Constructing a LiPON Layer on a 3D Lithium Metal Anode as an Artificial Solid Electrolyte Interphase with Long-Term Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of the LiB@LiPON Electrode
2.2. Materials Characterization
2.3. Electrochemical Measurements
3. Results and Discussion
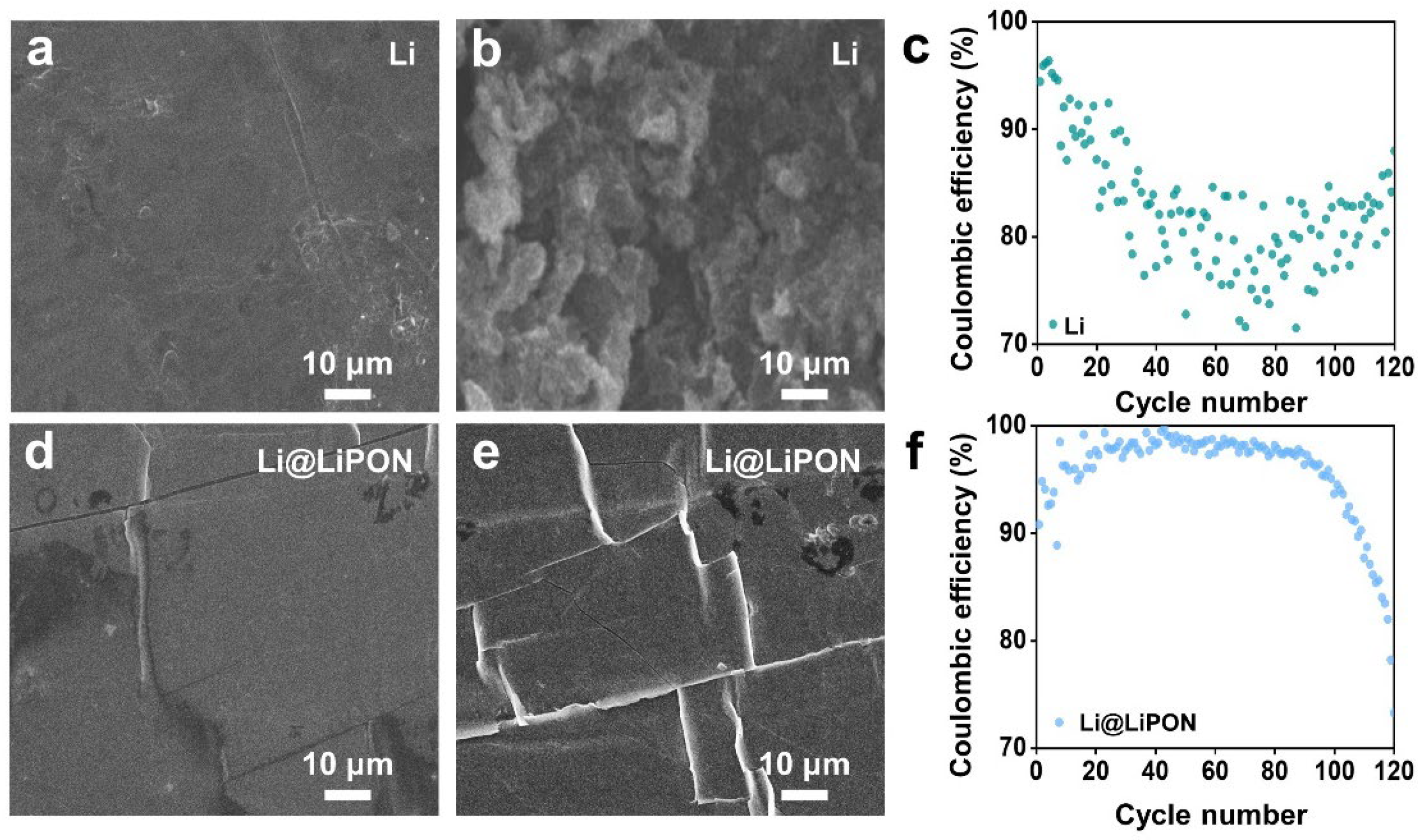
3.1. Morphology Revolution of LiPON on Pure Lithium Metal
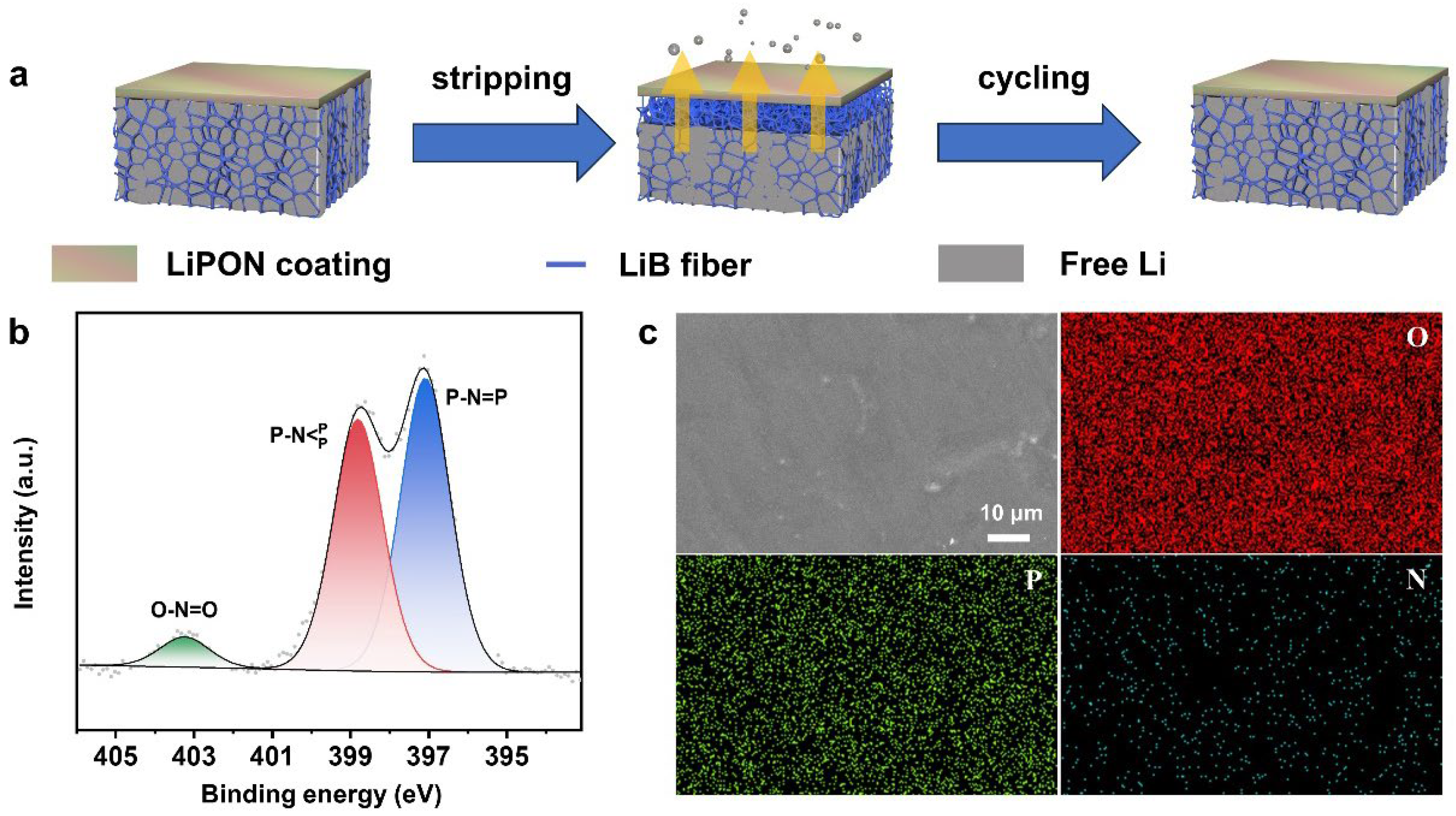
3.2. Characterizations of LiPON on LiB
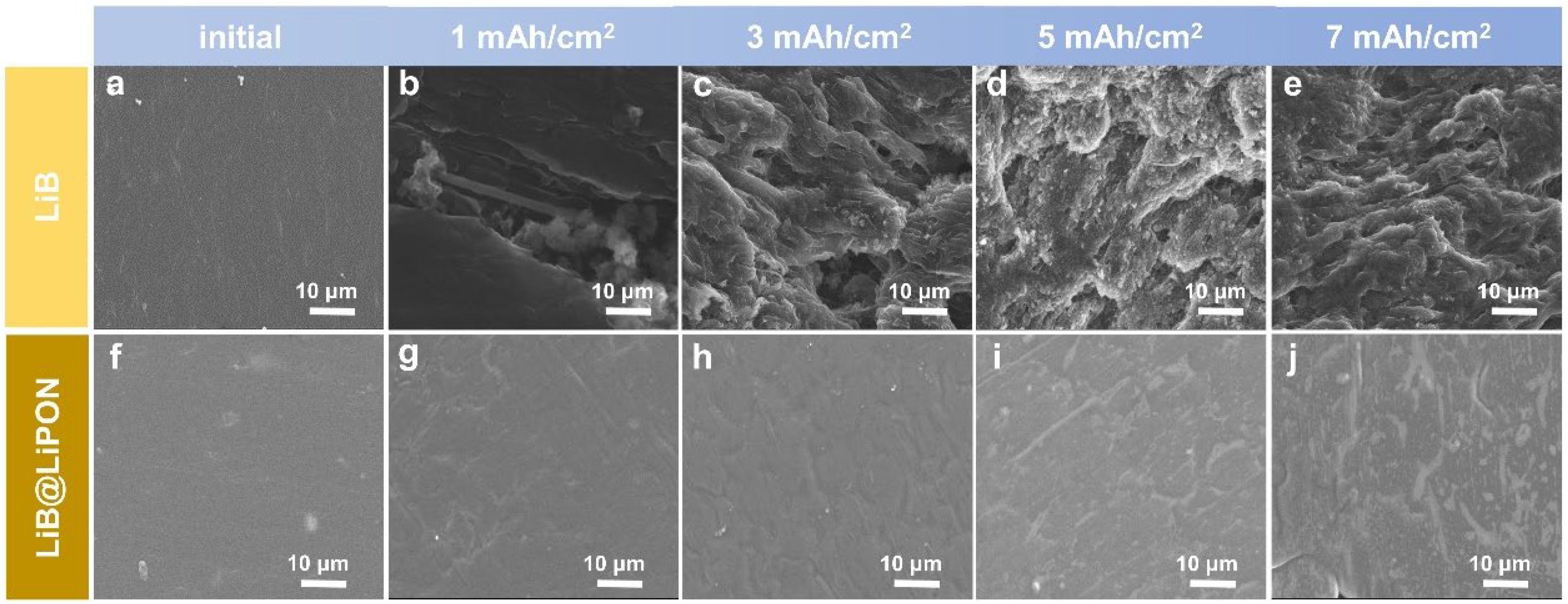
3.3. Morphology Revolution of LiB@LiPON during the First Charge Process
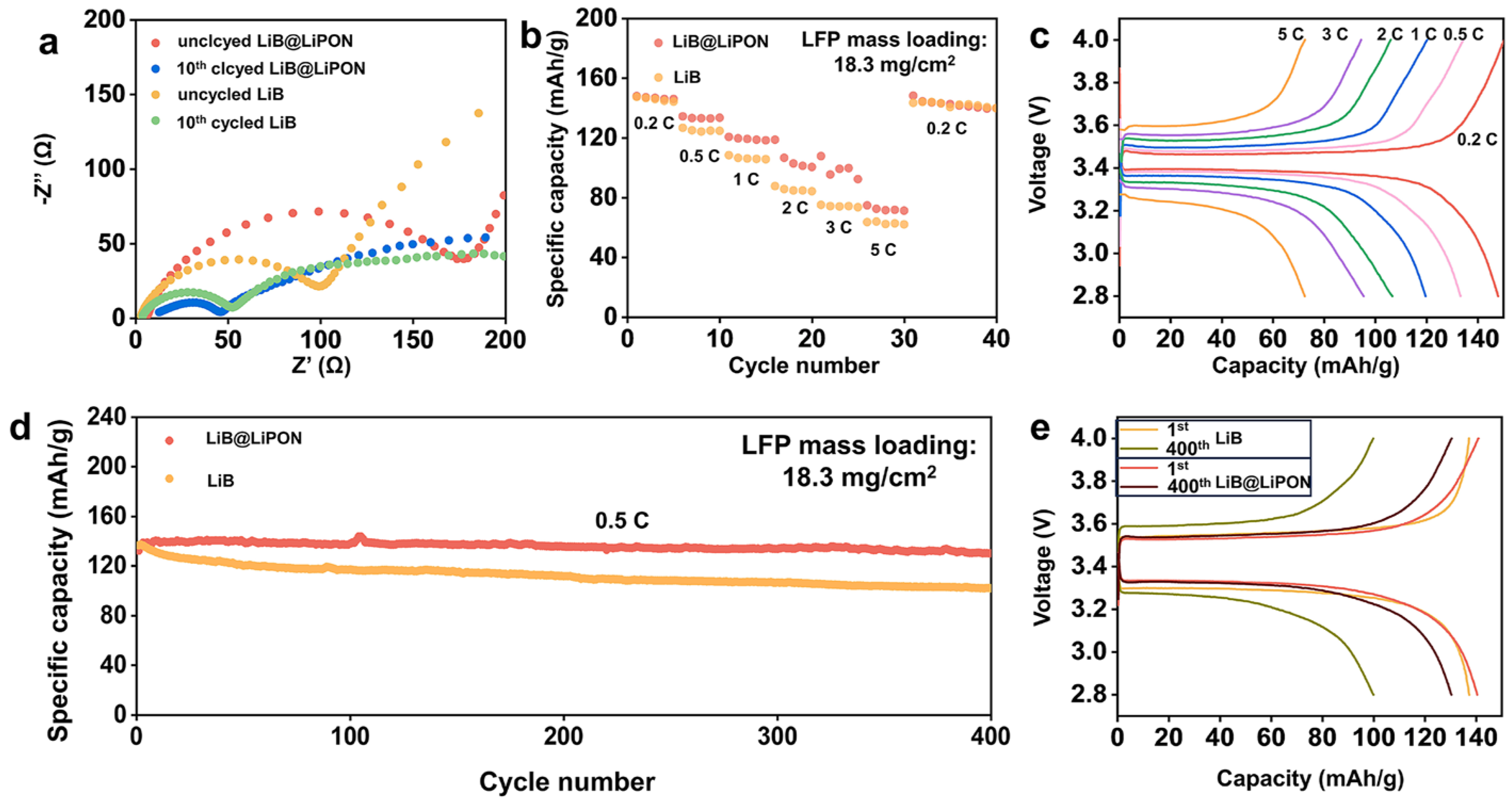
3.4. Electrochemical Performance of the LiB@LiPON Anode
3.5. Characterizations of the LiB@LiPON Anode after Cycling
3.6. Electrochemical Performances of the Full-Cells with the LiB@LiPON Anode
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Bao, Z.; Cui, Y.; Dufek, E.J.; Goodenough, J.B.; Khalifah, P.; Li, Q.; Liaw, B.Y.; Liu, P.; Manthiram, A.; et al. Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 2019, 4, 180–186. [Google Scholar] [CrossRef]
- Hu, M.; Tong, Z.; Cui, C.; Zhai, T.; Li, H. Facile, Atom-Economic, Chemical Thinning Strategy for Ultrathin Lithium Foils. Nano Lett. 2022, 22, 3047–3053. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.B.; Zhang, R.; Zhao, C.Z.; Zhang, Q. Toward Safe Lithium Metal Anode in Rechargeable Batteries: A Review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef]
- Niu, C.; Pan, H.; Xu, W.; Xiao, J.; Zhang, J.G.; Luo, L.; Wang, C.; Mei, D.; Meng, J.; Wang, X.; et al. Self-smoothing anode for achieving high-energy lithium metal batteries under realistic conditions. Nat. Nanotechnol. 2019, 14, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Huang, H.; Tong, J.; Li, W.; Liu, X.; Zhang, H.; Huang, H.; Zhou, W. Recent progress on the modification of high nickel content NCM: Coating, doping, and single crystallization. Interdiscip. Mater. 2022, 1, 330–353. [Google Scholar] [CrossRef]
- Huang, Y. The discovery of cathode materials for lithium-ion batteries from the view of interdisciplinarity. Interdiscip. Mater. 2022, 1, 323–329. [Google Scholar] [CrossRef]
- Kim, M.S.; Ryu, J.-H.; Deepika; Lim, Y.R.; Nah, I.W.; Lee, K.-R.; Archer, L.A.; Il Cho, W. Langmuir–Blodgett artificial solid-electrolyte interphases for practical lithium metal batteries. Nat. Energy 2018, 3, 889–898. [Google Scholar] [CrossRef]
- Cha, E.; Lee, H.; Choi, W. Improving Lithium-Metal Battery Performance under the Conditions of Lean Electrolyte through MoS2 Coating. ChemElectroChem 2020, 7, 890–892. [Google Scholar] [CrossRef]
- Shen, X.; Cheng, X.; Shi, P.; Huang, J.; Zhang, X.; Yan, C.; Li, T.; Zhang, Q. Lithium–matrix composite anode protected by a solid electrolyte layer for stable lithium metal batteries. J. Energy Chem. 2019, 37, 29–34. [Google Scholar] [CrossRef]
- Ghazi, Z.A.; Sun, Z.; Sun, C.; Qi, F.; An, B.; Li, F.; Cheng, H.M. Key Aspects of Lithium Metal Anodes for Lithium Metal Batteries. Small 2019, 15, e1900687. [Google Scholar] [CrossRef]
- Zhang, T.; Lu, H.; Yang, J.; Xu, Z.; Wang, J.; Hirano, S.I.; Guo, Y.; Liang, C. Stable Lithium Metal Anode Enabled by a Lithiophilic and Electron/Ion Conductive Framework. ACS Nano 2020, 14, 5618–5627. [Google Scholar] [CrossRef]
- Adhitama, E.; Bela, M.M.; Demelash, F.; Stan, M.C.; Winter, M.; Gomez-Martin, A.; Placke, T. On the Practical Applicability of the Li Metal-Based Thermal Evaporation Prelithiation Technique on Si Anodes for Lithium Ion Batteries. Adv. Energy Mater. 2023, 13, 2203256. [Google Scholar] [CrossRef]
- Ko, J.; Yoon, Y.S. Lithium fluoride layer formed by thermal evaporation for stable lithium metal anode in rechargeable batteries. Thin Solid Film. 2019, 673, 119–125. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, J.; Wen, X.; Zhao, Y.; Guo, J. Properties of Thin Lithium Metal Electrodes in Carbonate Electrolytes with Realistic Parameters. ACS Appl. Mater. Interfaces 2020, 12, 32863–32870. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Dai, A.; Fan, B.; Li, Y.; Shen, K.; Xiao, T.; Hou, G.; Cao, H.; Tao, X.; Tang, Y. Three-Dimensional Ordered Macro/Mesoporous Cu/Zn as a Lithiophilic Current Collector for Dendrite-Free Lithium Metal Anode. ACS Appl. Mater. Interfaces 2020, 12, 31542–31551. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yang, J.; Wu, C.; Huang, M.; Zhu, J.; Zeng, W.; Li, L.; Li, P.; Zhao, X.; Qiao, F.; et al. Stabilizing nucleation seeds in Li metal anode via ion-selective graphene oxide interfaces. Energy Storage Mater. 2023, 56, 572–581. [Google Scholar] [CrossRef]
- Guo, J.C.; Tan, S.J.; Zhang, C.H.; Wang, W.P.; Zhao, Y.; Wang, F.; Zhang, X.S.; Wen, R.; Zhang, Y.; Fan, M.; et al. A Self-Reconfigured, Dual-Layered Artificial Interphase Toward High-Current-Density Quasi-Solid-State Lithium Metal Batteries. Adv. Mater. 2023, 35, e2300350. [Google Scholar] [CrossRef]
- Kolesnikov, A.; Kolek, M.; Dohmann, J.F.; Horsthemke, F.; Börner, M.; Bieker, P.; Winter, M.; Stan, M.C. Galvanic Corrosion of Lithium-Powder-Based Electrodes. Adv. Energy Mater. 2020, 10, 2000017. [Google Scholar] [CrossRef]
- Qian, S.; Chen, H.; Zheng, M.; Zhu, Y.; Xing, C.; Tian, Y.; Yang, P.; Wu, Z.; Zhang, S. Complementary combination of lithium protection strategies for robust and longevous lithium metal batteries. Energy Storage Mater. 2023, 57, 229–248. [Google Scholar] [CrossRef]
- Hou, L.P.; Yao, N.; Xie, J.; Shi, P.; Sun, S.Y.; Jin, C.B.; Chen, C.M.; Liu, Q.B.; Li, B.Q.; Zhang, X.Q.; et al. Modification of Nitrate Ion Enables Stable Solid Electrolyte Interphase in Lithium Metal Batteries. Angew. Chem. Int. Ed. Engl. 2022, 61, e202201406. [Google Scholar] [CrossRef]
- Chang, C.; Yao, Y.; Li, R.; Guo, Z.H.; Li, L.; Pan, C.; Hu, W.; Pu, X. Self-healing single-ion-conductive artificial polymeric solid electrolyte interphases for stable lithium metal anodes. Nano Energy 2022, 93, 106871. [Google Scholar] [CrossRef]
- Chang, S.; Jin, X.; He, Q.; Liu, T.; Fang, J.; Shen, Z.; Li, Z.; Zhang, S.; Dahbi, M.; Alami, J.; et al. In Situ Formation of Polycyclic Aromatic Hydrocarbons as an Artificial Hybrid Layer for Lithium Metal Anodes. Nano Lett. 2022, 22, 263–270. [Google Scholar] [CrossRef]
- Zhao, Q.; Stalin, S.; Archer, L.A. Stabilizing metal battery anodes through the design of solid electrolyte interphases. Joule 2021, 5, 1119–1142. [Google Scholar] [CrossRef]
- Huang, Z.; Lai, J.-C.; Liao, S.-L.; Yu, Z.; Chen, Y.; Yu, W.; Gong, H.; Gao, X.; Yang, Y.; Qin, J.; et al. A salt-philic, solvent-phobic interfacial coating design for lithium metal electrodes. Nat. Energy 2023, 8, 577–585. [Google Scholar] [CrossRef]
- Lu, Q.; He, Y.B.; Yu, Q.; Li, B.; Kaneti, Y.V.; Yao, Y.; Kang, F.; Yang, Q.H. Dendrite-Free, High-Rate, Long-Life Lithium Metal Batteries with a 3D Cross-Linked Network Polymer Electrolyte. Adv. Mater. 2017, 29, 1604460. [Google Scholar] [CrossRef]
- Wu, J.; Rao, Z.; Liu, X.; Shen, Y.; Fang, C.; Yuan, L.; Li, Z.; Zhang, W.; Xie, X.; Huang, Y. Polycationic Polymer Layer for Air-Stable and Dendrite-Free Li Metal Anodes in Carbonate Electrolytes. Adv. Mater. 2021, 33, e2007428. [Google Scholar] [CrossRef]
- Zhu, B.; Jin, Y.; Hu, X.; Zheng, Q.; Zhang, S.; Wang, Q.; Zhu, J. Poly(dimethylsiloxane) Thin Film as a Stable Interfacial Layer for High-Performance Lithium-Metal Battery Anodes. Adv. Mater. 2017, 29, 1603755. [Google Scholar] [CrossRef] [PubMed]
- Stalin, S.; Chen, P.; Li, G.; Deng, Y.; Rouse, Z.; Cheng, Y.; Zhang, Z.; Biswal, P.; Jin, S.; Baker, S.P.; et al. Ultrathin zwitterionic polymeric interphases for stable lithium metal anodes. Matter 2021, 4, 3753–3773. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, H.; Hu, R.; Shen, Z.; Li, H.; Liu, J. Tough lithium-rich organic film via molecular layer deposition for highly stable lithium metal anode. J. Power Sources 2023, 555, 232395. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, J.; Huang, X.; Zhai, Z.; Tang, J.; You, J.; Shi, C.; Li, W.; Dai, P.; Zheng, W.; et al. Rigid and Flexible SEI Layer Formed Over a Cross-Linked Polymer for Enhanced Ultrathin Li Metal Anode Performance. Adv. Energy Mater. 2022, 12, 2103972. [Google Scholar] [CrossRef]
- Kang, D.; Hart, N.; Koh, J.; Ma, L.; Liang, W.; Xu, J.; Sardar, S.; Lemmon, J.P. Rearrange SEI with artificial organic layer for stable lithium metal anode. Energy Storage Mater. 2020, 24, 618–625. [Google Scholar] [CrossRef]
- Huang, K.; Song, S.; Xue, Z.; Niu, X.; Peng, X.; Xiang, Y. In-situ formation of LiF-rich solid-electrolyte interphases on 3D lithiophilic skeleton for stable lithium metal anode. Energy Storage Mater. 2023, 55, 301–311. [Google Scholar] [CrossRef]
- Ma, X.-X.; Shen, X.; Chen, X.; Fu, Z.-H.; Yao, N.; Zhang, R.; Zhang, Q. The Origin of Fast Lithium-Ion Transport in the Inorganic Solid Electrolyte Interphase on Lithium Metal Anodes. Small Struct. 2022, 3, 2200071. [Google Scholar] [CrossRef]
- Zheng, Y.; Xia, S.; Dong, F.; Sun, H.; Pang, Y.; Yang, J.; Huang, Y.; Zheng, S. High Performance Li Metal Anode Enabled by Robust Covalent Triazine Framework-Based Protective Layer. Adv. Funct. Mater. 2021, 31, 2006159. [Google Scholar] [CrossRef]
- Niu, J.; Wang, M.; Cao, T.; Cheng, X.; Wu, R.; Liu, H.; Zhang, Y.; Liu, X. Li metal coated with Li3PO4 film via atomic layer deposition as battery anode. Ionics 2021, 27, 2445–2454. [Google Scholar] [CrossRef]
- Chen, H.; Pei, A.; Lin, D.; Xie, J.; Yang, A.; Xu, J.; Lin, K.; Wang, J.; Wang, H.; Shi, F.; et al. Uniform High Ionic Conducting Lithium Sulfide Protection Layer for Stable Lithium Metal Anode. Adv. Energy Mater. 2019, 9, 1900858. [Google Scholar] [CrossRef]
- Liu, W.; Guo, R.; Zhan, B.; Shi, B.; Li, Y.; Pei, H.; Wang, Y.; Shi, W.; Fu, Z.; Xie, J. Artificial Solid Electrolyte Interphase Layer for Lithium Metal Anode in High-Energy Lithium Secondary Pouch Cells. ACS Appl. Energy Mater. 2018, 1, 1674–1679. [Google Scholar] [CrossRef]
- Li, N.W.; Yin, Y.X.; Yang, C.P.; Guo, Y.G. An Artificial Solid Electrolyte Interphase Layer for Stable Lithium Metal Anodes. Adv. Mater. 2016, 28, 1853–1858. [Google Scholar] [CrossRef]
- Kozen, A.C.; Pearse, A.J.; Lin, C.-F.; Noked, M.; Rubloff, G.W. Atomic Layer Deposition of the Solid Electrolyte LiPON. Chem. Mater. 2015, 27, 5324–5331. [Google Scholar] [CrossRef]
- Lacivita, V.; Westover, A.S.; Kercher, A.; Phillip, N.D.; Yang, G.; Veith, G.; Ceder, G.; Dudney, N.J. Resolving the Amorphous Structure of Lithium Phosphorus Oxynitride (Lipon). J. Am. Chem. Soc. 2018, 140, 11029–11038. [Google Scholar] [CrossRef]
- Li, L.; Liu, S.; Zhou, H.; Lei, Q.; Qian, K. All solid-state thin-film lithium-ion battery with Ti/ZnO/LiPON/LiMn2O4/Ti structure fabricated by magnetron sputtering. Mater. Lett. 2018, 216, 135–138. [Google Scholar] [CrossRef]
- Nowak, S.; Berkemeier, F.; Schmitz, G. Ultra-thin LiPON films—Fundamental properties and application in solid state thin film model batteries. J. Power Sources 2015, 275, 144–150. [Google Scholar] [CrossRef]
- Su, Y.; Falgenhauer, J.; Polity, A.; Leichtweiß, T.; Kronenberger, A.; Obel, J.; Zhou, S.; Schlettwein, D.; Janek, J.; Meyer, B.K. LiPON thin films with high nitrogen content for application in lithium batteries and electrochromic devices prepared by RF magnetron sputtering. Solid State Ion. 2015, 282, 63–69. [Google Scholar] [CrossRef]
- Wang, W.; Yue, X.; Meng, J.; Wang, J.; Wang, X.; Chen, H.; Shi, D.; Fu, J.; Zhou, Y.; Chen, J.; et al. Lithium phosphorus oxynitride as an efficient protective layer on lithium metal anodes for advanced lithium-sulfur batteries. Energy Storage Mater. 2019, 18, 414–422. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.-S.; Li, Q.-D.; Liu, Q.; Shi, P.-R.; Yu, J.; Lv, W.; Kang, F.; He, Y.-B.; Yang, Q.-H. A multifunctional artificial protective layer for producing an ultra-stable lithium metal anode in a commercial carbonate electrolyte. J. Mater. Chem. A 2021, 9, 7667–7674. [Google Scholar] [CrossRef]
- He, D.; Lu, J.; He, G.; Chen, H. Recent Advances in Solid-Electrolyte Interphase for Li Metal Anode. Front. Chem. 2022, 10, 916132. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, S.; Li, J.; Xie, B.; Ma, J.; Dong, S.; Cui, G. Robust Transport: An Artificial Solid Electrolyte Interphase Design for Anode-Free Lithium-Metal Batteries. Adv. Mater. 2023, 35, e2209404. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Chen, L.; Wang, T.; Hu, J.; Zhang, Q.; Zhang, H.; Nan, C.; Fan, L.Z. Self-Propagating Enabling High Lithium Metal Utilization Ratio Composite Anodes for Lithium Metal Batteries. Nano Lett. 2021, 21, 791–797. [Google Scholar] [CrossRef]
- Yang, Z.; Ruan, Q.; Xiong, Y.; Gu, X. Highly Stable Lithium Metal Anode Constructed by Three-Dimensional Lithiophilic Materials. Batteries 2022, 9, 30. [Google Scholar] [CrossRef]
- Hou, W.; Li, Y.; Li, S.; Liu, Z.; Ryan Galligan, P.; Xu, M.; Kim, J.-K.; Yuan, B.; Hu, R.; Luo, Z. Lithium dendrite suppression with Li3N-rich protection layer formation on 3D anode via ultra-low temperature nitriding. Chem. Eng. J. 2022, 441, 136067. [Google Scholar] [CrossRef]
- Wu, C.; Huang, H.; Lu, W.; Wei, Z.; Ni, X.; Sun, F.; Qing, P.; Liu, Z.; Ma, J.; Wei, W.; et al. Mg Doped Li-LiB Alloy with In Situ Formed Lithiophilic LiB Skeleton for Lithium Metal Batteries. Adv. Sci. 2020, 7, 1902643. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liang, Z.; Zhong, H.; Su, Y.; Wang, K.; Yang, Y. Bulk/Interfacial Synergetic Approaches Enable the Stable Anode for High Energy Density All-Solid-State Lithium–Sulfur Batteries. ACS Energy Lett. 2022, 7, 2761–2770. [Google Scholar] [CrossRef]
- Liu, Z.; Yin, J.; Meng, Z.; Chong, J.; Zhou, D. Electrochemical behavior and morphology of LiB compound anode materials. Trans. Nonferrous Met. Soc. China 2006, 16, 127–131. [Google Scholar] [CrossRef]
- Reyes Jiménez, A.; Nölle, R.; Wagner, R.; Hüsker, J.; Kolek, M.; Schmuch, R.; Winter, M.; Placke, T. A step towards understanding the beneficial influence of a LIPON-based artificial SEI on silicon thin film anodes in lithium-ion batteries. Nanoscale 2018, 10, 2128–2137. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dudney, N.J.; Nanda, J.; Liang, C. Artificial Solid Electrolyte Interphase to Address the Electrochemical Degradation of Silicon Electrodes. ACS Appl. Mater. Interfaces 2014, 6, 10083–10088. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mei, Y.; Gao, X.; Xiao, Y.; Tang, Z.; Xiang, X.; Deng, J. Inhibition of lithium dendrites by a LiPON interlayer with Li+ conductive and electron non-conductive dynamic properties based on Li7La3Zr2O12 solid electrolyte. J. Alloys Compd. 2024, 971, 172746. [Google Scholar] [CrossRef]
- Zhou, S.; Tian, R.; Wu, A.; Lin, L.; Huang, H. Fast Li+ migration in LiPON electrolytes doped by multi-valent Fe ions. J. Energy Chem. 2022, 75, 349–359. [Google Scholar] [CrossRef]
- Shrestha, S.; Kim, J.; Jeong, J.; Lee, H.J.; Kim, S.C.; Hah, H.J.; Oh, K.; Lee, S.-H. Effect of Amorphous LiPON Coating on Electrochemical Performance of LiNi0.8Mn0.1Co0.1O2 (NMC811) in All Solid-State Batteries. J. Electrochem. Soc. 2021, 168, 060537. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Q.; Yu, Y.; Zhu, Y.; Shen, C.; Gong, M.; Yan, K.; Xu, X. Constructing a LiPON Layer on a 3D Lithium Metal Anode as an Artificial Solid Electrolyte Interphase with Long-Term Stability. Batteries 2024, 10, 30. https://doi.org/10.3390/batteries10010030
Pan Q, Yu Y, Zhu Y, Shen C, Gong M, Yan K, Xu X. Constructing a LiPON Layer on a 3D Lithium Metal Anode as an Artificial Solid Electrolyte Interphase with Long-Term Stability. Batteries. 2024; 10(1):30. https://doi.org/10.3390/batteries10010030
Chicago/Turabian StylePan, Qianmu, Yongkun Yu, Yuxin Zhu, Chunli Shen, Minjian Gong, Kui Yan, and Xu Xu. 2024. "Constructing a LiPON Layer on a 3D Lithium Metal Anode as an Artificial Solid Electrolyte Interphase with Long-Term Stability" Batteries 10, no. 1: 30. https://doi.org/10.3390/batteries10010030



