Freeze-Drying-Assisted Preparation of High-Compaction-Density LiMn0.69Co0.01Fe0.3PO4 Cathode Materials with High-Capacity and Long Life-Cycle for Lithium Ion Batteries
Abstract
:1. Introduction
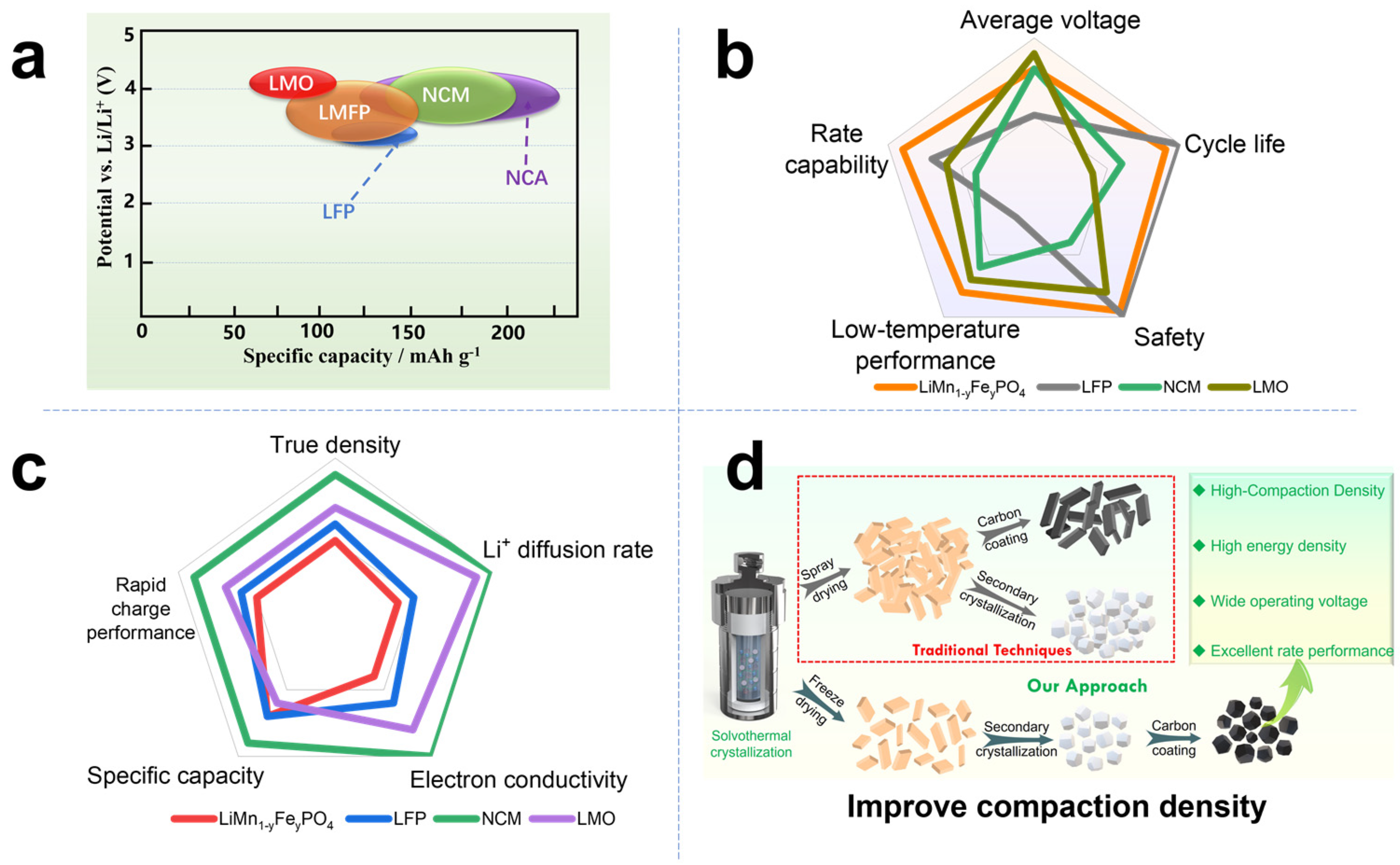
2. Materials and Methods
2.1. Materials and Synthesis
2.2. Characterization
2.3. Electrode Preparation and Electrochemistry
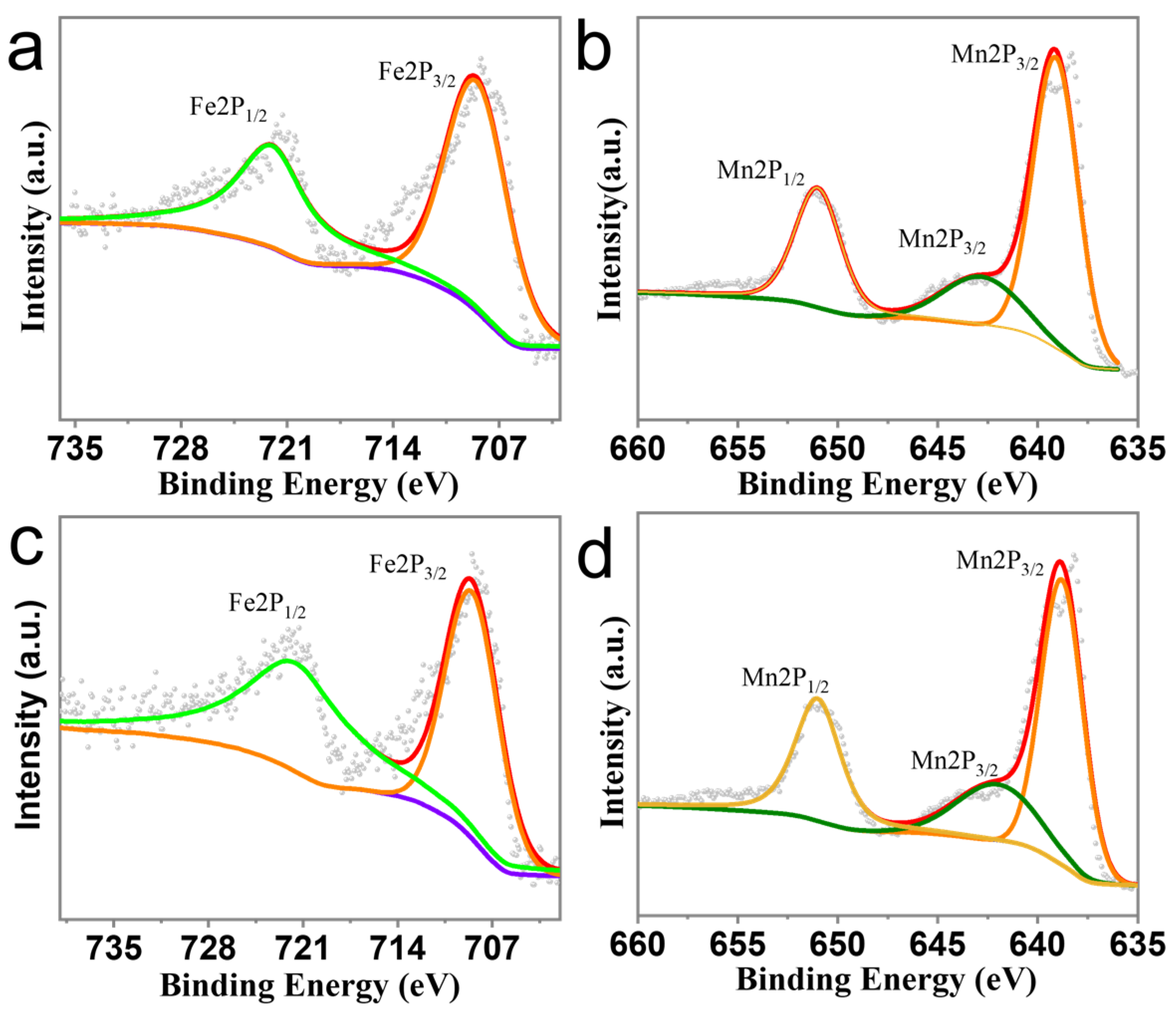
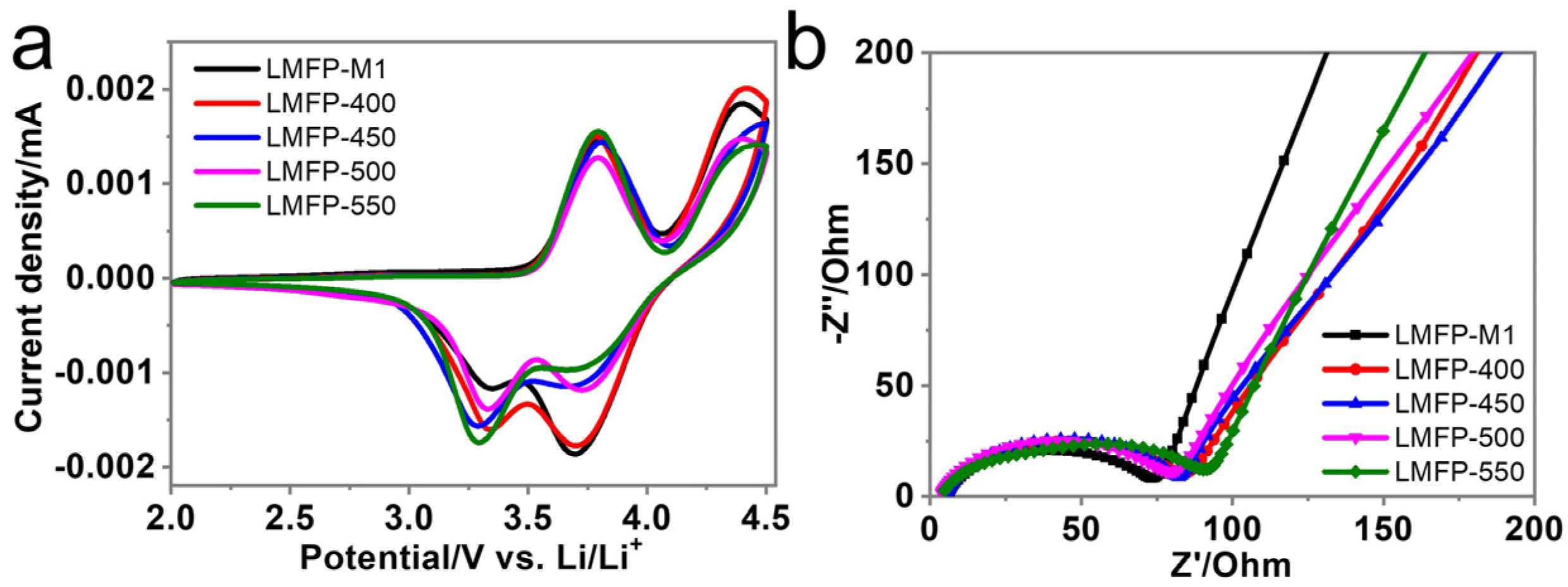
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Wu, Y.; Zhang, B.; Xiao, X.; Hu, Q.; Han, Q.; Wang, L.; Bei, F.; He, X. A Promising Solid-state Synthesis of LiMn1−yFeyPO4 Cathode for Lithium-ion Batteries. Small 2023, e2309629. [Google Scholar] [CrossRef]
- Renier, O.; Pellini, A.; Spooren, J. Advances in the Separation of Graphite from Lithium Iron Phosphate from End-of-Life Batteries Shredded Fine Fraction Using Simple Froth Flotation. Batteries 2023, 9, 589. [Google Scholar] [CrossRef]
- Padhi, A.K.; Nanjundaswamy, K.S.; Goodenough, J.B. Phospho-olivines as Positive-electrode Materials for Rechargeable Lithium Batteries. J. Electrochem. Soc. 1997, 144, 1188. [Google Scholar] [CrossRef]
- Malik, R.; Abdellahi, A.; Ceder, G. A Critical Review of the Li Insertion Mechanisms in LiFePO4 Electrodes. J. Electrochem. Soc. 2013, 160, A3179. [Google Scholar] [CrossRef]
- Wang, J.; Sun, X. Olivine LiFePO4: The Remaining Challenges for Future Energy Storage. Energy Environ. Sci. 2015, 8, 1110–1138. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, L.; Jiang, L.; Zhu, X.; Li, F.; Liu, X.; Mai, K.; Zhang, Z.; Fan, X.; Lv, X. Multifunctional Radical Polymers-enabled Rapid Charge/Discharge and High Capacity for Flexible Self-standing LiFePO4/PETM/SWNT Hybrid Electrodes. Chem. Eng. J. 2024, 482, 149008. [Google Scholar] [CrossRef]
- Chung, S.-Y.; Bloking, J.T.; Chiang, Y.-M. On the Electronic Conductivity of Phospho-olivines as Lithium Storage Electrodes. Nat. Mater. 2003, 2, 702–703. [Google Scholar] [CrossRef]
- Yang, Z.; Dai, Y.; Wang, S.; Yu, J. How to Make Lithium Iron Phosphate Better: A Review Exploring Classical Modification Approaches In-depth and Proposing Future Optimization Methods. J. Mater. Chem. A 2016, 4, 18210–18222. [Google Scholar] [CrossRef]
- Nwachukwu, I.M.; Nwanya, A.C.; Ekwealor AB, C.; Ezema, F. Research Progress in Solid-state Synthesized LiMnPO4 Cathode Material for Li-ion Battery Applications. Appl. Surf. Sci. Adv. 2023, 18, 100505. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, W.; Duan, J.; Qian, F.; Cao, Y.; He, J.; Wang, D.; Dong, P.; Zhang, J. Highly Efficient Synthesis of Nano LiMn0.90Fe0.10PO4/C Composite via Mechanochemical Activation Assisted Calcination. Ceram. Int. 2023, 49, 18483–18490. [Google Scholar]
- Zhou, F.; Kang, K.; Maxisch, T.; Ceder, G.; Morgan, D. The Electronic Structure and Band Gap of LiFePO4 and LiMnPO4. Solid State Commun. 2004, 132, 181–186. [Google Scholar] [CrossRef]
- Aravindan, V.; Gnanaraj, J.; Lee, Y.-S.; Madhavi, S. LiMnPO4–A next Generation Cathode Material for Lithium-ion Batteries. J. Mater. Chem. A 2013, 1, 3518–3539. [Google Scholar] [CrossRef]
- Lee, H.; Park, J.; Kim, M.; Choi, K.; Kim, K.; Im, D. High-Energy-Density Li-O2 Battery at Cell Scale with Folded Cell Structure. Joule 2018, 11, 016. [Google Scholar] [CrossRef]
- Shi, C.; Takeuchi, S.; Alexander, G.; Hamann, T.; O’Neill, J.; Dura, J.; Wachsman, E. High Sulfur Loading and Capacity Retention in Bilayer Garnet Sulfurized-Polyacrylonitrile/Lithium-Metal Batteries with Gel Polymer Electrolytes. Adv. Energy Mater. 2023, 13, 2301656. [Google Scholar] [CrossRef]
- Shi, C.; Hamann, T.; Takeuchi, S.; Alexander, G.; Nolan, A.; Limpert, M.; Fu, Z.; O’Neill, J.; Godbey, G.; Dura, J.; et al. 3D Asymmetric Bilayer Garnet-Hybridized High-Energy-Density Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2023, 15, 751–760. [Google Scholar] [CrossRef]
- Lu, X.; He, H.; Qiu, H.; Jiang, W.; Zhang, Y.; He, W. Ethylene Glycol Solvothermal Synthesis of LiMnPO4 Nanoparticles with High (200) Crystal Face Exposure for High Performance Lithium-ion Batteries. Mater. Sci. Eng. B 2024, 299, 117032. [Google Scholar] [CrossRef]
- Dong, Y.; Xie, H.; Song, J.; Xu, M.; Zhao, Y.; Goodenough, J. The Prepared and Electrochemical Property of Mg Doped LiMnPO4 Nanoplates as Cathode Materials for Lithium-ion Batteries. J. Electrochem. Soc. 2012, 159, A995. [Google Scholar] [CrossRef]
- Yonemura, M.; Yamada, A.; Takei, Y.; Sonoyama, N.; Kanno, R. Comparative Kinetic Study of Olivine LixMPO4 (M = Fe, Mn). J. Electrochem. Soc. 2004, 151, A1352. [Google Scholar] [CrossRef]
- Nishimura, S.-I.; Kobayashi, G.; Ohoyama, K.; Kanno, R.; Yashima, M.; Yamada, A. Experimental Visualization of Lithium Diffusion in LixFePO4. Nat. Mater. 2008, 7, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Kudo, Y.; Liu, K.-Y. Phase Diagram of Lix(MnyFe1−y)PO4(0 ≤ x, y ≤ 1). J. Electrochem. Soc. 2001, 148, A1153. [Google Scholar] [CrossRef]
- Yamada, A.; Chung, S. Crystal Chemistry of the Olivine-Type Li(MnyFe1−y)PO4 and (MnyFe1−y)PO4 as Possible 4 V Cathode Materials for Lithium Batteries. J. Electrochem. Soc. 2001, 148, A960–A967. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, L.; Zhang, S.; Zhao, Y.; Zhou, J.; Xie, H.; Goodenough, J. Two-phase Interface in LiMnPO4 Nanoplates. J. Power Sources 2012, 215, 116–121. [Google Scholar] [CrossRef]
- Zhu, K.; Zhang, W.; Du, J.; Liu, X.; Tian, J.; Ma, H.; Liu, S.; Shan, Z. Reaction Mechanism and Influence of the Experimental Variables for Solvothermal Synthesized LiMnPO4 Nanoplates. J. Power Sources 2015, 300, 139–146. [Google Scholar] [CrossRef]
- Esmezjan, L.; Mikhailova, D.; Etter, M.; Cabana, J.; Grey, C.; Indris, S.; Ehrenberg, H. Electrochemical Lithium Extraction and Insertion Process of Sol-gel Synthesized LiMnPO4 via Two-Phase Mechanism. J. Electrochem. Soc. 2019, 166, A1257–A1265. [Google Scholar] [CrossRef]
- Yamada, A.; Kudo, Y.; Liu, K.Y. Reaction Mechanism of the Olivine-type LixMn0.6Fe0.4PO4 (0 < x < 1). J. Electrochem. Soc. 2001, 148, A747–A754. [Google Scholar]
- Huang, W.; Tao, S.; Zhou, J.; Si, C.; Chen, X.; Huang, W.; Jin, C.; Chu, W.; Song, L.; Wu, Z. Phase Separations in LiFe1–xMnxPO4: A Random Stack Model for Efficient Cathode Materials. J. Phys. Chem. C 2013, 118, 796–803. [Google Scholar] [CrossRef]
- Lei, Z.; Naveed, A.; Lei, J.; Wang, J.; Yang, J.; Nuli, Y.; Meng, X.; Zhao, Y. High performance nano-sized LiMn1−xFexPO4 cathode materials for advanced lithium-ion batteries. RSC Adv. 2017, 7, 43708–43715. [Google Scholar] [CrossRef]
- Malik, R.; Zhou, F.; Ceder, G. Phase Diagram and Electrochemical Properties of Mixed Olivines from First-principles Calculations. Phys. Rev. B 2009, 79, 214201. [Google Scholar] [CrossRef]
- Dimesso, L.; Förster, C.; Jaegermann, W.; Khanderi, J.; Tempel, H.; Popp, A.; Engstler, J.; Schneider, J.; Sarapulova, A.; Mikhailova, D.; et al. Developments in Nanostructured LiMPO4 (M = Fe, Co, Ni, Mn) Composites Based on Three Dimensional Carbon Architecture. Chem. Soc. Rev. 2012, 41, 5068–5080. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Deng, W.; Xu, W.; Tian, Y.; Wang, A.; Wang, B.; Zou, G.; Hou, H.; Deng, W.; Ji, X. Olivine LiMnxFe1−xPO4 Cathode Materials for Lithium Ion Batteries: Restricted Factors of Rate Performances. J. Mater. Chem. A 2021, 9, 14214–14232. [Google Scholar] [CrossRef]
- Huang, S.; Lin, W.; Li, L.; Liu, P.; Huang, T.; Huang, Z.; Kong, J.; Xiong, W.; Yu, W.; Ye, S.; et al. Pathway for High-energy Density LiMnFePO4 Cathodes. Prog. Nat. Sci. Mater. Int. 2023, 33, 126–131. [Google Scholar] [CrossRef]
- Li, Y.; Xu, G.; Fan, S.M.; Ma, J.; Shi, X.; Long, Z.; Deng, W.; Fan, W.; Yang, S. Synthesis of Carbon-coated LiMn0.8Fe0.2PO4 Materials via an Aqueous Rheological Phase-assisted Solid-state Method. J. Solid State Electrochem. 2020, 24, 821–828. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, J.; Zhang, B.; Wu, Y.; Liu, J.; Yin, L.; Zhan, M.; Xiao, Y.; An, B.; Wang, L.; et al. Engineering Manganese-rich Phospho-olivine Cathode Materials with Exposed Crystal {010} Facets for Practical Li-ion Batteries. Chem. Eng. J. 2023, 454, 139986. [Google Scholar] [CrossRef]
- Jin, Y.; Tang, X.; Wang, Y.; Dang, W.; Huang, J.; Fang, X. High-tap Density LiFePO4 Microsphere Developed by Combined Computational and Experimental Approaches. CrystEngComm 2018, 20, 6695–6703. [Google Scholar] [CrossRef]
- Yang, S.; Ma, R.; Hu, M.; Xi, L.; Lu, Z.; Chung, C. Solvothermal Synthesis of Nano-LiMnPO4 from Li3PO4 Rod-like Precursor: Reaction Mechanism and Electrochemical Properties. J. Mater. Chem. 2012, 22, 25402–25408. [Google Scholar] [CrossRef]
- Zhang, X.; Hou, M.; Tamirate, A.G.; Zhu, H.; Wang, C.; Xia, Y. Carbon Coated Nano-sized LiMn0.8Fe0.2PO4 Porous Microsphere Cathode Material for Li-ion Batteries. J. Power Sources 2020, 448, 227438. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; An, L.; Zhao, X.; Wang, L.; Liang, G. High Energy Density LiFePO4/C Cathode Material Synthesized by Wet Ball Milling Combined with Spray Drying Method. J. Electrochem. Soc. 2017, 164, A3666. [Google Scholar] [CrossRef]
- Wang, X.; Wen, L.; Zheng, Y.; Liu, H.; Liang, G. Facile Synthesis and Electrochemical Properties of High Tap Density LiFePO4/C. Ionics 2019, 25, 4589–4596. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Zhang, H.; Liang, F.; Yao, Y.; Zhang, K. Freeze Drying under Vacuum Assisted Synthesis of LiFePO4@MWCNTs Composite with Phytic acid as Phosphorus Source for Advanced Li-storage. Vacuum 2021, 193, 110541. [Google Scholar] [CrossRef]
- Zhecheva, E.; Mladenov, M.; Zlatilova, P.; Koleva, V.; Stoyanova, R. Particle Size Distribution and Electrochemical Properties of LiFePO4 Prepared by a Freeze-drying Method. J. Phys. Chem. Solids 2010, 71, 848–853. [Google Scholar] [CrossRef]
- Palomares, V.; Goñi, A.; de Muro, L.G.; de Meatza, I.; Bengoechea, M.; Miguel, O.; Rojo, T. New Freeze-drying Method for LiFePO4 synthesis. J. Power Sources 2007, 171, 879–885. [Google Scholar] [CrossRef]
- Hadouchi, M.; Yaqoob, N.; Kaghazchi, P.; Tang, M.; Liu, J.; Sang, P.; Fu, Y.; Huang, Y.; Ma, J. Fast Sodium Intercalation in Na£FeV(PO), A Novel Sodium-Deficient NASICON Cathode for Sodium-ion Batteries. Energy Storage Mater. 2021, 35, 192–202. [Google Scholar] [CrossRef]
- Hou, J.; Hadouchi, M.; Sui, L.; Liu, J.; Tang, M.; Kan, W.; Avdeev, M.; Zhong, G.; Liao, Y.; Lai, Y.; et al. Unlocking Fast and Reversible Sodium Intercalation in NASICON Na4MnV(PO)3 by Fluorine Substitution. Energy Storage Mater. 2021, 42, 307–316. [Google Scholar] [CrossRef]
- Su, J.; Wei, B.Q.; Rong, J.; Yin, W.; Ye, Z.; Tian, X.; Ren, L.; Cao, M.; Hu, C. A General Solution-chemistry Route to the Synthesis LiMPO4 (M=Mn, Fe, and Co) Nanocrystals with [010] Orientation for Lithium ion Batteries. J. Solid State Chem. 2011, 184, 2909–2919. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, S.; Fan, C.L.; Yang, J.; Song, Z.; Zeng, X. High-performance LiMn0.8Fe0.2PO4/C Cathode Prepared by Using the Toluene-soluble Component of Pitch as a Carbon Source. Int. J. Energy Res. 2021, 45, 19103–19119. [Google Scholar] [CrossRef]
- Peng, L.; Zhang, X.; Fang, Z.; Zhu, Y.; Xie, Y.; Cha, J.; Yu, G. A General Facet-Controlled Synthesis of Single-Crystalline {010}-Oriented LiMPO4 (M = Mn, Fe, Co) Nanosheets. Chem. Mater. 2017, 29, 10526–10533. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, F. Probing the Morphology Dependence, Size Preference and Electron/ion Conductance of Manganese-based Lithium Transition-metal Phosphate as Cathode Materials for High-performance Lithium-ion Battery. J. Alloys Compd. 2021, 850, 156773. [Google Scholar] [CrossRef]
- Yang, H.; Fu, C.; Sun, Y.; Wang, L.; Liu, T. Fe-doped LiMnPO4@C Nanofibers with High Li-ion Diffusion Coefficient. Carbon 2019, 11, 067. [Google Scholar] [CrossRef]
- Mc Carthy, K.; Gullapalli, H.; Kennedy, T. Electrochemical Impedance Correlation Analysis for the Estimation of Li-ion Battery State of Charge, State of Health and Internal Temperature. J. Energy Storage 2022, 50, 104608. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, D.Y.; Qiao, J.; Chang, C. High Performance of LiMn1−xFexPO4/C (0 ≤ x ≤ 0.5) Nanoparticles Synthesized by Microwave-Assisted Solvothermal Method. Ionics 2018, 24, 689–696. [Google Scholar] [CrossRef]
- Deng, Z.; Wang, Q.; Peng, D.; Liu, H.; Chen, Y. Fast Precipitation-Induced LiFe0.5Mn0.5PO4/C Nanorods with A Fine Size and Large Exposure of the (010) Faces for High-Performance Lithium-ion Batteries. J. Alloys Compd. 2019, 794, 178–185. [Google Scholar] [CrossRef]
- Li, J.; Guo, C.; Qin, Y.; Ning, X. Ascorbic Acid-Assisted Solvothermal Synthesis of LiMn1−XFexPO4/C Nanoparticles for High Performance Li-ion Cathode Materials. Mater. Technol. 2020, 35, 9–10. [Google Scholar] [CrossRef]
- Lu, C.-H.; Subburaj, T.; Chiou, H.-T. Facile Sol-Gel Synthesis of LiMn0.5Fe0.5PO4 Cathode Materials Fostered by Bio-Derived Natural Agar. Ionics 2020, 26, 1051–1056. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, Y.; Zhang, Z.; Chen, X.; Guo, Y.; Xiao, D. A Phytic Acid Derived LiMn0.5Fe0.5PO4/Carbon Composite of High Energy Density for Lithium Rechargeable Batteries. Sci. Rep. 2019, 9, 6665. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Liu, X.; Zhu, B.; Wang, F. Solvothermal Synthesis of LiFe1/3Mn1/3Co1/3PO4 Solid Solution as Lithium Storage Cathode Materials. RSC Adv. 2017, 7, 14354. [Google Scholar] [CrossRef]
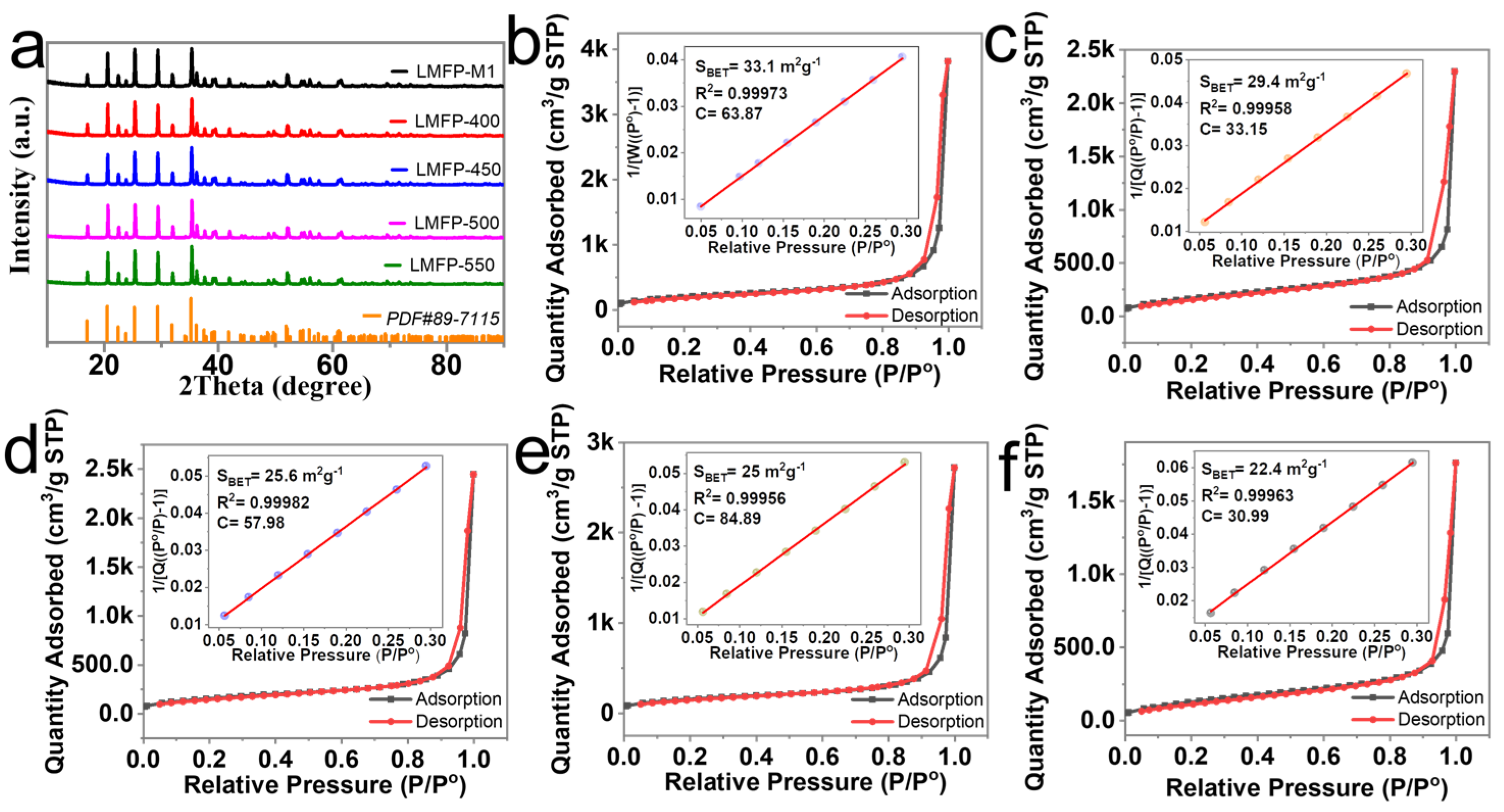



| Samples | LMFP-M1 | LMFP-400 | LMFP-450 | LMFP-500 | LMFP-550 | |
|---|---|---|---|---|---|---|
| Specific surface area (m2/g) | 33.1 | 29.4 | 25.6 | 25.0 | 22.4 | |
| After carbon coating | LMFP-M1/C | LMFP-400/C | LMFP-450/C | LMFP-500/C | LMFP-550/C | |
| Compaction density (g/cm³) | 1.96 | 2.18 | 2.27 | 2.34 | 2.43 | |
| Median Voltage (V) | 0.1 C | 4.028 | 4.025 | 3.996 | 4.009 | 3.995 |
| 0.2 C | 4.007 | 3.983 | 3.876 | 3.919 | 3.823 | |
| 1 C | 3.831 | 3.695 | 3.534 | 3.523 | 3.486 | |
| 2 C | 3.518 | 3.469 | 3.379 | 3.361 | 3.330 | |
| 5 C | 3.290 | 3.185 | 3.123 | 3.069 | 3.074 | |
| 10 C | 3.009 | 2.771 | 2.754 | 2.660 | 2.712 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Zheng, J.; Huang, H.; Li, H.; Zhang, H.; Li, L.; An, B.; Xiao, Y.; Sun, C. Freeze-Drying-Assisted Preparation of High-Compaction-Density LiMn0.69Co0.01Fe0.3PO4 Cathode Materials with High-Capacity and Long Life-Cycle for Lithium Ion Batteries. Batteries 2024, 10, 114. https://doi.org/10.3390/batteries10040114
Liu S, Zheng J, Huang H, Li H, Zhang H, Li L, An B, Xiao Y, Sun C. Freeze-Drying-Assisted Preparation of High-Compaction-Density LiMn0.69Co0.01Fe0.3PO4 Cathode Materials with High-Capacity and Long Life-Cycle for Lithium Ion Batteries. Batteries. 2024; 10(4):114. https://doi.org/10.3390/batteries10040114
Chicago/Turabian StyleLiu, Shaojun, Jingang Zheng, Hao Huang, Hongyang Li, Han Zhang, Lixiang Li, Baigang An, Yuanhua Xiao, and Chengguo Sun. 2024. "Freeze-Drying-Assisted Preparation of High-Compaction-Density LiMn0.69Co0.01Fe0.3PO4 Cathode Materials with High-Capacity and Long Life-Cycle for Lithium Ion Batteries" Batteries 10, no. 4: 114. https://doi.org/10.3390/batteries10040114





