-dimensional graphene composite for high performance lithium–sulfur batteries. J. Power Sources 2015, 275, 22–25. [Google Scholar] [CrossRef]
- Zhou, G.M.; Yin, L.C.; Wang, D.W.; Li, L.; Pei, S.F.; Gentle, I.R.; Li, F.; Cheng, H.M. Fibrous hybrid of graphene and sulfur nanocrystals for high-performance lithium–sulfur batteries. ACS Nano 2013, 7, 5367–5375. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-B.; Wang, Y.-N.; Wu, B.-R.; Xiong, Y.-K.; Liao, W.-L.; Wu, F.; Zhe, S. Preparation and electrochemical performance of activation graphene/sulfur complex cathode material for lithium-sulfur batteries. J. Inorg. Mater. 2014, 29, 627–632. [Google Scholar]
- Huang, X.; Sun, B.; Li, K.; Chen, S.; Wang, G. Mesoporous graphene paper imobilised sulfur as a flexible electrode for lithium–sulfur batteries. J. Mater. Chem. A 2013, 1, 13484–13489. [Google Scholar] [CrossRef]
- Xu, J.; Shui, J.; Wang, J.; Wang, M.; Liu, H.-K.; Dou, S.X.; Jeon, I.-Y.; Seo, J.-M.; Baek, J.-B.; Dai, L. Sulfur–graphene nanostructured cathodes via ball-milling for high-performance lithium–sulfur batteries. ACS Nano 2014, 8, 10920–10930. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-X.; Chou, S.-L.; Liu, H.-K.; Dou, S.-X. The Electrochemical properties of high-capacity sulphur/reduced graphene oxide with different electrolyte systems. J. Power Sources 2013, 244, 240–245. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Wang, Y.; Chen, J.; Zhou, H.; Huang, Y. Macroporous free-standing nano-sulfur/reduced graphene oxide paper as stable cathode for lithium-sulfur battery. Nano Energy 2015, 11, 678–686. [Google Scholar] [CrossRef]
- Zheng, S.; Wen, Y.; Zhu, Y.; Han, Z.; Wang, J.; Yang, J.; Wang, C. In situ sulfur reduction and intercalation of graphite oxides for Li–S battery cathodes. Adv. Energy Mater. 2014, 4, 1400482–1400491. [Google Scholar] [CrossRef]
- Evers, S.; Nazar, L.F. Graphene-enveloped sulfur in a one pot reaction: A cathode with good coulombic efficiency and high practical sulfur content. Chem. Commun. 2012, 48, 1233–1235. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Xu, G.-L.; Xu, Y.-F.; Sun, S.-G.; Zhang, X.; Qiu, Y.; Yang, S. A composite material of uniformly dispersed sulfur on reduced graphene oxide: Aqueous one-pot synthesis, characterization and excellent performance as the cathode in rechargeable lithium-sulfur batteries. Nano Res. 2012, 5, 726–738. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, J.; Zhang, J.; Su, Q.; Du, G. Graphene-wrapped sulfur nanospheres with ultra-high sulfur loading for high energy density lithium–sulfur batteries. Appl. Surf. Sci. 2015, 324, 399–404. [Google Scholar] [CrossRef]
- Wu, H.; Huang, Y.; Zong, M.; Fu, H.; Sun, X. Self-assembled graphene/sulfur composite as high current discharge cathode for lithium-sulfur batteries. Electrochim. Acta 2015, 163, 24–31. [Google Scholar] [CrossRef]
- Zhao, H.; Peng, Z.; Wang, W.; Chen, X.; Fang, J.; Xu, J. Reduced graphene oxide with ultrahigh conductivity as carbon coating layer for high performance sulphur@reduced graphene oxide cathode. J. Power Sources 2014, 245, 529–536. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, D.H.; Lv, W.; Wang, D.W.; Wei, W.; Zhou, G.M.; Wang, S.; Li, F.; Li, B.H.; Kang, F.; et al. A high-density graphene–sulfur assembly: A promising cathode for compact Li–S batteries. Nanoscale 2015, 7, 5592–5597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lv, W.; Zhang, W.; Zheng, X.; Wu, M.-B.; Wei, W.; Tao, Y.; Li, Z.; Yang, Q.-H. Reduction of graphene oxide by hydrogen sulfide: A promising strategy for pollutant control and as an electrode for Li–S Batteries. Adv. Energy Mater. 2014, 4, 1301565–1301569. [Google Scholar] [CrossRef]
- Xu, H.; Deng, Y.; Shi, Z.; Qian, Y.; Meng, Y.; Chen, G. Graphene-encapsulated sulfur (GES) composites with a core–shell structure as superior cathode materials for lithium–sulfur batteries. J. Mater. Chem. A 2013, 1, 15142–15149. [Google Scholar] [CrossRef]
- Xi, K.; Kidambi, P.R.; Chen, R.; Gao, C.; Peng, X.; Ducati, C.; Hofmann, S.; Kumar, R.V. Binder free three-dimensional sulphur/few-layer graphene foam cathode with enhanced high-rate capability for rechargeable lithium sulphur batteries. Nanoscale 2014, 6, 5746–5753. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.-Q.; Zhang, Q.; Huang, J.-Q.; Tian, G.-L.; Nie, J.-Q.; Peng, H.-J.; Wei, F. Unstacked double-layer templated graphene for high-rate lithium–sulphur batteries. Nat. Commun. 2014, 5, 3410–3417. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dong, Y.; Li, H.; Zhao, Z.; Wu, H.B.; Hao, C.; Liu, S.; Qiu, J.; Lou, X.W. Enhancing lithium-sulphur battery performance by strongly binding the discharge products on amino-functionalized reduced graphene oxide. Nat. Commun. 2014, 5, 5002–5009. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Yu, Z.; Gordin, M.L.; Wang, D. Advanced sulfur cathode enabled by highly crumpled nitrogen-doped graphene sheets for high-energy-density lithium–sulfur batteries. Nano Lett. 2016, 16, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-Q.; Zhang, Q.; Wei, F. Multi-functional separator/interlayer system for high-stable lithium-sulfur batteries: Progress and prospects. Energy Storage Mater. 2015, 1, 127–145. [Google Scholar] [CrossRef]
- Su, Y.S.; Manthiram, A. Lithium–sulphur batteries with a microporous carbon paper as a bifunctional interlayer. Nat. Commun. 2012, 3, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.H.; Manthiram, A. Carbonized eggshell membrane as a natural polysulphide reservoir for highly reversible Li–S batteries. Adv. Mater. 2014, 26, 1360–1365. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.H.; Manthiram, A. A natural carbonized leaf as polysulphide diffusion inhibitor for high-performance lithium–sulfur battery cells. ChemSusChem 2014, 7, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.R.; Zhang, K.; Fang, J.; Lai, Y.Q.; Li, Q.; Zhang, Z.A.; Li, J. High performance lithium sulphur batteries with a cassava-derived carbon sheet as a polysulphides inhibitor. New J. Chem. 2014, 38, 4549–4554. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Wang, Y.Y.; Peng, B.; Yu, W.T.; Wang, H.Y.; Wang, T.; Deng, B.W.; Chai, L.Y.; Zhang, K.; Wang, J.X. Preparation of a macroscopic, robust carbon-fiber monolith from filamentous fungi and its application in Li–S batteries. Green Chem. 2014, 16, 3926–3934. [Google Scholar] [CrossRef]
- Cao, Z.; Ma, C.; Yin, Y.; Zhang, J.; Ding, Y.; Shi, M.; Yang, S. Carbonized non-woven fabric films as adsorbing interlayers to enhance electrochemical performance of lithium–sulphur batteries. New J. Chem. 2015, 39, 9659–9664. [Google Scholar] [CrossRef]
- Chung, S.H.; Manthiram, A. A hierarchical carbonized paper with controllable thickness as a modulable interlayer system for high performance Li–S batteries. Chem. Commun. 2014, 50, 4184–4187. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.H.; Singhal, R.; Kalra, V.; Manthiram, A. Porous carbon mat as an electrochemical testing platform for investigating the polysulphide retention of various cathode configurations in Li–S cells. J. Phys. Chem. Lett. 2015, 6, 2163–2169. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zheng, M.; Lin, Z.; Zhao, B.; Zhang, S.; Yang, J.; Zhu, C.; Zhang, H.; Sun, D.; Shi, Y. Flexible cathodes and multifunctional interlayers based on carbonized bacterial cellulose for high-performance lithium–sulfur batteries. J. Mater. Chem. A 2015, 3, 10910–10918. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Y.; Kang, F. Porous carbon nanofiber paper as an effective interlayer for high-performance lithium-sulfur batteries. Electrochim. Acta 2015, 168, 271–276. [Google Scholar] [CrossRef]
- Wu, F.; Li, W.; Guan, L.; Ye, Y.; Qian, J.; Yang, X.; Xu, Y.; Chen, R. A polypyrrole-supported carbon paper acting as a polysulphide trap for lithium–sulfur batteries. RSC Adv. 2015, 5, 94479–94485. [Google Scholar] [CrossRef]
- Zhang, K.; Li, Q.; Zhang, L.; Fang, J.; Li, J.; Qin, F.; Zhang, Z.; Lai, Y. From filter paper to carbon paper and toward Li–S battery interlayer. Mater. Lett. 2014, 121, 198–201. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, G.; Lai, Y.; Li, J. A freestanding hollow carbon nanofiber/reduced graphene oxide interlayer for high-performance lithium–sulfur batteries. J. Alloy. Compd. 2016, 663, 501–506. [Google Scholar] [CrossRef]
- Zu, C.; Su, Y.S.; Fu, Y.; Manthiram, A. Improved lithium–sulfur cells with a treated carbon paper interlayer. Phys. Chem. Chem. Phys. 2013, 15, 2291–2297. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Su, Y.S.; Manthiram, A. Highly reversible lithium/dissolved polysulphide batteries with carbon nanotube electrodes. Angew. Chem. Int. Ed. 2013, 52, 6930–6935. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-Y.; Kim, H.M.; Lee, S.-K.; Lee, J.-H.; Abouimrane, A.; Khaleel, M.A.; Belharouak, I.; Manthiram, A.; Sun, Y.-K. High-energy, high-rate, lithium–sulphur batteries: Synergetic effect of hollow TiO2-webbed carbon nanotubes and a dual functional carbon-paper interlayer. Adv. Energy Mater. 2016, 6. [Google Scholar] [CrossRef]
- Su, Y.S.; Manthiram, A. A new approach to improve cycle performance of rechargeable lithium–sulfur batteries by inserting a free-standing MWCNT interlayer. Chem. Commun. 2012, 48, 8817–8819. [Google Scholar] [CrossRef] [PubMed]
- Song, J.X.; Yu, Z.X.; Xu, T.; Chen, S.R.; Sohn, H.; Regula, M.; Wang, D.H. Flexible freestanding sandwich-structured sulfur cathode with superior performance for lithium–sulfur batteries. J. Mater. Chem. A 2014, 2, 8623–8627. [Google Scholar] [CrossRef]
- Han, X.; Xu, Y.; Chen, X.; Chen, Y.-C.; Weadock, N.; Wan, J.; Zhu, H.; Liu, Y.; Li, H.; Rubloff, G.; et al. Reactivation of dissolved polysulphides in Li–S batteries based on atomic layer deposition of Al2O3 in nanoporous carbon cloth. Nano Energy 2013, 2, 1197–1206. [Google Scholar] [CrossRef]
- Xu, G.; Yuan, J.; Tao, X.; Ding, B.; Dou, H.; Yan, X.; Xiao, Y.; Zhang, X. Absorption mechanism of carbon-nanotube paper-titanium dioxide as a multifunctional barrier material for lithium-sulfur batteries. Nano Res. 2015, 8, 3066–3074. [Google Scholar] [CrossRef]
- Huang, J.-Q.; Zhang, B.; Xu, Z.-L.; Abouali, S.; Akbari Garakani, M.; Huang, J.; Kim, J.-K. Novel interlayer made from Fe3C/carbon nanofiber webs for high performance lithium–sulfur batteries. J. Power Sources 2015, 285, 43–50. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Chen, L. Reduced graphene oxide film as a shuttle-inhibiting interlayer in a lithium–sulfur battery. J. Power Sources 2013, 242, 65–69. [Google Scholar] [CrossRef]
- Han, K.; Shen, J.; Hao, S.; Ye, H.; Wolverton, C.; Kung, M.C.; Kung, H.H. Free-standing nitrogen-doped graphene paper as electrodes for high-performance lithium/dissolved polysulphide batteries. ChemSusChem 2014, 7, 2545–2553. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.H.; Manthiram, A. Bifunctional separator with a light-weight carbon-coating for dynamically and statically stable lithium-sulfur batteries. Adv. Funct. Mater. 2014, 24, 5299–5306. [Google Scholar] [CrossRef]
- Chung, S.-H.; Han, P.; Singhal, R.; Kalra, V.; Manthiram, A. Electrochemically stable rechargeable lithium–sulfur batteries with a microporous carbon nanofiber filter for polysulphide. Adv. Energy Mater. 2015, 5, 1500738–1500749. [Google Scholar] [CrossRef]
- Chung, S.H.; Manthiram, A. High-performance Li–S batteries with an ultra-lightweight MWCNT-coated separator. J. Phys. Chem. Lett. 2014, 5, 1978–1983. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Chung, S.H.; Manthiram, A. Effective stabilization of a high-loading sulphur cathode and a lithium-metal anode in Li–S batteries utilizing SWCNT-modulated separators. Small 2016, 12, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.-H.; Manthiram, A. A polyethylene glycol-supported microporous carbon coating as a polysulphide trap for utilizing pure sulfur cathodes in lithium–sulfur batteries. Adv. Mater. 2014, 26, 7352–7357. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Pei, S.; Li, L.; Wang, D.W.; Wang, S.; Huang, K.; Yin, L.C.; Li, F.; Cheng, H.M. A graphene–pure-sulfur sandwich structure for ultrafast, long-life lithium–sulfur batteries. Adv. Mater. 2014, 26, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Balach, J.; Jaumann, T.; Klose, M.; Oswald, S.; Eckert, J.; Giebeler, L. Functional mesoporous carbon-coated separator for long-life, high-energy lithium–sulfur batteries. Adv. Funct. Mater. 2015, 25, 5285–5291. [Google Scholar] [CrossRef]
- Balach, J.; Jaumann, T.; Klose, M.; Oswald, S.; Eckert, J.; Giebeler, L. Improved cycling stability of lithium–sulfur batteries using a polypropylene-supported nitrogen-doped mesoporous carbon hybrid separator as polysulphide adsorbent. J. Power Sources 2016, 303, 317–324. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, G.; Lai, Y.; Li, J.; Zhang, Z.; Chen, W. Nitrogen-doped porous hollow carbon sphere-decorated separators for advanced lithium–sulfur batteries. J. Power Sources 2015, 300, 157–163. [Google Scholar] [CrossRef]
- Zhao, D.; Qian, X.; Jin, L.; Yang, X.; Wang, S.; Shen, X.; Yao, S.; Rao, D.; Zhou, Y.; Xi, X. Separator modified by Ketjen black for enhanced electrochemical performance of lithium–sulfur batteries. RSC Adv. 2016, 6, 13680–13685. [Google Scholar] [CrossRef]
- Wang, Q.; Wen, Z.; Yang, J.; Jin, J.; Huang, X.; Wu, X.; Han, J. Electronic and ionic co-conductive coating on the separator towards high-performance lithium–sulfur batteries. J. Power Sources 2016, 306, 347–353. [Google Scholar] [CrossRef]
- Zhang, Y.; Miao, L.; Ning, J.; Xiao, Z.; Hao, L.; Wang, B.; Zhi, L. A graphene-oxide-based thin coating on the separator: an efficient barrier towards high-stable lithium–sulfur batteries. 2D Mater. 2015, 2, 024013. [Google Scholar] [CrossRef]
- Zhuang, T.Z.; Huang, J.Q.; Peng, H.J.; He, L.Y.; Cheng, X.B.; Chen, C.M.; Zhang, Q. Rational integration of polypropylene/graphene oxide/Nafion as ternary-layered separator to retard the shuttle of polysulphides for lithium–sulfur batteries. Small 2016, 12, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.-J.; Wang, D.-W.; Huang, J.-Q.; Cheng, X.-B.; Yuan, Z.; Wei, F.; Zhang, Q. Janus separator of polypropylene-supported cellular graphene framework for sulfur cathodes with high utilization in lithium–sulfur batteries. Adv. Sci. 2016, 3. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Ma, J.; Li, B.; Zuo, Y.; Xia, D. Enhanced cycle performance of lithium–sulfur batteries using a separator modified with a PVDF-C layer. ACS Appl. Mater. Interface 2014, 6, 20276–20281. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Lai, Y.; Zhang, Z.; Li, J.; Zhang, Z. Enhanced rate capability and cycle stability of lithium–sulfur batteries with a bifunctional MCNT@PEG-modified separator. J. Mater. Chem. A 2015, 3, 7139–7144. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, S.; Zhang, L.; Lin, R.; Wu, X.; Fang, H.; Ren, Y. Hollow spherical carbonized polypyrrole/sulphurcomposite cathode materials for lithium/sulphur cells with long cycle life. J. Power Sources 2014, 248, 337–342. [Google Scholar] [CrossRef]
- Jin, J.; Wen, Z.; Ma, G.; Lu, Y.; Rui, K. Mesoporous carbon/sulphur composite with polyaniline coating for llithium sulphur batteries. Solid State Ion. 2014, 262, 170–173. [Google Scholar] [CrossRef]
- Yang, Z.Z.; Wang, H.Y.; Lu, L.; Wang, C.; Zhong, X.B.; Wang, J.G.; Jiang, Q.C. Hierarchical TiO2 spheres as highly efficient polysulphide host for lithium-sulfur batteries. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- An, T.; Deng, D.; Lei, M.; Wu, Q.; Tian, Z.; Zheng, M.-S.; Dong, Q.-F. MnO modified carbon nanotubes as sulphur host with enhanced performance in Li/S batteries. J. Mater. Chem. A 2016, 4, 12858–12864. [Google Scholar] [CrossRef]



| Carbon Type | Porous Carbon | Carbon Nanospheres | CNTs | Graphene |
|---|---|---|---|---|
| Price | Cheap | Medium | Expensive | Expensive |
| Conductivity | Good | Good | Excellent | Excellent |
| BET surface area | High | Medium | Medium | Low |
| Pore volume | High | Medium | Low | High |
| Porous Carbon | Pore Size/BET Areas/Pore Volumes | Sulphur Content (wt%) | Reversible Capability (at nth Cycle and C-Rate) | Reference | |
|---|---|---|---|---|---|
| Microporous Carbon | Bamboo carbon | 0.6–0.75 nm/791.80 m2·g−1/0.38 cm3·g−1 | 50 | 550 mA·h·g−1 (150th, 0.5C) | [14] |
| MLC-2 | 0.6 nm/576 m2·g−1/− | 70 | 612 mA·h·g−1 (500th, 0.5C) | [37] | |
| Nitrogen-doped microporous carbons | <2 nm/1900 m2·g−1/1.2 cm3·g−1 | 40 | 1054 mA·h·g−1 (100th, 0.5C) | [39] | |
| Carbon FDU | 0.46 nm/876 m2·g−1/0.93 cm3·g−1 | 40 | 608 mA·h·g−1 (170th, 500 mA·g−1) | [40] | |
| PAN derived carbon | <2 nm/738 m2·g−1/− | 53.7 | 740 mA·h·g−1 (100th, 0.05C) | [41] | |
| Micro-activated carbon | 1.87 nm/1696 m2·g−1/0.79 cm3·g−1 | 54.27 | 500 mA·h·g−1 (50th, 3200 mA·g−1) | [42] | |
| Graphitic carbon | <2 nm/791.80 m2·g−1/0.38 cm3·g−1 | 88.9 | 448.6 mA·h·g−1 (200th, 0.5 C) | [43] | |
| Microporous carbon | 0.5 nm/−/0.217 cm3·g−1 | 31 | 600 mA·h·g−1 (4020th, 400 mA·g−1) | [44] | |
| Carbon spheres | 0.7 nm/843.5 m2·g−1/− | 42 | 650 mA·h·g−1 (500th, 400 mA·g−1) | [45] | |
| Carbon microspheres | 1.0 nm/915 m2·g−1/0.46 cm3·g−1 | 40 | 720 mA·h·g−1 (100th,100 mA·g−1) | [46] | |
| Flower-shaped activated porous carbon | <2 nm/2539 m2·g−1/1.48 cm3·g−1 | 60 | 600 mA·h·g−1 (50th, 1600 mA·g−1) | [47] | |
| Mesoporous carbon | Meso-active carbon | 2.5 nm/1080 m2·g−1/− | 30 | 440 mA·h·g−1 (25th, 0.3 mA·cm−2) | [48] |
| CMK-3 | 3.3 nm/1976 m2·g−1/2.1 cm3·g−1 | 70 | ∼800 mA·h·g−1 (20th, 168 mA·g−1) | [49] | |
| Mesoporous carbon | 12 nm/1100 m2·g−1/2.3 cm3·g−1 | 70 | ∼500 mA·h·g−1 (40th, 0.2C) | [51] | |
| CMK-3 with aniline groups | 2–3 nm/639.6 m2·g−1/0.7 cm3·g−1 | 42 | 920 mA·h·g−1 (100th, 0.2C) | [52] | |
| CMK-3 with DNA | 3.6 nm/1117 m2·g−1/1.37 cm3·g−1 | 56 | 771 mA·h·g−1 (100th, 0.1C) | [53] | |
| Ordered mesoporous carbon | 5.6/2.3 nm/2102 m2·g−1/2.0 cm3·g−1 | 60 | 400 mA·h·g−1 (400th, 0.5C) | [57] | |
| Bimodal porous carbons | 5.6/2.0 nm/2300 m2·g−1/2.0 cm3·g−1 | 50 | 550 mA·h·g−1 (100th, 1C) | [58] | |
| Tri-modal mesoporous carbon | 2.5/9/15 nm/2371 m2·g−1/2.85 cm3·g−1 | 70 | 700 mA·h·g−1 (300th, 0.5C) | [59] | |
| Porous biomass-derived carbon | 20–30 nm/949.85 m2·g−1/3.14 cm3·g−1 | 81.29 | 483 mA·h·g−1 (300th, 1C) | [60] | |
| Porous carbon-2 | 4 nm/735.2 m2·g−1/1.56 cm3·g−1 | 58.12 | 730 mA·h·g−1 (105th, 600 mA·g−1) | [61] | |
| Mesoporous carbon | 22 nm/1175 m2·g−1/4.8 cm3·g−1 | 50 | 840 mA·h·g−1 (100th, 0.1 C) | [62] | |
| Mesoporous carbon nanospheres | 3.5 nm/857 m2·g−1/− | 20 | 400 mA·h·g−1 (50th, 1.8C) | [64] | |
| Silica templated mesoporous carbon-20a | 19.9 nm/974 m2·g−1/1.96 cm3·g−1 | 60 | 835 mA·h·g−1 (50th, 0.25C) | [65] | |
| Spherical ordered mesoporous carbon | 6.0/3.1 nm/2445 m2·g−1/2.32 cm3·g−1 | 70 | 830 mA·h·g−1 (100th, 0.1C) | [67] | |
| Sulphur–functionalized mesoporous carbons | 5.0 nm/1044 m2·g−1/1.4 cm3·g−1 | - | ∼300 mA·h·g−1 (100th, 0.1C) | [68] | |
| Porous spherical carbon | 5.16 nm/2008.12 m2·g−1/3.40 cm3·g−1 | 59 | ∼850 mA·h·g−1 (50th, 0.1C) | [69] | |
| Nitrogen-doped mesoporous carbon | 2–20 nm/1474.97 m2·g−1/2.08 cm3·g−1 | 70 | 800 mA·h·g−1 (100th, 0.70 mA·cm−2) | [70] | |
| Mesoporous carbon foam | 2–10 nm/1478.55 m2·g−1/2.28 cm3·g−1 | 57.22 | 878 mA·h·g−1 (50th, 0.05C) | [72] | |
| Layered Ti2AlC–derived carbon | 3–15 nm/1264 m2·g−1/1.89 cm3·g−1 | 50 | 724 mA·h·g−1 (200th, 0.5C) | [73] | |
| Mesoporous carbon | 10 nm/215 m2·g−1/− | 36 | 836 mA·h·g−1 (100th, 230 mA·g−1) | [74] | |
| HPC | MOF-5 derived hierarchically porous carbon | 0–200 nm/1945 m2·g−1/1.92 cm3·g−1 | 51 | 662.3 mA·h·g−1 (40th, 400 mA·g−1) | [38] |
| Aspergillus flavus derived carbon | 1.5–3.5 nm/2459.6 m2·g−1/1.30 cm3·g−1 | 56.7 | 800 mA·h·g−1 (120th, 0.5C) | [75] | |
| Hierarchically ordered porous carbon | 9/300 nm/850 m2·g−1/1.4 cm3·g−1 | 50 | 473.6 mA·h·g−1 (100th, 1C) | [77] | |
| PAN derived HPC | 0–100 nm/1473.2 m2·g−1/− | 57 | 745 mA·h·g−1 (84th, 40 mA·g−1) | [79] | |
| Hierarchically porous carbon | <2 nm, 2–4 nm, 7.3 nm/1566.1 m2·g−1/0.503 cm3·g−1 (micropore volume ) | 51.5 | ∼350 mA·h·g−1 (50th, 2500 mA·g−1) | [80] | |
| Hierarchical porous honeycomb carbon | 0–100 nm/614.4 m2·g−1/1.34 cm3·g−1 | 66.3 | 564 mA·h·g−1 (100th, 2C) | [81] | |
| Hierarchically porous carbon monoliths | 0–100 nm/1426 m2·g−1/3.097 cm3·g−1 | 75 | 469 mA·h·g−1 (25th, −) | [82] | |
| Sulphur–infiltrated porous carbon microspheres | 0–100 nm/2485 m2·g−1/5.1 cm3·g−1 | 60 | 904 mA·h·g−1 (100th, 1C) | [83] | |
| Mg(OH)2 templated-hierarchically porous carbon | 0–100 nm/902.5 m2·g−1/2.60 cm3·g−1 | 84 | 562 mA·h·g−1 (100th, 1C) | [84] | |
| Hierarchically pore-structured carbon pillars | 0–25 nm/951 m2·g−1/0.484 cm3·g−1 | 58 | 456 mA·h·g−1 (40th, 0.1C) | [85] | |
| Multi-chambered micro/mesoporous carbon nanocubes | 0–90 nm/2425 m2·g−1/3.72 cm3·g−1 | 72 | 530 mA·h·g−1 (1000th, 5C) | [86] | |
| Composite Name | Sulphur Content in the Composite (wt%) | Reversible Capability (at nth Cycle and C-Rate) | Reference |
|---|---|---|---|
| MWCNTs–S | − | ~500 mA·h·g−1 (60th, 2 mA·cm−2) | [116] |
| S–coated-MWCNTs | 68 | ~650 mA·h·g−1 (60th, 100 mA·g−1) | [117] |
| Nano-S/MWCNTs | 60 | 810 mA·h·g−1 (30th, 300 mA·g−1) | [118] |
| S–MWCNTs | 75 | 854 mA·h·g−1 (30th, 100 mA·g−1) | [119] |
| Precipitated S/MWCNTs | 58.3 | 800 mA·h·g−1 (50th, 0.05C) | [120] |
| S-impregnated disordered CNTs | 40 | ~750 mA·h·g−1 (100th, 0.25C) | [121] |
| MWCNTs–S | 75 | 592 mA·h·g−1 (50th, 200 mA·g−1) | [122] |
| S–MWCNTs/KB | 85 | 420 mA·h·g−1 (100th, 1C) | [123] |
| Aligned CNTs/S | 90 | ~650 mA·h·cm−3 (90th, 0.1C) | [124] |
| S–CNTs | 65 | 740 mA·h·g−1 (100th, 0.1C) | [125] |
| CNTs–S | ~81 | 863 mA·h·g−1 (1000th, 5C) | [126] |
| CNTs–S | 90 | 600 mA·h·g−1 (19th, 0.02C) | [127] |
| VA-CNTs/S | 70 | ~800 mA·h·g−1 (40th, 0.08C) | [128] |
| S–coated CNTs | 40 | ~400 mA·h·g−1 (100th, 0.1C) | [129] |
| PD-coated S–MWCNTs–COOH–PAA | 83 | 640 mA·h·g−1 (500th, 1000 mA·g−1) | [130] |
| PAN–S@MWCNTs | 30 | 530 mA·h·g−1 (50th, 1C) | [131] |
| MWCNTs@Meso-C/S | 58.27 | 540 mA·h·g−1 (50th, 0.5 C) | [132] |
| GS–MWCNTs@S | 70 | 844 mA·h·g−1 (100th, 0.2 C) | [133] |
| VGCFs–S–MWCNTs | − | 716 mA·h·g−1 (40th, 335 mA·g−1) | [134] |
| MWCNTs–S–PAN | 35.1 | 491.5 mA·h·g−1 (100th, 0.5 C) | [135] |
| RGO@MWCNTs–W/S | 68.9 | 891.5 mA·h·g−1 (200th, 1 C) | [136] |
| Mesoporous carbon–CNTs–S | 75 | 740 mA·h·g−1 (50th, 0.84 mA·cm−2) | [137] |
| S/MWCNTs nanomicrosphere | 57 | 780 mA·h·g−1 (200th, 1000 mA·g−1) | [138] |
| Hierarchical CNTs@S coaxial nanocables | 50 | 713 mA·h·g−1 (150th, 1C) | [139] |
| S/PNCNTs | − | 933 mA·h·g−1 (50th, 1C) | [140] |
| Composite Name | Reducing Agent/Method | Sulphur Content in Graphene/RGO Composite (wt%) | Reversible Capability (at nth Cycle and C-Rate) | Reference |
|---|---|---|---|---|
| RGO–S | Hydrothermal | 22 | ~550 mA·h·g−1 (40th, 50 mA·g−1) | [148] |
| S–graphene | 75.2 | 662 mA·h·g−1 (100th, 1000 mA·g−1) | [149] | |
| Dense S–graphene | 69.6 | 770 mA·h·g−1 (300th, 0.5C) | [150] | |
| S–GS | 80 | 4.53 mA·h·cm−2 (300th, 0.1C) | [151] | |
| S@3D–graphene | 73 | 700 mA·h·g−1 (100th, 0.1C) | [152] | |
| Graphene–S | 63 | 541 mA·h·g−1 (100th, 750 mA·g−1) | [153] | |
| AG/S | Ar Thermal Pyrolysis | 65 | 717.1 mA·h·g−1 (200th, 1000 mA·g−1) | [154] |
| MGP–S | 55 | 689 mA·h·g−1 (50th, 0.1C) | [155] | |
| S–GnPs–CP | 70 | ~700 mA·h·g−1 (500th, 2C) | [156] | |
| S/RGO | N2 Thermal Pyrolysis | 60 | 625 mA·h·g−1 (200th, 0.1C) | [157] |
| S–RGO | H2/Ar Thermal Pyrolysis | 63 | 658 mA·h·g−1 (200th, 500 mA·g−1) | [158] |
| RGO/S | Vacuum Thermal Pyrolysis | 52 | ~880 mA·h·g−1 (220th, 100 mA·g−1) | [159] |
| GSC | Na2S~2.4 | 87 | 550 mA·h·g−1 (50th, 0.2C) | [160] |
| SGC | Na2S | 63.6 | 440 mA·h·g−1 (500th, 0.75C) | [161] |
| S–nanosphere@graphene | Hydrazine | 91 | 430 mA·h·g−1 (100th, 0.2C) | [162] |
| RGO/S | HI | 80 | 720 mA·h·g−1 (50th, 0.5 mA·cm−2) | [163] |
| S@RGO | 85 | 480 mA·h·g−1 (200th, 0.05C) | [164] | |
| HDGS | H2S | 32 | ~100 mA·h·cm−2 (300th, 0.8C) | [165] |
| HRGO/S | ~50 | ~525 mA·h·g−1 (100th, 500 mA·g−1) | [166] | |
| GES | Urea | 83.3 | 523.1 mA·h·g−1 (500th, 3C) | [167] |
| Carbon Interlayer Type | Sulphur Loading in the Electrode | Reversible Capability (at nth Cycle and C-Rate) | Reference |
|---|---|---|---|
| Nitrogen and phosphorous dual-doped graphene | 70% | 638 mA·h·g−1(500th, 1C) | [7] |
| Bamboo carbon fiber | 70% | 605.7 mA·h·g−1(300th, 1C) | [18] |
| MCP | 70% | 1000 mA·h·g−1(100th, 1C) | [173] |
| Carbonized eggshell membrane | 3.0–3.2 mg·cm−2 | 1000 mA·h·g−1(100th, 0.1C) | [174] |
| Carbonized leaf | 1.3 mg·cm−2 | 800 mA·h·g−1(150th, 0.5C) | [175] |
| Cassava-derived carbon sheet | 1.6 mg·cm−2 | 811 mA·h·g−1(100th, 0.5C) | [176] |
| Filamentous fungi-derived carbon | 60% | 650 mA·h·g−1(100th, 0.5C) | [177] |
| Carbonized non-woven fabrics films | 2 mg·cm−2 | 677 mA·h·g−1(100th, 1C) | [178] |
| Hierarchical carbonized paper | 1.1 mg·cm−2 | 780 mA·h·g−1(200th, 2C) | [179] |
| Porous carbon mat | 1.45 mg·cm−2 | 527 mA·h·g−1(400th, 1C) | [180] |
| Carbonized bacterial cellulose | 81% | 620 mA·h·g−1(300th, 800 mA·g−1) | [181] |
| PI-based ACNFs | 2.5 ± 0.1 mg·cm−2 | 897 mA·h·g−1(100th, 0.1C) | [182] |
| Polypyrrole-supported carbon | 70% | 555 mA·h·g−1(200th, 0.5C) | [183] |
| Filter paper derived carbon | 70% | 560 mA·h·g−1(50th, 1C) | [184] |
| CNF/RGO | 1.40 mg·cm−2 | 533.6 mA·h·g−1(400th, 1C) | [185] |
| Carbon paper | 60% | 929 mA·h·g−1(100th, 1C) | [186] |
| MWCNTs | 1.20 mg·cm−2 | 1200 mA·h·g−1(100th, 1C) | [187] |
| TiO2–CNTs | 1.8 mg·cm−2 | 848 mA·h·g−1(100th, 5C) | [188] |
| MWCNTs | 70% | 804 mA·h·g−1(100th, 1C) | [189] |
| KB in PTFE latex | 4.0 mg·cm−2 | 1000 mA·h·g−1(150th, 0.5C) | [190] |
| Al2O3–porous carbon cloth | 1.2 mg·cm−2 | 766 mA·h·g−1(40th, 40 mA·g−1) | [191] |
| Carbon-nanotube paper–titanium dioxide | 70% | 575.8 mA·h·g−1(250th, 0.5C) | [192] |
| Fe3C/CNF web | 70% | 893 mA·h·g−1(100th, 200 mA·g−1) | [193] |
| RGO–carbon black | 80% | 895 mA·h·g−1(100th, 0.1C) | [194] |
| Nitrogen-doped graphene | 0.4–0.5 mg·cm−2 | ~400 mA·h·g−1(100th, 2C) | [195] |
| Carbon Modified Separator | Sulphur Content in the Electrode | Reversible Capability (at nth Cycle and C-Rate) | Reference |
|---|---|---|---|
| Super P modified separator | 1.1–1.3 mg·cm−2 | 701 mA·h·g−1 (200th, 2C) | [196] |
| ACNF modified separator | 2.1–2.3 mg·cm−2 | 819 mA·h·g−1 (200th, 2C) | [197] |
| MWCNTs modified separator | 70% | 621 mA·h·g−1 (300th, 1C) | [198] |
| SWCNTs modulated separator | 6.3 mg·cm−2 | 500 mA·h·g−1 (100th, 0.2C) | [199] |
| PEG–microporous carbon modified separator | 70% | 596 mA·h·g−1 (500th, 0.2C) | [200] |
| Graphene modified separator | 70% | 680 mA·h·g−1 (300th, 1500 mA·g−1) | [201] |
| Mesoporous carbon modified separator | 1.55 mg·cm−2 | 591 mA·h·g−1 (500th, 2C) | [202] |
| Polypropylene–nitrogen-doped mesoporous carbon modified separator | 3.95 mg·cm−2 | 566 mA·h·g−1 (1200th, 0.5C) | [203] |
| Nitrogen-doped hollow porous carbon spheres modified separator | 1.6 mg·cm−2 | 542 mA·h·g−1 (500th, 1C) | [204] |
| KB modified separator | 64% | 815 mA·h·g−1 (100th, 1C) | [205] |
| Acetylene black–CNTs modified separator | 1.5–2.0 mg·cm−2 | 830 mA·h·g−1 (150th, 0.5C) | [206] |
| GO modified separator | 1.2–1.4 mg·cm−2 | 750 mA·h·g−1 (100th, 1C) | [207] |
| Polypropylene/GO/Nafion modified separator | 60% | ~750 mA·h·g−1 (200th, 0.5C) | [208] |
| Polypropylene–cellular graphene modified separator | 1.2 mg·cm−2 | 800 mA·h·g−1 (250th, 0.2C) | [209] |
| Polyvinylidene fluoride–carbon modified separator | 7 mg·cm−2 | 669 mA·h·g−1 (500th, 0.5C) | [210] |
| MWCNTs@PEG modified separator | 60% | 490 mA·h·g−1 (500th, 1C) | [211] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, X.; Hencz, L.; Zhang, S. Recent Development of Carbonaceous Materials for Lithium–Sulphur Batteries. Batteries 2016, 2, 33. https://doi.org/10.3390/batteries2040033
Gu X, Hencz L, Zhang S. Recent Development of Carbonaceous Materials for Lithium–Sulphur Batteries. Batteries. 2016; 2(4):33. https://doi.org/10.3390/batteries2040033
Chicago/Turabian StyleGu, Xingxing, Luke Hencz, and Shanqing Zhang. 2016. "Recent Development of Carbonaceous Materials for Lithium–Sulphur Batteries" Batteries 2, no. 4: 33. https://doi.org/10.3390/batteries2040033





