The Impact of a Combined Battery Thermal Management and Safety System Utilizing Polymer Mini-Channel Cold Plates on the Thermal Runaway and Its Propagation
Abstract
:1. Introduction
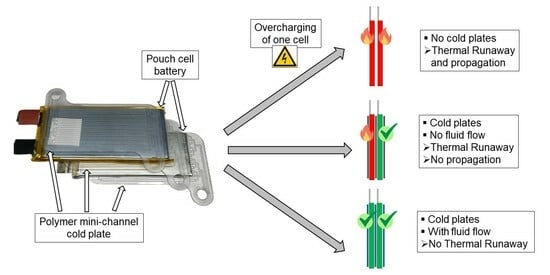
- Is there a difference in battery cell behavior between thermally conditioned and non-conditioned cells when subjected to overcharge-induced TR up to the cell fire? Is there a significant temporal impact on the process chain recognizable, especially before the TR and cell fire?
- Which heat dissipation rate is achievable using the polymer mini-channel cold plates?
- If the TR and cell fire are reached, is there a detectable preventive influence of the polymer cold plates on TRP to the adjacent cells?
2. Theory
2.1. Overcharge-Induced Thermal Runaway
2.2. Cold Plate Thermal Management
3. Materials and Methods
3.1. Battery Cell
3.2. Polymer Mini-Channel Cold Plates
3.3. Experimental Design
3.3.1. Structure of the Battery Assembly
3.3.2. Experimental Setup and Procedure
3.3.3. Measurement Plan
4. Results and Discussion
4.1. Overcharging without Thermal Conditioning
4.1.1. Temperature and Voltage Characteristics
4.1.2. Visualization of the Cell Fire
4.1.3. Evaluation of the Results
4.2. Overcharging with Thermal Conditioning
4.2.1. Temperature and Voltage Characteristics and Comparison
4.2.2. Evaluation of Results and Comparison
4.3. Overcharging with Malfunctioning Thermal Management
4.3.1. Temperature and Voltage Characteristics
4.3.2. Visualization of the Cell Fire with Thermal Management and Safety System
4.3.3. Impact of Polymer-Based Cold Plates as Thermal Barriers
4.4. Performance of the MCHS
5. Conclusions
6. Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Symbol | Name | Unit |
| Area | m2 | |
| Heat capacity | J/kg K | |
| Current | A | |
| Thermal conductivity | W/m K | |
| length | m | |
| Mass flow rate | kg/m3 | |
| Time | s | |
| Temperature | K | |
| Electric charge | Ah | |
| Heat | J | |
| Heat flow rate | W | |
| Electric resistance | ||
| Open circuit voltage | V | |
| Electric voltage | V |
Subscripts
| DC | Direct current |
| eff | effective |
| el | electric |
| Fluid,in | Fluid inlet |
| Fluid,out | Fluid outlet |
| i | internal |
| irrev | irreversible |
| layer,j | layer number j |
| OCV | Open Circuit Voltage |
| rev | reversible |
| parallel | |
| ⊥ | perpendicular |
Abbreviation
| Exp. | Experimental |
| Ind. | Induction |
| Max. | Maximum |
| Num. | Numerical |
| OC | Overcharged |
| Std. dev. | Standard deviation |
| Temp. | Temperature |
Acronyms
| ABS | Acrylonitrile butadiene styrene |
| CC | Constant Current |
| CCCV | Constant Current Constant Voltage |
| BEV | Battery electric vehicle |
| BTMS | Battery thermal management system |
| EIS | Electrochemical Impedance Spectroscopy |
| EUCAR | European Council for Automotive Research & Development |
| FT-IR | Fourier Transform-Infrared Spectrometer |
| MCHS | Mini-channel heat sink or heat source |
| NMC | Nickel-Manganese-Cobalt |
| OVGU | Otto-von-Guericke University |
| PCM | Phase Change Material |
| SEI | Solid Electrolyte Interphase |
| SOC | State of charge |
| TCs | Thermocouples |
| TIM | Thermal Interface Material |
| TPS | Transient Plane Source |
| TR | Thermal runaway |
| TRP | Thermal runaway propagation |
Appendix A
Appendix A.1. Equations
Appendix A.2. Figures and Tables


| Exp. | Ambient Temp. | Non-Linear Increase of Voltage/ Begin Stage II | Voltage Drop/ Begin Stage IV | TR/ Begin Stage V | Duration from Voltage Increase to Drop/ Stages II + III | Duration from Voltage Drop to TR/ Stage IV | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (°C) | (V) | Time (min) | SOC (%) | Upper (V) | Lower (V) | Diff. (V) | Time (min) | SOC (%) | Time (min) | SOC (%) | Time (min) | Time (min) | |
| #1 | 8 | 4.89 | 30.1 | 150 | 7.22 | 6.05 | 1.17 | 46.7 | 178 | 49.7 | 183 | 16.6 | 3.0 |
| #2 | 10 | 4.89 | 27.8 | 146 | 6.25 | 5.25 | 1.00 | 43.7 | 173 | 50.0 | 183 | 15.9 | 6.3 |
| #3 | 8 | 4.85 | 26.1 | 143 | 6.84 | 5.93 | 0.91 | 43.8 | 173 | 45.0 | 175 | 17.7 | 1.2 |
| #4 | 10 | 4.96 | 24.8 | 141 | 8.33 | 6.91 | 1.42 | 39.0 | 165 | 40.5 | 168 | 14.2 | 1.5 |
| #5 | 12 | 4.91 | 26.4 | 144 | 5.77 | 5.28 | 0.49 | 42.5 | 171 | 50.0 | 183 | 16.1 | 7.5 |
| #6 | 11 | 4.80 | 29.1 | 149 | 6.83 | 6.23 | 0.60 | 40.6 | 168 | 41.7 | 170 | 11.5 | 1.1 |
| #7 | 14 | 4.86 | 29.2 | 149 | 8.52 | 6.08 | 2.44 | 45.2 | 175 | 46.5 | 178 | 16.0 | 1.3 |
| #8 | 10 | 4.88 | 25.3 | 142 | 6.51 | 5.69 | 0.82 | 44.0 | 173 | 50.3 | 184 | 18.7 | 6.3 |
| #9 | 7 | 4.76 | 24.3 | 140 | 6.17 | 5.43 | 0.74 | 43.2 | 172 | 55.2 | 192 | 18.9 | 12.0 |


References
- Mallick, S.; Gayen, D. Thermal behaviour and thermal runaway propagation in lithium-ion battery systems—A critical review. J. Energy Storage 2023, 62, 106894. [Google Scholar] [CrossRef]
- Li, W.; Zhou, Y.; Zhang, H.; Tang, X. A Review on Battery Thermal Management for New Energy Vehicles. Energies 2023, 16, 4845. [Google Scholar] [CrossRef]
- Bandhauer, T.M.; Garimella, S.; Fuller, T.F. A Critical Review of Thermal Issues in Lithium-Ion Batteries. J. Electrochem. Soc. 2011, 158, R1. [Google Scholar] [CrossRef]
- Tomaszewska, A.; Chu, Z.; Feng, X.; O’Kane, S.; Liu, X.; Chen, J.; Ji, C.; Endler, E.; Li, R.; Liu, L.; et al. Lithium-ion battery fast charging: A review. eTransportation 2019, 1, 100011. [Google Scholar] [CrossRef]
- Newman, J.; Balsara, N.P. Electrochemical Systems, 4th ed.; Wiley: Hoboken, NJ, USA, 2021; ISBN 9781119514596. [Google Scholar]
- Gu, W.B.; Wang, C.Y. Thermal-Electrochemical Modeling of Battery Systems. J. Electrochem. Soc. 2000, 147, 2910. [Google Scholar] [CrossRef]
- Bernardi, D.; Pawlikowski, E.; Newman, J. A General Energy Balance for Battery Systems. J. Electrochem. Soc. 1985, 132, 5–12. [Google Scholar] [CrossRef]
- Heubner, C.; Schneider, M.; Lämmel, C.; Michaelis, A. Local Heat Generation in a Single Stack Lithium Ion Battery Cell. Electrochim. Acta 2015, 186, 404–412. [Google Scholar] [CrossRef]
- Hu, Y.; Choe, S.-Y.; Garrick, T.R. Measurement of heat generation rate and heat sources of pouch type Li-ion cells. Appl. Therm. Eng. 2021, 189, 116709. [Google Scholar] [CrossRef]
- Duan, J.; Tang, X.; Dai, H.; Yang, Y.; Wu, W.; Wei, X.; Huang, Y. Building Safe Lithium-Ion Batteries for Electric Vehicles: A Review. Electrochem. Energy Rev. 2020, 3, 1–42. [Google Scholar] [CrossRef]
- Kleiner, K.; Jakes, P.; Scharner, S.; Liebau, V.; Ehrenberg, H. Changes of the balancing between anode and cathode due to fatigue in commercial lithium-ion cells. J. Power Sources 2016, 317, 25–34. [Google Scholar] [CrossRef]
- Korthauer, R. Lithium-Ion Batteries: Basics and Applications; Springer: Berlin/Heidelberg, Germany, 2019; ISBN 9783662530719. [Google Scholar]
- Liu, J.; Huang, Z.; Sun, J.; Wang, Q. Heat generation and thermal runaway of lithium-ion battery induced by slight overcharging cycling. J. Power Sources 2022, 526, 231136. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, J.; Zhu, X.; Wang, H.; Huang, L.; Wang, Y.; Xu, S. Overcharge-to-thermal-runaway behavior and safety assessment of commercial lithium-ion cells with different cathode materials: A comparison study. J. Energy Chem. 2021, 55, 484–498. [Google Scholar] [CrossRef]
- Liu, B.; Jia, Y.; Yuan, C.; Wang, L.; Gao, X.; Yin, S.; Xu, J. Safety issues and mechanisms of lithium-ion battery cell upon mechanical abusive loading: A review. Energy Storage Mater. 2020, 24, 85–112. [Google Scholar] [CrossRef]
- Cai, T.; Valecha, P.; Tran, V.; Engle, B.; Stefanopoulou, A.; Siegel, J. Detection of Li-ion battery failure and venting with Carbon Dioxide sensors. eTransportation 2021, 7, 100100. [Google Scholar] [CrossRef]
- Wang, Q.; Ping, P.; Zhao, X.; Chu, G.; Sun, J.; Chen, C. Thermal runaway caused fire and explosion of lithium ion battery. J. Power Sources 2012, 208, 210–224. [Google Scholar] [CrossRef]
- Battery Pack Directed Venting System. Available online: https://patents.google.com/patent/US8557416B2/en (accessed on 12 November 2023).
- Vehicle Battery Pack Thermal Barrier. Available online: https://patents.google.com/patent/US8875828B2/en (accessed on 12 November 2023).
- Bai, Q.; Li, K.; Zan, J.; Liu, J.; Ou, J.; Liu, J. Influence of Insulation Material Thickness on Spread of Thermal Runaway in Battery Packs. Processes 2023, 11, 1321. [Google Scholar] [CrossRef]
- Arrhenius, S. XXXI. On the influence of carbonic acid in the air upon the temperature of the ground. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1896, 41, 237–276. [Google Scholar] [CrossRef]
- Jalkanen, K.; Karppinen, J.; Skogström, L.; Laurila, T.; Nisula, M.; Vuorilehto, K. Cycle aging of commercial NMC/graphite pouch cells at different temperatures. Appl. Energy 2015, 154, 160–172. [Google Scholar] [CrossRef]
- Hunt, I.A.; Zhao, Y.; Patel, Y.; Offer, J. Surface Cooling Causes Accelerated Degradation Compared to Tab Cooling for Lithium-Ion Pouch Cells. J. Electrochem. Soc. 2016, 163, A1846–A1852. [Google Scholar] [CrossRef]
- Gungor, S.; Gocmen, S.; Cetkin, E. A review on battery thermal management strategies in lithium-ion and post-lithium batteries for electric vehicles. J. Therm. Eng. 2023, 9, 1078–1099. [Google Scholar] [CrossRef]
- Liu, J.; Chen, H.; Huang, S.; Jiao, Y.; Chen, M. Recent Progress and Prospects in Liquid Cooling Thermal Management System for Lithium-Ion Batteries. Batteries 2023, 9, 400. [Google Scholar] [CrossRef]
- Xia, G.; Cao, L.; Bi, G. A review on battery thermal management in electric vehicle application. J. Power Sources 2017, 367, 90–105. [Google Scholar] [CrossRef]
- Shahid, S.; Agelin-Chaab, M. A review of thermal runaway prevention and mitigation strategies for lithium-ion batteries. Energy Convers. Manag. X 2022, 16, 100310. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A. Identification and Mitigation of Shortcomings in Direct and Indirect Liquid Cooling-Based Battery Thermal Management System. Energies 2023, 16, 3857. [Google Scholar] [CrossRef]
- Sarvar-Ardeh, S.; Rashidi, S.; Rafee, R.; Karimi, N. A review on the applications of micro-/mini-channels for battery thermal management. J. Therm. Anal. Calorim. 2023, 148, 7959–7979. [Google Scholar] [CrossRef]
- Wilke, S.; Schweitzer, B.; Khateeb, S.; Al-Hallaj, S. Preventing thermal runaway propagation in lithium ion battery packs using a phase change composite material: An experimental study. J. Power Sources 2017, 340, 51–59. [Google Scholar] [CrossRef]
- Jiaqiang, E.; Han, D.; Qiu, A.; Zhu, H.; Deng, Y.; Chen, J.; Zhao, X.; Zuo, W.; Wang, H.; Chen, J.; et al. Orthogonal experimental design of liquid-cooling structure on the cooling effect of a liquid-cooled battery thermal management system. Appl. Therm. Eng. 2018, 132, 508–520. [Google Scholar] [CrossRef]
- Tang, A.; Li, J.; Lou, L.; Shan, C.; Yuan, X. Optimization design and numerical study on water cooling structure for power lithium battery pack. Appl. Therm. Eng. 2019, 159, 113760. [Google Scholar] [CrossRef]
- Li, Q.; Shi, H.; Xie, G.; Xie, Z.; Liu, H. Parametric study and optimization on novel fork-type mini-channel network cooling plates for a Li-ion battery module under high discharge current rates. Int. J. Energy Res. 2021, 45, 17784–17804. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, Z.; Mei, W.; Jia, Z.; Song, L.; Wang, Q. Preventing effect of different interstitial materials on thermal runaway propagation of large-format lithium iron phosphate battery module. J. Energy Storage 2023, 63, 107082. [Google Scholar] [CrossRef]
- Feng, X.; He, X.; Ouyang, M.; Lu, L.; Wu, P.; Kulp, C.; Prasser, S. Thermal runaway propagation model for designing a safer battery pack with 25 Ah LiNixCoyMnzO2 large format lithium ion battery. Appl. Energy 2015, 154, 74–91. [Google Scholar] [CrossRef]
- Tsao, C.-W.; DeVoe, D.L. Bonding of thermoplastic polymer microfluidics. Microfluid Nanofluid 2009, 6, 1–16. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, J.; Guo, Z.; Wang, H.; Sun, Z.; Mei, X. Design and Optimization of a Novel Microchannel Battery Thermal Management System Based on Digital Twin. Energies 2022, 15, 1421. [Google Scholar] [CrossRef]
- Rui, X.; Feng, X.; Wang, H.; Yang, H.; Zhang, Y.; Wan, M.; Wei, Y.; Ouyang, M. Synergistic effect of insulation and liquid cooling on mitigating the thermal runaway propagation in lithium-ion battery module. Appl. Therm. Eng. 2021, 199, 117521. [Google Scholar] [CrossRef]
- Zhang, W.; Liang, Z.; Yin, X.; Ling, G. Avoiding thermal runaway propagation of lithium-ion battery modules by using hybrid phase change material and liquid cooling. Appl. Therm. Eng. 2021, 184, 116380. [Google Scholar] [CrossRef]
- Zhang, T.; Gao, Q.; Gu, Y.; Li, Y. Studies on thermal management of lithium-ion battery using non-metallic heat exchanger. Appl. Therm. Eng. 2021, 182, 116095. [Google Scholar] [CrossRef]
- Kshetrimayum, K.S.; Yoon, Y.-G.; Gye, H.-R.; Lee, C.-J. Preventing heat propagation and thermal runaway in electric vehicle battery modules using integrated PCM and micro-channel plate cooling system. Appl. Therm. Eng. 2019, 159, 113797. [Google Scholar] [CrossRef]
- Rao, Z.; Wang, Q.; Huang, C. Investigation of the thermal performance of phase change material/mini-channel coupled battery thermal management system. Appl. Energy 2016, 164, 659–669. [Google Scholar] [CrossRef]
- Ruiz, V.; Pfrang, A.; Kriston, A.; Omar, N.; van den Bossche, P.; Boon-Brett, L. A review of international abuse testing standards and regulations for lithium ion batteries in electric and hybrid electric vehicles. Renew. Sustain. Energy Rev. 2018, 81, 1427–1452. [Google Scholar] [CrossRef]
- Feng, X.; Zheng, S.; Ren, D.; He, X.; Wang, L.; Liu, X.; Li, M.; Ouyang, M. Key Characteristics for Thermal Runaway of Li-ion Batteries. Energy Procedia 2019, 158, 4684–4689. [Google Scholar] [CrossRef]
- Ren, D.; Feng, X.; Lu, L.; He, X.; Ouyang, M. Overcharge behaviors and failure mechanism of lithium-ion batteries under different test conditions. Appl. Energy 2019, 250, 323–332. [Google Scholar] [CrossRef]
- Essl, C.; Golubkov, A.W.; Fuchs, A. Comparing Different Thermal Runaway Triggers for Two Automotive Lithium-Ion Battery Cell Types. J. Electrochem. Soc. 2020, 167, 130542. [Google Scholar] [CrossRef]
- Larsson, F.; Mellander, B.-E. Abuse by External Heating, Overcharge and Short Circuiting of Commercial Lithium-Ion Battery Cells. J. Electrochem. Soc. 2014, 161, A1611–A1617. [Google Scholar] [CrossRef]
- Chen, Y. Recent advances of overcharge investigation of lithium-ion batteries. Ionics 2022, 28, 495–514. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, Z.; Wang, Z.; Zhang, L.; Zhu, X.; Dorrell, D.D. Thermal runaway behavior during overcharge for large-format Lithium-ion batteries with different packaging patterns. J. Energy Storage 2019, 25, 100811. [Google Scholar] [CrossRef]
- Noh, H.-J.; Youn, S.; Yoon, C.S.; Sun, Y.-K. Comparison of the structural and electrochemical properties of layered Li[NixCoyMnz]O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries. J. Power Sources 2013, 233, 121–130. [Google Scholar] [CrossRef]
- Liu, Q.; Du, C.; Shen, B.; Zuo, P.; Cheng, X.; Ma, Y.; Yin, G.; Gao, Y. Understanding undesirable anode lithium plating issues in lithium-ion batteries. RSC Adv. 2016, 6, 88683–88700. [Google Scholar] [CrossRef]
- Broussely, M.; Biensan, P.; Bonhomme, F.; Blanchard, P.; Herreyre, S.; Nechev, K.; Staniewicz, R.J. Main aging mechanisms in Li ion batteries. J. Power Sources 2005, 146, 90–96. [Google Scholar] [CrossRef]
- Vetter, J.; Novák, P.; Wagner, M.R.; Veit, C.; Möller, K.-C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing mechanisms in lithium-ion batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Bugga, R.V.; Smart, M.C. Lithium Plating Behavior in Lithium-Ion Cells. ECS Trans. 2010, 25, 241–252. [Google Scholar] [CrossRef]
- Kim, C.-S.; Jeong, K.M.; Kim, K.; Yi, C.-W. Effects of Capacity Ratios between Anode and Cathode on Electrochemical Properties for Lithium Polymer Batteries. Electrochim. Acta 2015, 155, 431–436. [Google Scholar] [CrossRef]
- Arai, J.; Nakahigashi, R.; Sugiyama, T. Study on Cycle Life of Lithium-Ion Batteries Using in Situ7 li Solid-State Nuclear Magnetic Resonance. ECS Trans. 2015, MA2015-03, 449. [Google Scholar] [CrossRef]
- Grimsmann, F.; Brauchle, F.; Gerbert, T.; Gruhle, A.; Parisi, J.; Knipper, M. Impact of different aging mechanisms on the thickness change and the quick-charge capability of lithium-ion cells. J. Energy Storage 2017, 14, 158–162. [Google Scholar] [CrossRef]
- Petzl, M.; Kasper, M.; Danzer, M.A. Lithium plating in a commercial lithium-ion battery—A low-temperature aging study. J. Power Sources 2015, 275, 799–807. [Google Scholar] [CrossRef]
- Hossain, S.; Kim, Y.-K.; Saleh, Y.; Loutfy, R. Overcharge studies of carbon fiber composite-based lithium-ion cells. J. Power Sources 2006, 161, 640–647. [Google Scholar] [CrossRef]
- Purushothaman, B.K.; Landau, U. Rapid Charging of Lithium-Ion Batteries Using Pulsed Currents A Theoretical Analysis. J. Electrochem. Soc. 2006, 153, A533–A542. [Google Scholar] [CrossRef]
- Arora, P.; Doyle, M.; White, R.E. Mathematical Modeling of the Lithium Deposition Overcharge Reaction in Lithium-Ion Batteries Using Carbon-Based Negative Electrodes. J. Electrochem. Soc. 1999, 146, 3543–3553. [Google Scholar] [CrossRef]
- Burns, J.C.; Stevens, D.A.; Dahn, J.R. In-Situ Detection of Lithium Plating Using High Precision Coulometry. J. Electrochem. Soc. 2015, 162, A959–A964. [Google Scholar] [CrossRef]
- Aurbach, D. A short review of failure mechanisms of lithium metal and lithiated graphite anodes in liquid electrolyte solutions. Solid State Ion. 2002, 148, 405–416. [Google Scholar] [CrossRef]
- An, S.J.; Li, J.; Daniel, C.; Mohanty, D.; Nagpure, S.; Wood, D.L. The state of understanding of the lithium-ion-battery graphite solid electrolyte interphase (SEI) and its relationship to formation cycling. Carbon 2016, 105, 52–76. [Google Scholar] [CrossRef]
- Palacín, M.R. Understanding ageing in Li-ion batteries: A chemical issue. Chem. Soc. Rev. 2018, 47, 4924–4933. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, R.; Key, B.; Chen, H.; Best, A.S.; Hollenkamp, A.F.; Grey, C.P. In situ NMR observation of the formation of metallic lithium microstructures in lithium batteries. Nat. Mater. 2010, 9, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Aurbach, D.; Zinigrad, E.; Teller, H.; Dan, P. Factors Which Limit the Cycle Life of Rechargeable Lithium (Metal) Batteries. J. Electrochem. Soc. 2000, 147, 1274. [Google Scholar] [CrossRef]
- Streich, D.; Guéguen, A.; Mendez, M.; Chesneau, F.; Novák, P.; Berg, E.J. Online Electrochemical Mass Spectrometry of High Energy Lithium Nickel Cobalt Manganese Oxide/Graphite Half- and Full-Cells with Ethylene Carbonate and Fluoroethylene Carbonate Based Electrolytes. J. Electrochem. Soc. 2016, 163, A964–A970. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Xiong, D.J.; Petibon, R.; Du, C.Y.; Dahn, J.R. Gas Evolution during Unwanted Lithium Plating in Li-Ion Cells with EC-Based or EC-Free Electrolytes. J. Electrochem. Soc. 2016, 163, A3010–A3015. [Google Scholar] [CrossRef]
- Feng, X.; Fang, M.; He, X.; Ouyang, M.; Lu, L.; Wang, H.; Zhang, M. Thermal runaway features of large format prismatic lithium ion battery using extended volume accelerating rate calorimetry. J. Power Sources 2014, 255, 294–301. [Google Scholar] [CrossRef]
- Fleischhammer, M.; Waldmann, T.; Bisle, G.; Hogg, B.-I.; Wohlfahrt-Mehrens, M. Interaction of cyclic ageing at high-rate and low temperatures and safety in lithium-ion batteries. J. Power Sources 2015, 274, 432–439. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhao, F.; Wang, W.; Zhao, Y.; Liang, Z.; Yan, D. Overcharge failure investigation of lithium-ion batteries. Electrochim. Acta 2015, 178, 682–688. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Z.; Wang, Y.; Wang, H.; Wang, C.; Tong, L.; Yi, M. Overcharge investigation of large format lithium-ion pouch cells with Li(Ni0.6Co0.2Mn0.2)O2 cathode for electric vehicles: Thermal runaway features and safety management method. Energy 2019, 169, 868–880. [Google Scholar] [CrossRef]
- Hestenes, J.C.; Sadowski, J.T.; May, R.; Marbella, L.E. Transition Metal Dissolution Mechanisms and Impacts on Electronic Conductivity in Composite LiNi0.5Mn1.5O4 Cathode Films. ACS Mater. Au 2023, 3, 88–101. [Google Scholar] [CrossRef]
- Yamaki, J.-I.; Tobishima, S.-I. Rechargeable lithium anodes. In Handbook of Battery Materials; Daniel, C., Besenhard, J.O., Besenhard, J.O., Eds.; Wiley: Weinheim, Germany, 2012; pp. 377–404. ISBN 9783527326952. [Google Scholar]
- Gireaud, L.; Grugeon, S.; Laruelle, S.; Yrieix, B.; Tarascon, J.-M. Lithium metal stripping/plating mechanisms studies: A metallurgical approach. Electrochem. Commun. 2006, 8, 1639–1649. [Google Scholar] [CrossRef]
- Aurbach, D.; Cohen, Y. Morphological Studies of Li Deposition Processes in LiAsF6/PC Solutions by In Situ Atomic Force Microscopy. J. Electrochem. Soc. 1997, 144, 3355–3360. [Google Scholar] [CrossRef]
- Ohsaki, T.; Kishi, T.; Kuboki, T.; Takami, N.; Shimura, N.; Sato, Y.; Sekino, M.; Satoh, A. Overcharge reaction of lithium-ion batteries. J. Power Sources 2005, 146, 97–100. [Google Scholar] [CrossRef]
- Nowak, S.; Winter, M. Review—Chemical Analysis for a Better Understanding of Aging and Degradation Mechanisms of Non-Aqueous Electrolytes for Lithium Ion Batteries: Method Development, Application and Lessons Learned. J. Electrochem. Soc. 2015, 162, A2500–A2508. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Z.; Yan, W. Identification and characteristic analysis of powder ejected from a lithium ion battery during thermal runaway at elevated temperatures. J. Hazard. Mater. 2020, 400, 123169. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Rafiz, K.; Liu, J.; Jin, Y.; Lin, J.Y.S. Effects of lithium dendrites on thermal runaway and gassing of LiFePO4 batteries. Sustain. Energy Fuels 2020, 4, 2342–2351. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Jiang, L.; Luo, Z.; Wu, T.; Shao, L.; Sun, J.; Liu, C.; Li, G.; Cao, K.; Wang, Q. Overcharge Behavior and Early Warning Analysis of LiNi0.5Co0.2Mn0.3O2/C Lithium-Ion Battery with High Capacity. J. Electrochem. Soc. 2019, 166, A1055–A1062. [Google Scholar] [CrossRef]
- Feng, X.; He, X.; Ouyang, M.; Wang, L.; Lu, L.; Ren, D.; Santhanagopalan, S. A Coupled Electrochemical-Thermal Failure Model for Predicting the Thermal Runaway Behavior of Lithium-Ion Batteries. J. Electrochem. Soc. 2018, 165, A3748–A3765. [Google Scholar] [CrossRef]
- Richter, F.; Kjelstrup, S.; Vie, P.J.; Burheim, O.S. Thermal conductivity and internal temperature profiles of Li-ion secondary batteries. J. Power Sources 2017, 359, 592–600. [Google Scholar] [CrossRef]
- Oehler, D.; Bender, J.; Seegert, P.; Wetzel, T. Investigation of the Effective Thermal Conductivity of Cell Stacks of Li-Ion Batteries. Energy Technol. 2021, 9, 2000722. [Google Scholar] [CrossRef]
- Wassiliadis, N.; Steinsträter, M.; Schreiber, M.; Rosner, P.; Nicoletti, L.; Schmid, F.; Ank, M.; Teichert, O.; Wildfeuer, L.; Schneider, J.; et al. Quantifying the state of the art of electric powertrains in battery electric vehicles: Range, efficiency, and lifetime from component to system level of the Volkswagen ID.3. eTransportation 2022, 12, 100167. [Google Scholar] [CrossRef]
- Kurmaev, R.K.; Struchkov, V.S.; Novak, V.V. Experience in the development of an effective thermal management system for the high-voltage battery of the vehicle. IOP Conf. Ser. Mater. Sci. Eng. 2020, 819, 12020. [Google Scholar] [CrossRef]
- Parrish, R.; Elankumaran, K.; Gandhi, M.; Nance, B.; Meehan, P.; Milburn, D.; Siddiqui, S.; Brenz, A. Voltec Battery Design and Manufacturing. In Proceedings of the SAE 2011 World Congress & Exhibition, Detroit, MI, USA, 12–14 April 2011; SAE Technical Paper. SAE International: Warrendale, PA, USA, 2011. [Google Scholar] [CrossRef]
- Schmidt, A.; Oehler, D.; Weber, A.; Wetzel, T.; Ivers-Tiffée, E. A multi scale multi domain model for large format lithium-ion batteries. Electrochim. Acta 2021, 393, 139046. [Google Scholar] [CrossRef]
- Darcovich, K.; MacNeil, D.D.; Recoskie, S.; Cadic, Q.; Ilinca, F. Comparison of cooling plate configurations for automotive battery pack thermal management. Appl. Therm. Eng. 2019, 155, 185–195. [Google Scholar] [CrossRef]
- Loges, A.; Herberger, S.; Seegert, P.; Wetzel, T. A study on specific heat capacities of Li-ion cell components and their influence on thermal management. J. Power Sources 2016, 336, 341–350. [Google Scholar] [CrossRef]
- Paarmann, S.; Schuld, K.; Wetzel, T. Inhomogeneous Aging in Lithium-Ion Batteries Caused by Temperature Effects. Energy Technol. 2022, 10, 2200384. [Google Scholar] [CrossRef]
- Werner, D.; Paarmann, S.; Wiebelt, A.; Wetzel, T. Inhomogeneous Temperature Distribution Affecting the Cyclic Aging of Li-Ion Cells. Part II: Analysis and Correlation. Batteries 2020, 6, 12. [Google Scholar] [CrossRef]
- Kong, L.; Aalund, R.; Alipour, M.; Stoliarov, S.I.; Pecht, M. Evaluating the Manufacturing Quality of Lithium Ion Pouch Batteries. J. Electrochem. Soc. 2022, 169, 40541. [Google Scholar] [CrossRef]
- IEC 62620; Secondary Cells and Batteries Containing Alkaline or Other Non-Acid Electrolytes—Secondary Lithium Cells and Batteries for Use in Industrial Applications. International Electrotechnical Commission (IEC): Geneva, Switzerland, 2014.
- Onda, K.; Kameyama, H.; Hanamoto, T.; Ito, K. Experimental Study on Heat Generation Behavior of Small Lithium-Ion Secondary Batteries. J. Electrochem. Soc. 2003, 150, A285. [Google Scholar] [CrossRef]
- Abdul-Quadir, Y.; Laurila, T.; Karppinen, J.; Jalkanen, K.; Vuorilehto, K.; Skogström, L.; Paulasto-Kröckel, M. Heat generation in high power prismatic Li-ion battery cell with LiMnNiCoO2 cathode material. Int. J. Energy Res. 2014, 38, 1424–1437. [Google Scholar] [CrossRef]
- Alihosseini, Y.; Zabetian Targhi, M.; Heyhat, M.M.; Ghorbani, N. Effect of a micro heat sink geometric design on thermo-hydraulic performance: A review. Appl. Therm. Eng. 2020, 170, 114974. [Google Scholar] [CrossRef]
- Liu, H.; Li, P. Even distribution/dividing of single-phase fluids by symmetric bifurcation of flow channels. Int. J. Heat Fluid Flow 2013, 40, 165–179. [Google Scholar] [CrossRef]
- Li, P.; Coopamah, D.; Ki, J.-P. Uniform distribution of species in fuel cells using a multiple flow bifurcation design. In Proceedings of the ASME 2008 6th International Conference on Fuel Cell Science, Engineering and Technology, Denver, CO, USA, 16–18 June 2008; pp. 897–902. [Google Scholar] [CrossRef]
- Kandlikar, S.G.; Grande, W.J. Evolution of Microchannel Flow Passages—Thermohydraulic Performance and Fabrication Technology. Heat Transf. Eng. 2003, 24, 3–17. [Google Scholar] [CrossRef]
- López Bonilla, J.; Fond, B.; Graichen, H.; Hamann, J.; Beyrau, F.; Boye, G. Thermal characterization of high-performance battery cells during charging and discharging using optical temperature measurement methods. In Proceedings of the FISITA World Congress 2021—Technical Programme, Prague, Czech Republic, 13–17 September 2021; ISBN 9781916025929. [Google Scholar]
- Graichen, H.-C.; Sauerhering, J.; Stamann, O.; Beyrau, F.; Boye, G. Influence of Adhesive Tapes as Thermal Interface Materials on the Thermal Load of a Compact Electrical Machine. WEVJ 2022, 13, 42. [Google Scholar] [CrossRef]
- Graichen, H.-C.; Sauerhering, J.; Reuber, F.; Dlugosch, A.; Beyrau, F.; Chinni, M.; Boye, G. Advanced thermal management optimization of a liquid cooled compact electrical engine with air gap winding. In Proceedings of the FISITA World Congress 2021—Technical Programme, Prague, Czech Republic, 13–17 September 2021; ISBN 9781916025929. [Google Scholar]
- Feng, X.; Ren, D.; He, X.; Ouyang, M. Mitigating Thermal Runaway of Lithium-Ion Batteries. Joule 2020, 4, 743–770. [Google Scholar] [CrossRef]
- Vorwerk, P.; Hahn, S.-K.; Daniel, C.; Krause, U.; Keutel, K. Detection of Critical Conditions in Pouch Cells Based on Their Expansion Behavior. Batteries 2022, 8, 42. [Google Scholar] [CrossRef]
- Liu, P.; Yang, L.; Xiao, B.; Wang, H.; Li, L.; Ye, S.; Li, Y.; Ren, X.; Ouyang, X.; Hu, J.; et al. Revealing Lithium Battery Gas Generation for Safer Practical Applications. Adv. Funct. Mater. 2022, 32, 2208586. [Google Scholar] [CrossRef]
- Lin, H.; Chua, D.; Salomon, M.; Shiao, H.-C.; Hendrickson, M.; Plichta, E.; Slane, S. Low-Temperature Behavior of Li-Ion Cells. Electrochem. Solid-State Lett. 2001, 4, A71. [Google Scholar] [CrossRef]
- Huang, C.-K.; Sakamoto, J.S.; Wolfenstine, J.; Surampudi, S. The Limits of Low-Temperature Performance of Li-Ion Cells. J. Electrochem. Soc. 2000, 147, 2893. [Google Scholar] [CrossRef]
- Yaqub, A.; Lee, Y.-J.; Hwang, M.J.; Pervez, S.A.; Farooq, U.; Choi, J.-H.; Kim, D.; Choi, H.-Y.; Cho, S.-B.; Doh, C.-H. Low temperature performance of graphite and LiNi0.6Co0.2Mn0.2O2 electrodes in Li-ion batteries. J. Mater. Sci. 2014, 49, 7707–7714. [Google Scholar] [CrossRef]
- Li, J.; Yuan, C.F.; Guo, Z.H.; Zhang, Z.A.; Lai, Y.Q.; Liu, J. Limiting factors for low-temperature performance of electrolytes in LiFePO4/Li and graphite/Li half cells. Electrochim. Acta 2012, 59, 69–74. [Google Scholar] [CrossRef]
- Misiewicz, C.; Lundström, R.; Ahmed, I.; Lacey, M.J.; Brant, W.R.; Berg, E.J. Online electrochemical mass spectrometry on large-format Li-ion cells. J. Power Sources 2023, 554, 232318. [Google Scholar] [CrossRef]
















| Source/ Year | Type of Examination | Type of Cell/ Configuration | Type of BTMS | Findings |
|---|---|---|---|---|
| [37]/ 2022 | Num./exp., Cycling/cooling | Pouch, 10 Ah/ module | Direct cooling by immersion, ABS micro-channel plates as flow separator/ turbulator between cells | Micro-channel plate improves cooling performance in narrow space by reducing the temperature gradient in the system |
| [38]/ 2021 | Num./exp., TR ind./cooling | Prismatic, 37 Ah/ module | Indirect bottom cooling by aluminum mini-channel cold plate and insulation by aerogel layers between cells | Singular liquid cooling or insulation fails to mitigate TRP; coupling of cooling and insulating with 1 mm layers avoids TRP |
| [39]/ 2021 | Num./exp., Cycling and TR ind./cooling | Prismatic, 25 Ah/ array of two cells | Indirect cooling aluminum plate-fin and fluid cooling channels, embedded in PCM layer between cells | Cycling: coupled cooling results in more uniform temperatures than singular techniques; TRP: induction by overheating only prevented by coupled cooling, not by singular techniques; PCM of high thermal conductivity lowers system temperature but increases TRP probability |
| [40]/ 2021 | Num./exp., Cycling/cooling | 18650 Cylindrical, Unknown cell capacity/ 50 Ah pack | Indirect cooling by polymer macro-channel tubes, connected at one side with the cells | Heat dissipation requirements met at low discharge rates; <5 °C temperature gradient in the system up to 2 C |
| [41]/ 2019 | Num., TR ind./cooling | 18650 Cylindrical, 1.5 Ah/ module | Indirect cooling by aluminum micro-channel cold plates embedded in a PCM matrix between battery rows | TRP induced by nail penetration is prevented by coupled cooling, not by singular techniques |
| [42]/ 2016 | Num., Cycling/cooling | Prismatic, unknown capacity/ Module | Indirect cooling by aluminum mini-channel tubes coupled into PCM layer between cells | Coupled cooling lowers maximum temperatures; decreasing temperatures with rising thermal conductivity of the PCM |
| Parameter | Value |
|---|---|
| Voltage limits (V) | 3–4.2 |
| Nominal capacity (Ah) | 12.5 |
| Weight (g) | 260 ± 15 |
| Energy density (Wh/kg) | 173 |
| Max. charge/discharge current | 1 C |
| Operating temperatures (°C) | 0~50 |
| Width/Length/Thickness (mm) | 88/192/7.6 |
| ) @ 1 kHz and 50% SOC | 3.5 |
| Cycle stability (-) | 2000 |
| Device | Model | Comment | |||
|---|---|---|---|---|---|
| Power Supply | Delta Elektronika SM15–200 D | Range: 0–15 V, 0–200 A | |||
| Refrigeration thermostat | Huber Ministat 240 | Range: −45–200 °C; Cooling power 550 W @ 0 °C | |||
| High-speed camera | Photron Fastcam Mini UX100 | 1.280 × 1024 pixels, 250 fps frame rate | |||
| Sensors | Model | Range | Max. uncertainty | ||
| Temperature | Sheath TC Type K, 1 m × 1 mm | Up to 1300 °C short-term | After calibration: ±0.2 K | ||
| Mass flow | Krohne Optimass 6400 C | 0–450 kg/h | ±0.05% of the value | ||
| Pressure | Yokogawa EJX110A | 5–1000 mbar | ±0.04% of the value | ||
| Voltage | Voltcraft VC 950 Datalogger | 0.001 mV–1000 V | ±0.03% of the value | ||
| Data acquisition system | |||||
| Rack: NI cDAQ-9174; Modules: NI-9203 Current Input, NI-9213 Thermocouple | |||||
| Test Scenario | Experiment Number | Fluid Inlet Temperature (°C) | Comment |
|---|---|---|---|
| Overcharging, no thermal conditioning | #1–9 | - | Base reference |
| Overcharging, thermal conditioning with MCHS | #1*–4* | 5, 20, 30 | Regular cooling |
| Overcharging, malfunctioning thermal conditioning with MCHS at critical conditions | #5*c | 40 | Interruption of overcharging |
| #6*c | 40 | Interruption of conditioning |
| Exp. #1–9 | Non-Linear Increase of Voltage/ Begin Stage II | Voltage Drop/ Begin Stage IV | TR/ Begin Stage V | Duration from Voltage Increase to Drop/ Stages II + III | Duration from Voltage Drop to TR/ Stage IV | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (V) | Time (min) | SOC (%) | Upper (V) | Lower (V) | Diff. (V) | Time (min) | SOC (%) | Time (min) | SOC (%) | Time (min) | Time (min) | |
| Mean | 4.87 | 27.0 | 145 | 6.94 | 5.87 | 1.07 | 43.2 | 172 | 47.7 | 179 | 16.2 | 4.5 |
| Std. Dev. | 0.06 | 2.0 | 3 | 0.89 | 0.50 | 0.55 | 2.2 | 4 | 4.4 | 7 | 2.3 | 3.6 |
| Exp. | Fluid Inlet Temp. | Starting Temp. of Cell | Non-Linear Increase of Voltage/ Begin Stage II | Voltage Drop/ Begin Stage IV | Duration from Voltage Increase to Drop/ Stage II + III | Max. Temp. OC Cell | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (°C) | (°C) | (V) | Time (min) | SOC (%) | Upper (V) | Lower (V) | Diff. (V) | Time (min) | SOC (%) | Time (min) | (°C) | |
| #1* | 5 | 9 | 4.91 | 23.7 | 139 | 5.83 | 5.30 | 0.53 | 42.9 | 172 | 19.2 | 59 |
| #2* | 5 | 6 | 4.75 | 22.7 | 138 | 5.79 | 5.45 | 0.34 | 43.7 | 173 | 20.9 | 55 |
| #3* | 20 | 17 | 4.76 | 28.6 | 148 | 5.52 | 5.05 | 0.47 | 43.0 | 172 | 14.5 | 47 |
| #4* | 30 | 25 | 4.73 | 28.2 | 148 | 5.67 | 4.82 | 0.85 | 42.8 | 171 | 14.7 | 48 |
| Exp. | Fluid Inlet Temp. | Starting Temp. of Cell | Non-Linear Increase of Voltage/ Begin Stage II | Voltage Drop/ Begin Stage IV | Duration from Voltage Increase to Drop/ Stage II + III | Max. Temp. OC Cell | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (°C) | (°C) | (V) | Time (min) | SOC (%) | Upper (V) | Lower (V) | Diff. (V) | Time (min) | SOC (%) | Time (min) | (°C) | |
| #5*c | 40 | 33 | 4.77 | 28.6 | 148 | 5.69 | 4.87 | 0.82 | 39.2 | 165 | 10.6 | 62 |
| #6*c | 40 | 34 | 5.01 | 33.9 | 156 | 5.99 | 5.02 | 0.97 | 44.0 | 173 | 10.1 | 59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graichen, H.-C.; Boye, G.; Sauerhering, J.; Köhler, F.; Beyrau, F. The Impact of a Combined Battery Thermal Management and Safety System Utilizing Polymer Mini-Channel Cold Plates on the Thermal Runaway and Its Propagation. Batteries 2024, 10, 1. https://doi.org/10.3390/batteries10010001
Graichen H-C, Boye G, Sauerhering J, Köhler F, Beyrau F. The Impact of a Combined Battery Thermal Management and Safety System Utilizing Polymer Mini-Channel Cold Plates on the Thermal Runaway and Its Propagation. Batteries. 2024; 10(1):1. https://doi.org/10.3390/batteries10010001
Chicago/Turabian StyleGraichen, Henrik-Christian, Gunar Boye, Jörg Sauerhering, Florian Köhler, and Frank Beyrau. 2024. "The Impact of a Combined Battery Thermal Management and Safety System Utilizing Polymer Mini-Channel Cold Plates on the Thermal Runaway and Its Propagation" Batteries 10, no. 1: 1. https://doi.org/10.3390/batteries10010001